A) \[sp-sp\]
B) \[p-p\]
C) \[s{{p}^{2}}-s{{p}^{2}}\]
D) \[s{{p}^{3}}-s{{p}^{3}}\]
Correct Answer: C
Solution :
The molecular orbital picture of benzene shows that in it all the six carbon atoms are \[s{{p}^{2}}\] hybridized. Out of these three \[s{{p}^{2}}\] hybrid orbitals of each C atom, two orbitals overlap with \[s{{p}^{2}}\] hybrid orbitals of adjacent C atoms to form six \[CC\] single bonds. The remaining \[s{{p}^{2}}\]orbital of each C atom overlaps with s-orbital of each hydrogen atom to form six C ? H single sigma bonds. Each C atom is now left with one unhybridisedp-orbital perpendicular to the plane of the ring.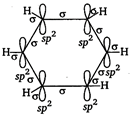
You need to login to perform this action.
You will be redirected in
3 sec
