question_answer 1) A ray of light travels from an optically denser to a rarer medium. The critical angle for the two media is C. The maximum possible deviation of the ray will be:
A)
2C
done
clear
B)
\[\frac{\pi }{2}-C\]
done
clear
C)
\[\pi \]-C
done
clear
D)
\[\pi -2C\]
done
clear
View Answer play_arrow
question_answer 2) When one of the slits of Youngs experiment is covered with a transparent sheet of thickness 4.8 mm, the central shifts to a position originally occupies by the 30th bright fringe. What should be the thickness of the sheet if the central fringe has to shift to the position occupied by 20th bright fringe?
A)
1.6 mm
done
clear
B)
3.8 mm
done
clear
C)
3.2 mm
done
clear
D)
7.6 mm
done
clear
View Answer play_arrow
question_answer 3) Light passes successively through two polarimeter tubes each of length 0.29 m. The first tube contains dextro rotatory solution of concentration \[60kg{{m}^{-3}}\]and specific rotation \[0.01\text{ }rad\text{ }{{m}^{2}}k{{g}^{-1}}.\] The second tube contains laevorotatory solution of concentration \[30\text{ }kg\text{ }{{m}^{-5}}\] and specific rotation \[0.02\text{ }rad\text{ }{{m}^{2}}k{{g}^{-1}}.\] The net rotation produced is:
A)
\[0{}^\circ \]
done
clear
B)
\[15{}^\circ \]
done
clear
C)
\[10{}^\circ \]
done
clear
D)
\[20{}^\circ \]
done
clear
View Answer play_arrow
question_answer 4) \[{{\upsilon }_{O}}\]and \[{{\upsilon }_{E}}\] represent the velocities, \[\mu O\,and\,\mu E\]the refractive indices of ordinary and extraordinary rays for a double refracting crystal. Then:
A)
\[\upsilon O\le {{v}_{E}},{{\mu }_{O}}\le {{\mu }_{E}},\]if the crystal is quartz
done
clear
B)
\[{{\upsilon }_{O}}\ge {{\upsilon }_{E}},{{\mu }_{O}}\le {{\mu }_{E}},\]if the crystal is calcite
done
clear
C)
\[{{\upsilon }_{0}}\ge {{v}_{E}},{{\mu }_{0}}\ge {{\mu }_{E}},\]if the crystal is quartz
done
clear
D)
\[{{\upsilon }_{0}}\le {{v}_{E}},{{\mu }_{0}}\ge {{\mu }_{E}},\]if the crystal is calcite
done
clear
View Answer play_arrow
question_answer 5) A racing car moving towards a cliff, sounds its horn. The driver observes that the sound reflected from the cliff has a pitch one octave higher than the actual sound of the horn. If v is the velocity of sound, then the velocity of the car is:
A)
\[\upsilon /2\]
done
clear
B)
\[\upsilon /\sqrt{2}\]
done
clear
C)
\[\upsilon /4\]
done
clear
D)
\[\upsilon /3\]
done
clear
View Answer play_arrow
question_answer 6) The de-Broglie wavelength of an electron in the first Bohr orbit is:
A)
equal to half the circumference of the first orbit
done
clear
B)
equal to one fourth the circumference of the first orbit
done
clear
C)
equal to the circumference of the first orbit
done
clear
D)
equal to twice the circumference of the first orbit
done
clear
View Answer play_arrow
question_answer 7) Out of the following statements which is not true?
A)
Infrared radiations arise due to minor electron transitions in atoms.
done
clear
B)
Infrared radiations are used for long distance photography.
done
clear
C)
Sun is the natural source of infrared radiation.
done
clear
D)
Infrared radiations are detected by using a bolometer.
done
clear
View Answer play_arrow
question_answer 8) In nuclear fission the percentage of mass converted into energy is about:
A)
0.01%
done
clear
B)
10%
done
clear
C)
1%
done
clear
D)
0.1%
done
clear
View Answer play_arrow
question_answer 9) If \[{{l}_{1}},{{l}_{2}},{{l}_{3}}\] are the lengths of the emitter, base and collector of a transistor, then:
A)
\[{{l}_{3}}<{{l}_{2}}<{{l}_{1}}\]
done
clear
B)
\[{{l}_{1}}={{l}_{2}}={{l}_{3}}\]
done
clear
C)
\[{{l}_{3}}<{{l}_{1}}<{{l}_{2}}\]
done
clear
D)
\[{{l}_{3}}<{{l}_{1}}<{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 10) When the conductivity of a semiconductor is only due to breaking of covalent bonds, the semiconductor is called:
A)
intrinsic
done
clear
B)
extrinsic
done
clear
C)
p - type
done
clear
D)
n - type
done
clear
View Answer play_arrow
question_answer 11) A very large number of balls are thrown vertically upwards in quick successions in such a way that the next ball is thrown when the previous one is at the maximum height. If the maximum height is 5 m, the number of balls thrown per minute is: (take\[g=10\text{ }m/{{s}^{2}}\])
A)
80
done
clear
B)
120
done
clear
C)
40
done
clear
D)
60
done
clear
View Answer play_arrow
question_answer 12) The light reflected by a plane mirror may form a real image:
A)
if the rays incident on the mirror are converging
done
clear
B)
if the rays incident on the mirror are diverging
done
clear
C)
under no circumstances
done
clear
D)
if the object is placed very close to the mirror
done
clear
View Answer play_arrow
question_answer 13)
A convex is made up of three different materials as shown in the figure. For a point object places on its axis, the number of images formed are:
A)
5
done
clear
B)
1
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 14) Light appears to travel in straight lines because:
A)
light consists of very small particles
done
clear
B)
the frequency of light is very small
done
clear
C)
the velocity of light is different for different colours
done
clear
D)
the wavelength of light is very small
done
clear
View Answer play_arrow
question_answer 15) In Youngs double slit experiment, the central bright fringe can be identified:
A)
as it a narrower than other bright fringes
done
clear
B)
by using white light instead of monochromatic light
done
clear
C)
as it has a greater intensity than other bright fringes
done
clear
D)
as it is wider than other bright fringes.
done
clear
View Answer play_arrow
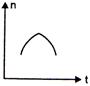
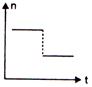
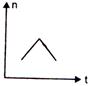
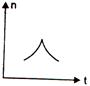
question_answer 16) A railway engine whistling at a constant frequency moves with a constant speed. It goes past a stationary observer beside the railway track. Which of the following graphs best represent the variation of frequency of the sound n heard by the observer with the time t?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 17) The tension of a stretched string is increased by 69%. In order to keep its frequency of vibration constant, its length must be increased by:
A)
30%
done
clear
B)
20%
done
clear
C)
69%
done
clear
D)
\[\sqrt{69}%\]
done
clear
View Answer play_arrow
question_answer 18) Under the same conditions of temperature and pressure, the velocity of sound in oxygen and hydrogen are \[{{\upsilon }_{O}}\]and \[{{\upsilon }_{H}}.\]Then:
A)
\[{{v}_{O}}=4\upsilon H\]
done
clear
B)
\[{{\upsilon }_{H}}=4{{\upsilon }_{O}}\]
done
clear
C)
\[{{\upsilon }_{O}}={{\upsilon }_{H}}\]
done
clear
D)
\[{{\upsilon }_{H}}=16{{v}_{O}}\]
done
clear
View Answer play_arrow
question_answer 19) 64 small drops of mercury, each of radius r and charge q coalesce to form a big drop. The ratio of the surface density of charge of each small drop with of the big drop is:
A)
64 : 1
done
clear
B)
1 : 64
done
clear
C)
1 : 4
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 20) Two capacitors of capacitances 3 \[\mu F\]and 6 \[\mu F\]are charged to a potential of 12 V each. They are now connected to each other, with the positive plate to each joined to the negative plate to the other. The potential difference across each will be:
A)
4 V
done
clear
B)
6 V
done
clear
C)
zero
done
clear
D)
3 V
done
clear
View Answer play_arrow
question_answer 21) The resultant of two forces, one double the other in magnitude, is perpendicular to the smaller of the two forces. The angle between the two forces is:
A)
\[120{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[150{}^\circ \]
done
clear
View Answer play_arrow
question_answer 22)
The load versus elongation graph for four wires of the same materials is shown in the figure.
A)
OC
done
clear
B)
OD
done
clear
C)
OA
done
clear
D)
OB
done
clear
View Answer play_arrow
question_answer 23) One kilogram of ice at 0°C is mixed with one kilogram of water at 80°C. The final temperature of the mixture is (take: specific heat of water \[=4200\text{ }J\text{ }k{{g}^{-1}}{{K}^{-1}},\] latent heat of ice\[=336\text{ }kJ\text{ }k{{g}^{-1}}\]).
A)
\[40{}^\circ C\]
done
clear
B)
\[60{}^\circ C\]
done
clear
C)
\[0{}^\circ C\]
done
clear
D)
\[50{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 24) A Carnots engine is made to work between \[200{}^\circ C\] and \[0{}^\circ C\] first and then between \[0{}^\circ C\] and \[-200{}^\circ C\]. The ratio of efficiencies of the engine in the two cases is:
A)
1 : 173
done
clear
B)
1.73 : 1
done
clear
C)
1 : 2
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 25) An object is placed 12 cm to the left of a converging lens of focal length 8 cm. Another converging lens of 6 cm focal length is placed at a distance of 30 cm. to the right of the first lens. The second lens will produce:
A)
a virtual enlarged image
done
clear
B)
no image
done
clear
C)
a real inverted image
done
clear
D)
a real enlarged image
done
clear
View Answer play_arrow
question_answer 26) The resistance of an incandescent lamp is:
A)
smaller when switched on
done
clear
B)
greater when switched off
done
clear
C)
the same whether it is switch off or switch on
done
clear
D)
greater when switched on
done
clear
View Answer play_arrow
question_answer 27) A superconductor exhibits perfect:
A)
ferromagnetism
done
clear
B)
ferrimagnetism
done
clear
C)
diamagnetism
done
clear
D)
paramagnetism
done
clear
View Answer play_arrow
question_answer 28) A magnet is dropped down an infinitely long vertical copper tube. Then:
A)
the magnet moves with continuously decreasing velocity and ultimately comes to rest
done
clear
B)
the magnet moves with continuously increasing velocity and ultimately acquires a constant terminal Velocity
done
clear
C)
the magnet moves with continuously increasing velocity and acceleration
done
clear
D)
the magnet moves with continuously increasing velocity but constant acceleration.
done
clear
View Answer play_arrow
question_answer 29) Whenever a hydrogen atom emits a photon in the Balmer series, it:
A)
may emit another photon in the Paschen series
done
clear
B)
need not emit any more photon
done
clear
C)
may emit another photon in the Balmer series
done
clear
D)
must emit another photon in the Lyman series.
done
clear
View Answer play_arrow
question_answer 30) The SI unit of radioactivity is:
A)
Rutherford
done
clear
B)
Roentgen
done
clear
C)
Becqueral
done
clear
D)
Curie
done
clear
View Answer play_arrow
question_answer 31) An ammeter and a voltmeter are joined in series to a cell. Their readings are A and V respectively. If a resistance is now joined in parallel with the voltmeter, then:
A)
A will decrease, V will increase
done
clear
B)
A will increase, V will decrease
done
clear
C)
both A and V will increase
done
clear
D)
both A and V will increase
done
clear
View Answer play_arrow
question_answer 32)
If each of the resistance of the network shown in figure is R, the equivalent resistance between A and B is:
A)
3 R
done
clear
B)
5 R
done
clear
C)
R/2
done
clear
D)
R
done
clear
View Answer play_arrow
question_answer 33) A cell supplies a current of 0.9 A through a 20. resistor and a current of 0.3 A through a 70. resistor. The internal resistance of the cell is:
A)
1.2\[\Omega \]
done
clear
B)
2.0\[\Omega \]
done
clear
C)
0.5\[\Omega \]
done
clear
D)
1.0\[\Omega \]
done
clear
View Answer play_arrow
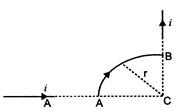
question_answer 34)
A wire carrying current i is shaped as shown. Section AB is a quarter circle of radius r. The magnetic field is directed:
A)
perpendicular to the plane of the paper and directed into the paper
done
clear
B)
at an angle \[\pi \]/4 to the plane of the paper
done
clear
C)
along the bisector of the angle ACB away from AB
done
clear
D)
along the bisector of ACB towards AB.
done
clear
View Answer play_arrow
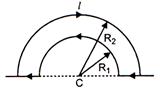
question_answer 35)
The wire loop formed by joining two semicircular sections of radii R1 and R2 and centre C, carries a current I as shown. The magnetic field at C has a magnitude:
A)
\[\frac{{{\mu }_{0}}I}{2}\left( \frac{1}{{{R}_{1}}}-\frac{1}{{{R}_{2}}} \right)\]
done
clear
B)
\[\frac{{{\mu }_{0}}I}{4}\left( \frac{1}{{{R}_{1}}}-\frac{1}{{{R}_{2}}} \right)\]
done
clear
C)
\[\frac{{{\mu }_{0}}I}{2}\left( \frac{1}{{{R}_{1}}}+\frac{1}{{{R}_{2}}} \right)\]
done
clear
D)
\[\frac{{{\mu }_{0}}I}{4}\left( \frac{1}{{{R}_{1}}}+\frac{1}{{{R}_{2}}} \right)\]
done
clear
View Answer play_arrow
question_answer 36) To increase both the resolving power and magnifying power of a telescope:
A)
the focal length of the objective has to be increased
done
clear
B)
both the focal length and aperture of the objective has to be increased
done
clear
C)
the wavelength of light has to be decreased
done
clear
D)
the aperture of the objective has to be increased
done
clear
View Answer play_arrow
question_answer 37) Forty one tuning forks are arranged in increasing order of frequencies such that every fork gives 5 beats with the next. The last fork has a frequency that is double the frequency of the first fork is:
A)
210
done
clear
B)
400
done
clear
C)
205
done
clear
D)
200
done
clear
View Answer play_arrow
question_answer 38) In a stationary wave all the particles:
A)
in the region between two nodes vibrate in same phase
done
clear
B)
on either side of a node vibrate in same phase
done
clear
C)
of the medium vibrate in same phase
done
clear
D)
in the region between two antinodes vibrate in same phase.
done
clear
View Answer play_arrow
question_answer 39) A cylindrical tube, open at both ends, has a fundamental frequency \[{{f}_{0}}\]in air. The tube is dipped vertically into water such that half of its length is inside water. The fundamental frequency of the air column now is:
A)
\[{{f}_{0}}\]
done
clear
B)
\[3{{f}_{0}}/4\]
done
clear
C)
\[2{{f}_{0}}\]
done
clear
D)
\[{{f}_{0}}/2\]
done
clear
View Answer play_arrow
question_answer 40) A man x can hear upto 10 kHz and another man y upto 2 kHz. A note of frequency 500 Hz is produced before them from a stretched string then both will hear sounds of:
A)
different pitch but same quality
done
clear
B)
same pitch but different quality
done
clear
C)
same pitch and same quality
done
clear
D)
different pitch and different quality.
done
clear
View Answer play_arrow
question_answer 41) When 100 V D.C. is applied across a coil, a current of 1 A flows through it. When 100 V A.C. of 50 Hz is applied to the same coil only 0-5 A flows. The inductance of the coil is:
A)
5.5 mH
done
clear
B)
0.55 mH
done
clear
C)
55 mH
done
clear
D)
0.55 H
done
clear
View Answer play_arrow
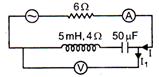
question_answer 42)
In the circuit shown in the figure, the A.C. source gives a voltage V = 20 cos (2000 t). Neglecting source resistance, the voltmeter and ammeter reading will be:
A)
1.68 V, 0.47 A
done
clear
B)
0 V, 0.47 A
done
clear
C)
5.6 V, 1.4 A
done
clear
D)
0V, 1.4 A
done
clear
View Answer play_arrow
question_answer 43) In the Bohr model of the hydrogen atom, let R, V and E represent the radius of the orbit, the speed of electron and the total energy of the electron respectively. Which of the following quantity is proportional to the quantum number n?
A)
E / V
done
clear
B)
R / E
done
clear
C)
V R
done
clear
D)
R E
done
clear
View Answer play_arrow
question_answer 44) In a sample of radioactive material, what percentage of the initial number of active nuclei will decay during one mean life?
A)
63 %
done
clear
B)
69.3 %
done
clear
C)
37 %
done
clear
D)
50 %
done
clear
View Answer play_arrow
question_answer 45) A caesium photocell, with a steady potential difference of 60V across it, is illuminated by a bright point source of light 50 cm away. When the same light is places 1 cm. away, the photoelectrons emitted from the cell:
A)
are half as numerous
done
clear
B)
are one quarter as numerous
done
clear
C)
each carry one quarter of their previous energy
done
clear
D)
each carry one quarter of their previous momentum.
done
clear
View Answer play_arrow
question_answer 46) The equation y = A cos2 \[\left[ 2\pi nt-2\pi \frac{x}{y} \right]\]represents a wave with:
A)
amplitude A/2, frequency 2n and wavelength \[\lambda \]
done
clear
B)
amplitude A/2, frequency In and wavelength \[\lambda /2\]
done
clear
C)
amplitude A, frequency n and wavelength \[\lambda \]
done
clear
D)
amplitude A, frequency 2n and wavelength 2\[\lambda \]
done
clear
View Answer play_arrow
question_answer 47) A light points fixed to one prong of a tuning fork touches a vertical plate. The fork is set vibrating and the plate is allowed to fall freely. If eight oscillations are counted when the plate falls through 10 cm, the frequency of the tuning fork is:
A)
280 Hz
done
clear
B)
360 Hz
done
clear
C)
56 Hz
done
clear
D)
560 Hz
done
clear
View Answer play_arrow
question_answer 48) Three point charges are placed at the comers of an equilateral triangle. Assuming only electrostatic forces are acting. Then the system:
A)
will be in equilibrium if the charges rotate about the centre of the triangle
done
clear
B)
can never be in equilibrium
done
clear
C)
will be in equilibrium if the charges have the same magnitudes but different signs
done
clear
D)
will be in equilibrium if the charges have different magnitude and different signs.
done
clear
View Answer play_arrow
question_answer 49) Two copper balls, each weighing 10 g are kept in air 10cm apart. If one electron from every 10 atoms is transferred from one ball to the other, the coulomb force between them is: (atomic weight of copper is 63-5)
A)
\[2.0\times {{10}^{4}}N\]
done
clear
B)
\[2.0\times {{10}^{10}}N\]
done
clear
C)
\[2.0\text{ }{{10}^{6}}N\]
done
clear
D)
\[2.0\times {{10}^{8}}N\]
done
clear
View Answer play_arrow
question_answer 50) What fraction of the energy drawn from the charging battery is stored in a capacitor?
A)
75%
done
clear
B)
100%
done
clear
C)
25%
done
clear
D)
50%
done
clear
View Answer play_arrow
question_answer 51) A projectile is moving at \[20\text{ }m{{s}^{-1}}\]at its highest point, where it breaks into equal parts due to an internal explosion. One part moves vertically up at \[30\text{ }m{{s}^{-1}}\]with respect to the ground. Then the other part will move at:
A)
\[20\text{ }m{{s}^{-1}}\]
done
clear
B)
10 \[\sqrt{31}m{{s}^{-1}}\]
done
clear
C)
\[50\text{ }m{{s}^{-1}}\]
done
clear
D)
\[30\text{ }m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 52) A body is projected vertically upwards from the surface of a planet of radius R with a velocity equal to half the escape velocity for that planet. The maximum height attained by the body is:
A)
R/2
done
clear
B)
R/3
done
clear
C)
R/5
done
clear
D)
R/4
done
clear
View Answer play_arrow
question_answer 53) From the top of a tower a stone is thrown up which reaches the ground in a time \[{{t}_{1}}.\]A second stone thrown down with the same speed reaches the ground in a time \[{{t}_{2}}.\]A third stone released from rest from the same between reaches the ground in a time t3 Then:
A)
\[\frac{1}{{{t}_{3}}}=\frac{1}{{{t}_{2}}}-\frac{1}{{{t}_{1}}}\]
done
clear
B)
\[{{t}_{3}}^{2}={{t}_{1}}^{2}-{{t}_{2}}^{2}\]
done
clear
C)
\[{{t}_{3}}=\frac{{{t}_{1}}+{{t}_{2}}}{2}\]
done
clear
D)
\[{{t}_{3}}=\sqrt{{{t}_{1}}{{t}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 54)
Five rods of same dimensions are arranged as shown in the figure. They
A)
\[{{k}_{1}}{{k}_{4}}={{k}_{2}}{{k}_{3}}\]
done
clear
B)
\[{{k}_{1}}={{k}_{4}}\,and\,{{k}_{2}}={{k}_{3}}\]
done
clear
C)
\[\frac{{{k}_{1}}}{{{k}_{4}}}=\frac{{{k}_{2}}}{{{k}_{3}}}\]
done
clear
D)
\[{{k}_{1}}{{k}_{2}}={{k}_{3}}{{k}_{4}}\]
done
clear
View Answer play_arrow
question_answer 55) The energy spectrum of a black body exhibits a maximum around a wavelength\[{{\lambda }_{0}}\]. The temperature of the black body is now changed such that the energy is maximum around a wavelength 3\[{{\lambda }_{0}}\]/4. The power radiated by the black body will now increase by a factor of:
A)
64/27
done
clear
B)
256/81
done
clear
C)
4/3
done
clear
D)
16/9
done
clear
View Answer play_arrow
question_answer 56) n identical bulbs, each designated to draw a power P from a certain voltage supply. The total power which they will draw is:
A)
P/n
done
clear
B)
P/n2
done
clear
C)
np
done
clear
D)
P
done
clear
View Answer play_arrow
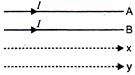
question_answer 57)
A and B are two conductors carrying a current I in the same direction, x and y are two electron beams moving in the same direction. Then there will be:
A)
attraction between A and B, repulsion between x and y
done
clear
B)
repulsion between A and B, attraction between x and y
done
clear
C)
attraction between A and B and x and y
done
clear
D)
repulsion between A and B and x and y
done
clear
View Answer play_arrow
question_answer 58)
The current-voltage graph for a given metallic conductor at two different temperature \[{{T}_{1}}\]and \[{{T}_{2}}\]are as shown in the figure. Then:
A)
\[{{T}_{1}}<{{T}_{2}}\]
done
clear
B)
nothing can be said about\[{{T}_{1}}\] and \[{{T}_{2}}\]
done
clear
C)
\[{{T}_{1}}={{T}_{2}}\]
done
clear
D)
\[{{T}_{1}}>{{T}_{2}}\]
done
clear
View Answer play_arrow
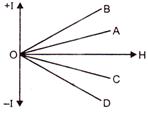
question_answer 59)
The variation of the intensity of magnetisation (I) with respect to the magnetising field (H) in a diamagnetic substance is described by the graph:
A)
OC
done
clear
B)
OD
done
clear
C)
OA
done
clear
D)
OB
done
clear
View Answer play_arrow
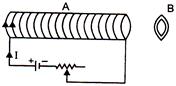
question_answer 60)
An aluminium ring B faces an electromagnet A. The current J through A can be altered. Then which of the following statements is correct?
A)
If I decreases, A will repel B
done
clear
B)
Whether I increases or decreases, B will not experience any force
done
clear
C)
If I increases, A will repel B
done
clear
D)
If I increases, A will attract B
done
clear
View Answer play_arrow
question_answer 61) Glacial acetic acid is obtained by:
A)
chemically separating acetic acid
done
clear
B)
treating vinegar with dehydrating agents
done
clear
C)
crystallising, separating and melting acetic acid
done
clear
D)
distilling vinegar.
done
clear
View Answer play_arrow
question_answer 62) An organic acid without a carboxylic acid group is:
A)
picric acid
done
clear
B)
oxalic acid
done
clear
C)
vinegar
done
clear
D)
ascorbic acid
done
clear
View Answer play_arrow
question_answer 63) An ester used as medicine is:
A)
ethyl benzoate
done
clear
B)
methyl salicylate
done
clear
C)
methyl acetate
done
clear
D)
ethyl acetate
done
clear
View Answer play_arrow
question_answer 64) Reaction of aniline with benzaldehyde is:
A)
polymerisation
done
clear
B)
condensation
done
clear
C)
addition
done
clear
D)
substitution
done
clear
View Answer play_arrow
question_answer 65) Halo alkane in the presence of alcoholic \[KOH\] undergoes:
A)
elimination
done
clear
B)
polymerisation
done
clear
C)
dimerisation
done
clear
D)
substitution
done
clear
View Answer play_arrow
question_answer 66) The simplest way to check whether a system is colloidal is by:
A)
electrodialysis
done
clear
B)
finding out particle size
done
clear
C)
Tyndall effect
done
clear
D)
Brownian movement.
done
clear
View Answer play_arrow
question_answer 67) An example for a strong electrolyte is :
A)
urea
done
clear
B)
ammonium hydroxide
done
clear
C)
sugar
done
clear
D)
sodium acetate
done
clear
View Answer play_arrow
question_answer 68) An example of a salt that will not hydrolyse is:
A)
\[C{{H}_{3}}COON{{H}_{4}}\]
done
clear
B)
\[C{{H}_{3}}COOK\]
done
clear
C)
\[N{{H}_{4}}Cl\]
done
clear
D)
\[KCl\]
done
clear
View Answer play_arrow
question_answer 69) \[{{C}^{14}}\] is:
A)
a natural non-radioactive isotope
done
clear
B)
an artificial non-radioactive isotope
done
clear
C)
an artificial radioactive isotope
done
clear
D)
a natural radioactive isotope.
done
clear
View Answer play_arrow
question_answer 70) A cuprous ore among the following is:
A)
cuprite
done
clear
B)
malachite
done
clear
C)
chalcopyrites
done
clear
D)
azurite
done
clear
View Answer play_arrow
question_answer 71) van der Waals equation for n moles of a gas is:
A)
\[\left( P+\frac{{{n}^{2}}a}{{{V}^{2}}} \right)(V-nb)=RT\]
done
clear
B)
\[\left( P+\frac{na}{{{V}^{2}}} \right)(V-nb)=RT\]
done
clear
C)
\[\left( P+\frac{a}{{{V}^{2}}} \right)(V-nb)=nRT\]
done
clear
D)
\[\left( P+\frac{{{n}^{2}}a}{{{V}^{2}}} \right)(V-b)=RT\]
done
clear
View Answer play_arrow
question_answer 72) Smallest among these species is:
A)
lithium
done
clear
B)
lithium ion
done
clear
C)
hydrogen
done
clear
D)
helium
done
clear
View Answer play_arrow
question_answer 73) Sodium chloride is an ionic compound where as hydrpgen chloride gas is mainly convalent because:
A)
electronegativity difference in the case of hydrogen is less than \[2.1\].
done
clear
B)
hydrogen chloride is a gas
done
clear
C)
hydrogen is a non-metal
done
clear
D)
sodium is reactive.
done
clear
View Answer play_arrow
question_answer 74) Covalent compounds have low melting -point because:
A)
covalent molecules are held by weak van der Waals force of attraction
done
clear
B)
covalent bond is less exothermic
done
clear
C)
covalent bond is weaker than ionic bond
done
clear
D)
covalent molecules have definite shape.
done
clear
View Answer play_arrow
question_answer 75) A neutral fertilizer among these compounds is:
A)
ammonium nitrate
done
clear
B)
urea
done
clear
C)
CAN
done
clear
D)
ammonium sulphate
done
clear
View Answer play_arrow
question_answer 76) A condensation polymer among the following polymers is:
A)
teflon
done
clear
B)
polysterene
done
clear
C)
PVC
done
clear
D)
decron
done
clear
View Answer play_arrow
question_answer 77) A compound that undergoes bromination easily is:
A)
toluene
done
clear
B)
benzoic acid
done
clear
C)
phenol
done
clear
D)
benzene
done
clear
View Answer play_arrow
question_answer 78) A sugar that is not a disacharide among the following is:
A)
galactose
done
clear
B)
lactose
done
clear
C)
maltose
done
clear
D)
sucrose
done
clear
View Answer play_arrow
question_answer 79) Drying soil invariably contains :
A)
butyric acid
done
clear
B)
stearic acid
done
clear
C)
lauric acid
done
clear
D)
linoleic acid
done
clear
View Answer play_arrow
question_answer 80) Hetrocyclic amino acid among these compound is:
A)
lysine
done
clear
B)
tyrosine
done
clear
C)
proline
done
clear
D)
serine
done
clear
View Answer play_arrow
question_answer 81) Aluminium oxide is not reduced by chemical reactions since:
A)
reducing agent contaminate
done
clear
B)
the process pollute the environment
done
clear
C)
Aluminium oxide is highly stable
done
clear
D)
Aluminium oxide is reactive.
done
clear
View Answer play_arrow
question_answer 82) Iron loses magnetic property at:
A)
melting point
done
clear
B)
1000 K
done
clear
C)
Curie point
done
clear
D)
boiling point
done
clear
View Answer play_arrow
question_answer 83) An example for a double salt is:
A)
potassium ferricyanide
done
clear
B)
cobalt hexamine chloride
done
clear
C)
cuprous sulphate
done
clear
D)
Mohrs salt.
done
clear
View Answer play_arrow
question_answer 84) The set of compounds in which the reactivity of halogen atom in the ascending order is:
A)
vinyl chloride, chloroethane, chlorobenzene
done
clear
B)
vinyl chloride, chlorobenzene, chloroethane
done
clear
C)
chloroethane, chlorobenzene, vinyl chloride
done
clear
D)
chlorobenzene, vinyl chloride, chloroethane.
done
clear
View Answer play_arrow
question_answer 85) Methanal is:
A)
\[C{{H}_{3}}CHO\]
done
clear
B)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{2}}OH\]
done
clear
D)
\[HCHO\]
done
clear
View Answer play_arrow
question_answer 86) In the case of auto catalysis:
A)
product catalysis
done
clear
B)
solvent catalysis
done
clear
C)
reactant catalysis
done
clear
D)
heat produced in the reaction catalysis.
done
clear
View Answer play_arrow
question_answer 87) Which of the following statements is true?
A)
\[\Delta E\] is always greater than \[\Delta H\]
done
clear
B)
\[\Delta E\] is always less than \[\Delta H\]
done
clear
C)
\[\Delta E\] may be lesser or greater or equal to \[\Delta H\]
done
clear
D)
\[\Delta E\] is always proportional to \[\Delta H\].
done
clear
View Answer play_arrow
question_answer 88) Order of reaction is decided by :
A)
molecularity
done
clear
B)
pressure
done
clear
C)
temperature
done
clear
D)
mechanism of reaction as well as relative concentration of reactants
done
clear
View Answer play_arrow
question_answer 89) Half-life of a reaction is found to be inversely proportional to the cube of initial concentration. The order of reaction is:
A)
\[5\]
done
clear
B)
\[2\]
done
clear
C)
\[4\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 90) Which among the following statement is false?
A)
The adsorption may be monolayered or multilayered.
done
clear
B)
Particle size of adsorbent will not affect the amount of adsorption.
done
clear
C)
Increase of pressure increases amount of adsorption.
done
clear
D)
Increase of temperature may decrease the amount of adsorption.
done
clear
View Answer play_arrow
question_answer 91) Purine derivative among the following bases is:
A)
cytosine
done
clear
B)
guanine
done
clear
C)
uracil
done
clear
D)
thymine.
done
clear
View Answer play_arrow
question_answer 92) A drug that is antipyretic as well as analgesic is:
A)
penicillin
done
clear
B)
chloroquin
done
clear
C)
para acetamidophenol
done
clear
D)
chloropromazine hydrochloride
done
clear
View Answer play_arrow
question_answer 93) For a /-orbital the values of m are:
A)
\[-1,\,0,\,+1\]
done
clear
B)
\[0,+1,+2,+3\]
done
clear
C)
\[-2,-1,0,+1,+2\]
done
clear
D)
\[-3,-2,-1,0,+1,+2,+3\]
done
clear
View Answer play_arrow
question_answer 94) Chloride ion and potassium ion are isoelectronic. Then:
A)
potassium ion is relatively bigger
done
clear
B)
depends on the other cation and anion
done
clear
C)
their size are same
done
clear
D)
chloride ion is bigger than potassium ion.
done
clear
View Answer play_arrow
question_answer 95) In a reversible reaction a catalyst will affect:
A)
the rate of forward reaction and reverse reaction
done
clear
B)
neither the forward reaction nor the rate of reverse reaction
done
clear
C)
the rate of forward reaction
done
clear
D)
the rate of reverse reaction.
done
clear
View Answer play_arrow
question_answer 96) Pentavalence in phosphorous is more stable when compared to that of nitrogen even through they belong to same group is due to:
A)
reactivity of phosphorous
done
clear
B)
inert nature of nitrogen
done
clear
C)
dissimilar electronic configuration
done
clear
D)
larger size of phosphorous atom.
done
clear
View Answer play_arrow
question_answer 97) Aqueous solutions of hydrogen sulphide and sulphurdioxide when mixed together, yield:
A)
sulphur trioxide and water
done
clear
B)
hydrogen and sulphurous acid
done
clear
C)
sulphur and water
done
clear
D)
hydrogen peroxide and sulphur.
done
clear
View Answer play_arrow
question_answer 98) A salt producing hydrocarbon among these compounds is:
A)
ethane
done
clear
B)
methane
done
clear
C)
ethene
done
clear
D)
ethyne.
done
clear
View Answer play_arrow
question_answer 99) Octane number is zero for:
A)
n-octane
done
clear
B)
iso- ocatne
done
clear
C)
n-heptane
done
clear
D)
iso-heptane
done
clear
View Answer play_arrow
question_answer 100) The intensive property among these quantities is:
A)
enthalpy
done
clear
B)
mass/volume
done
clear
C)
mass
done
clear
D)
volume
done
clear
View Answer play_arrow
question_answer 101) 30g of Mg and 30g of oxygen are reacted and the residual mixture contains:
A)
\[45g\]of \[MgO\]and \[15g\]of \[{{O}_{2}}\]
done
clear
B)
\[50g\]of \[MgO\] and \[10g\]of \[{{O}_{2}}\]
done
clear
C)
\[60g\] of \[MgO\] only
done
clear
D)
\[40g\] of \[MgO\] and \[20g\] of \[{{O}_{2}}\].
done
clear
View Answer play_arrow
question_answer 102) \[3g\]of oxide of a metal is converted to chloride completely and it yielded 5 g of chloride. The equivalent weight of metal is:
A)
\[12\]
done
clear
B)
\[20\]
done
clear
C)
\[33.25\]
done
clear
D)
\[2.325\]
done
clear
View Answer play_arrow
question_answer 103) \[20ml\]of \[0.25\text{ }N\]strong acid and \[30ml\text{ }0.2\text{ }N\]of strong base are mixed; the resulting solution is:
A)
\[0.02N\]acidic
done
clear
B)
\[0.025\]N basic
done
clear
C)
\[0.02\text{ }N\]basic
done
clear
D)
\[0.025\text{ }N\]acidic
done
clear
View Answer play_arrow
question_answer 104) The rate of forward reaction is two times that of reverse reaction at a given temperature and identical concentration. \[{{K}_{equilibrium}}\]is:
A)
\[2.5\]
done
clear
B)
\[2.0\]
done
clear
C)
\[0.5\]
done
clear
D)
\[1.5\]
done
clear
View Answer play_arrow
question_answer 105) A chemical reaction was carried out at 300 K and 280 K. The rate constants were found to be \[{{K}_{1}}\] and \[{{K}_{2}}\]respectively. Then:
A)
\[{{K}_{2}}=0.25{{K}_{1}}\]
done
clear
B)
\[{{K}_{2}}=0.5{{K}_{1}}\]
done
clear
C)
\[{{K}_{2}}=4{{K}_{1}}\]
done
clear
D)
\[{{K}_{2}}=2{{K}_{1}}\]
done
clear
View Answer play_arrow
question_answer 106) Ammonium ion is:
A)
neither an acid nor base
done
clear
B)
both an acid and a base
done
clear
C)
a conjugate acid
done
clear
D)
a conjugate base
done
clear
View Answer play_arrow
question_answer 107) Water is a:
A)
amphoteric acid
done
clear
B)
aprotic solvent
done
clear
C)
protophobic solvent
done
clear
D)
photophilic solvent
done
clear
View Answer play_arrow
question_answer 108) A smuggler could not carry gold by depositing iron on gold surface since:
A)
gold has higher standard reduction potential than iron
done
clear
B)
gold has lower standard reduction potential than iron
done
clear
C)
gold is denser
done
clear
D)
iron rusts.
done
clear
View Answer play_arrow
question_answer 109) A is an aqueous acid; B is an aqueous base. They are diluted separately, then:
A)
pH of A decreases and pH of B increases
done
clear
B)
pH of A increases and pH of B decreases till pH in each case is 7
done
clear
C)
pH of A and B increase
done
clear
D)
pH of B and A decrease.
done
clear
View Answer play_arrow
question_answer 110) \[_{Z}{{X}^{M}}{{+}_{2}}H{{e}^{4}}{{\to }_{15}}{{P}^{30}}+o{{n}^{1}},\]Then:
A)
\[Z=12,\text{ }M=27\]
done
clear
B)
\[Z=13,\text{ }M=27\]
done
clear
C)
\[Z=12,M=17\]
done
clear
D)
\[Z=13,M=28\]
done
clear
View Answer play_arrow
question_answer 111) Kinetic theory of gases presumes that collision between the molecules to be perfectly elastic because:
A)
collisions will not split the molecule
done
clear
B)
the molecules are tiny
done
clear
C)
the molecules are rigid
done
clear
D)
the temperature remains constant irrespective of collisions.
done
clear
View Answer play_arrow
question_answer 112) Bio-gas production is more useful when compared to the direct use of dung because:
A)
both fuel value and fertilizer value are effectively utilized
done
clear
B)
production of bio-gas involves less lab our
done
clear
C)
fuel is quickly produced
done
clear
D)
the fertilizer produced is a fluid.
done
clear
View Answer play_arrow
question_answer 113) Compounds with high heat of formation are less stable because:
A)
high temperature is required to synthesise them
done
clear
B)
molecules of such compounds are distorted
done
clear
C)
it is difficult to synthesise them
done
clear
D)
energy rich state leads to instability.
done
clear
View Answer play_arrow
question_answer 114) In the case of a radio isotope the value of \[{{T}_{1/2}}\] and \[\lambda \] are identical in magnitude. The value is:
A)
\[0.693\]
done
clear
B)
\[{{(0.693)}^{1/2}}\]
done
clear
C)
\[1/0.693\]
done
clear
D)
\[{{(0.693)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 115) Heat of neutralisation of weak acid and strong base is less than the heat of neutralisation of strong acid and strong base due to:
A)
energy has to be spent for the total dissociation of weak acid
done
clear
B)
salt of weak acid and strong base is not stable
done
clear
C)
incomplete dissociation of weak acid
done
clear
D)
incomplete neutralisation of weak acid.
done
clear
View Answer play_arrow
question_answer 116) A radio isotope will not emit :
A)
gamma and alpha rays simultaneously
done
clear
B)
gamma rays only
done
clear
C)
alpha and beta rays simultaneously
done
clear
D)
beta and gamma rays simultaneously.
done
clear
View Answer play_arrow
question_answer 117) Heat treatment alters the properties of steel due to:
A)
chemical reaction on heating
done
clear
B)
partial rusting
done
clear
C)
change in the residual energy
done
clear
D)
change in the lattice structure due to differential rate of cooling.
done
clear
View Answer play_arrow
question_answer 118) Chemical bond implies:
A)
attraction and repulsion
done
clear
B)
attraction and repulsion balanced at a particular distance
done
clear
C)
attraction
done
clear
D)
repulsion.
done
clear
View Answer play_arrow
question_answer 119) Grignard reagent is not prepared in aqueous medium but prepared in either medium. Because:
A)
the reagent reacts with water
done
clear
B)
the reagent becomes inactive in water
done
clear
C)
it is insoluble in water
done
clear
D)
the reagent is highly reactive in water.
done
clear
View Answer play_arrow
question_answer 120) A lone pair of electrons in an atom implies:
A)
a pair of valence electrons not involved in bonding
done
clear
B)
a pair of electrons involved in bonding
done
clear
C)
a pair of electrons
done
clear
D)
a pair of valence electrons
done
clear
View Answer play_arrow
question_answer 121) Which of the following is a part of our brain?
A)
Corpora allata
done
clear
B)
Corpora adiposa
done
clear
C)
Corpora bigemina
done
clear
D)
Corpora quadrigemina
done
clear
View Answer play_arrow
question_answer 122) One growth ring of plants consists of:
A)
only autumn wood
done
clear
B)
spring wood and early wood
done
clear
C)
only spring wood
done
clear
D)
spring wood and autumn wood
done
clear
View Answer play_arrow
question_answer 123) Which of the following gases contributes to global warming?
A)
Carbon dioxide
done
clear
B)
Nitrogen dioxide
done
clear
C)
Carbon monoxide
done
clear
D)
Sulphur dioxide
done
clear
View Answer play_arrow
question_answer 124) Which of the odd type of vegetable in a basket containing the following?
A)
Beet roots
done
clear
B)
Potatoes
done
clear
C)
Carrots
done
clear
D)
Radishes
done
clear
View Answer play_arrow
question_answer 125) Vessels are the major water conducting cells in:
A)
dicots only
done
clear
B)
pteridophytes and gymnosprems
done
clear
C)
angiosperms
done
clear
D)
monocots only
done
clear
View Answer play_arrow
question_answer 126) Turtles are:
A)
pisces
done
clear
B)
reptiles
done
clear
C)
molluscans
done
clear
D)
arthropods
done
clear
View Answer play_arrow
question_answer 127) Haploid plants can be produced by:
A)
pollen culture
done
clear
B)
cotyledon culture
done
clear
C)
embryo culture
done
clear
D)
meristem culture
done
clear
View Answer play_arrow
question_answer 128) Movement of \[C{{O}_{2}}\] and \[{{O}_{2}}\] across the alveoli and capillaries takes place by:
A)
imbibitions
done
clear
B)
carrier transport
done
clear
C)
diffusion
done
clear
D)
active transport
done
clear
View Answer play_arrow
question_answer 129) A monohybrid cross between two plants, one having 24 cm. long internodes and the other having 12 cm. long internodes, produced F1 hybrids all having 18 cm. long internodes. This is a case of:
A)
recessive dominance
done
clear
B)
multiple allelism
done
clear
C)
complete dominance
done
clear
D)
incomplete dominance
done
clear
View Answer play_arrow
question_answer 130) A bipolar neuron has:
A)
1 dendrite and 1 axon
done
clear
B)
2 axons and 2 dendrites
done
clear
C)
2 dendrites and 1 axon
done
clear
D)
2 axons and 1 dendrite
done
clear
View Answer play_arrow
question_answer 131) The nature of a fruit developing from a flower depends on the type of:
A)
gynoecium
done
clear
B)
pollination
done
clear
C)
fertilization
done
clear
D)
androecium
done
clear
View Answer play_arrow
question_answer 132) Some blue-green algae can be used as biofertilizer because:
A)
they are photosynthetic
done
clear
B)
they have mucilage
done
clear
C)
they grow everywhere
done
clear
D)
they can fix nitrogen
done
clear
View Answer play_arrow
question_answer 133) The process which cannot takes place in the absence of virus is:
A)
translocation
done
clear
B)
transduction
done
clear
C)
transformation
done
clear
D)
conjugation
done
clear
View Answer play_arrow
question_answer 134) One molecule of an enzyme is needed to convert 2 molecules of a substrate into products in 5 minutes. 10 molecules of the enzyme and 25 molecules of the substrate are mixed in a test tube. After 10 minutes the test tube will be having:
A)
products and enzyme
done
clear
B)
products, enzyme and 5 molecules of unreacted substrate
done
clear
C)
products and 5 molecules of unreacted substrate
done
clear
D)
products only
done
clear
View Answer play_arrow
question_answer 135) In angiosperms, triple fusion results in the formation of:
A)
secondary nucleus
done
clear
B)
polar nucleus
done
clear
C)
primary endosperm nucleus
done
clear
D)
zygotic nucleus
done
clear
View Answer play_arrow
question_answer 136) If the chyme of a person who had orally consumed only starch as food is analysed before it enters the duodenum, it will show the presence of:
A)
starch, dextrin and maltose
done
clear
B)
starch, dextrin and glucose
done
clear
C)
dextrin and maltose
done
clear
D)
dextrin and glucose
done
clear
View Answer play_arrow
question_answer 137) The total number of canines in the permanent dental set of humans is:
A)
8
done
clear
B)
12
done
clear
C)
6
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 138) What should be the blood types of parents in the children having only A and B types of blood?
A)
A and 0
done
clear
B)
AB and A
done
clear
C)
AB and 0
done
clear
D)
A and B
done
clear
View Answer play_arrow
question_answer 139) The number of nephrons in a kidney is equal to:
A)
the number of Bowmans capsules
done
clear
B)
sum of Bowmans capsules and malpighian capsules
done
clear
C)
sum of Bowmans capsules and glomeruli
done
clear
D)
double the number of Bowmans capsules
done
clear
View Answer play_arrow
question_answer 140) Chargaffs rule is applicable to:
A)
single stranded RNA
done
clear
B)
single stranded DNA and RNA
done
clear
C)
single stranded DNA
done
clear
D)
double stranded DNA
done
clear
View Answer play_arrow
question_answer 141) Diabetes insipidus is due to:
A)
hypersecretion of insulin
done
clear
B)
hyposecretion of vasopressin
done
clear
C)
hyperesecretion of vasopressin
done
clear
D)
hyposecretion of insulin
done
clear
View Answer play_arrow
question_answer 142) Choose the diagram which correctly shows the anaphase I stage:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 143) Choose the schemmatic diagram which properly represents pulmonary circulation in humans.
A)
left auricle \[\xrightarrow[\text{blood}]{\text{oxygenated}}\] lungs\[\xrightarrow[\text{blood}]{\text{deoxygenated}}\] right ventricle
done
clear
B)
left auricle \[\xrightarrow[\text{blood}]{\text{deoxygenated}}\] lungs \[\xrightarrow[\text{blood}]{\text{oxygenated}}\] right ventricle
done
clear
C)
right ventricle \[\xrightarrow[\text{blood}]{\text{deoxygenated}}\] lungs\[\xrightarrow[\text{blood}]{\text{oxygenated}}\] left auricle
done
clear
D)
right ventricle \[\xrightarrow[\text{blood}]{\text{oxygenated}}\] lungs\[\xrightarrow[\text{blood}]{\text{deoxygenated}}\] left auricle
done
clear
View Answer play_arrow
question_answer 144) Water lost by transpiration is:
A)
rich in dissolved minerals
done
clear
B)
rich in solutes
done
clear
C)
pure water
done
clear
D)
rich in dissolved salts
done
clear
View Answer play_arrow
question_answer 145) What will be the direction of net osmotic movement of water if solution A, enclosed in a semipermeable membrane, having an osmotic potential of - 30 bars and turgor pressure of 5 bars is submerged in a solution B with an osmotic potential of -10 bars and 0 turgor pressure?
A)
B to A
done
clear
B)
equal movement in both directions
done
clear
C)
A to B
done
clear
D)
no movement
done
clear
View Answer play_arrow
question_answer 146) Cockroaches are :
A)
ureotelic
done
clear
B)
ureotelic or ammonotelic
done
clear
C)
uricotelic
done
clear
D)
ammonotelic
done
clear
View Answer play_arrow
question_answer 147) A gamete normally contains:
A)
many alleles of a gene
done
clear
B)
all alleles of a gene
done
clear
C)
two alleles of a gene
done
clear
D)
one allele of a gene
done
clear
View Answer play_arrow
question_answer 148) The most abundant granulocytes in human blood is:
A)
neutrophils
done
clear
B)
monocytes
done
clear
C)
basophils
done
clear
D)
eosinophils
done
clear
View Answer play_arrow
question_answer 149) Metastasis is associated with:
A)
crown gall tumour
done
clear
B)
both malignant and benign tumours
done
clear
C)
benign tumours
done
clear
D)
malignant tumours
done
clear
View Answer play_arrow
question_answer 150) The phase of cell cycle during which DNA + polymerase is functionally active is:
A)
S
done
clear
B)
\[{{G}_{2}}\]
done
clear
C)
G1
done
clear
D)
M
done
clear
View Answer play_arrow
question_answer 151)
Match the biochemical processes given under Column I with their respective cellular locations given under Column II. From the answer choose the one which gives the correct combination of alphabets: Column I Column II A Krebs cycle I Stroma B Glycolysis J Grana C Calvin cycle K Mitochondrial matrix L Cytoplasm
A)
A = L, B = K, C = J
done
clear
B)
A = K, B = L, C = I
done
clear
C)
A = K, B = L, C = J
done
clear
D)
A = L, B = K, C = I
done
clear
View Answer play_arrow
question_answer 152) According to Lamarckism, long necked Giraffes evolved because:
A)
humans preferred only long necked ones
done
clear
B)
of stretching of necks over many generations by short necked ones
done
clear
C)
nature selected only long necked ones
done
clear
D)
short necks suddenly changed into long necks
done
clear
View Answer play_arrow
question_answer 153) An ascomycetes fungus is:
A)
Agaricus
done
clear
B)
Plenrotes
done
clear
C)
Phytophthora
done
clear
D)
Yeast
done
clear
View Answer play_arrow
question_answer 154)
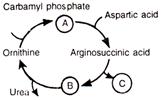
In the skeletal form of ornithine cycle given, some intermediate products indicated by albhabets. Choose the answer in which these alphabets are properly matched with the names of corresponding products:
A)
A = arginine, B = succinic acid, C = fumaric acid
done
clear
B)
A = citrulline, B = arginine, C = succinic acid
done
clear
C)
A = citrulline, B = fumaric acid, C = arginine
done
clear
D)
A = citrulline, B = arginine, C = fumaric acid
done
clear
View Answer play_arrow
question_answer 155)
Choose correct description of the flower depicted in the floral diagram given below:
A)
united, valvate sepals; free, imbricate petals ; free stamens; unilocular ovary with marginal placenta
done
clear
B)
united, valvate sepals; free, imbricate petals; epipetalous stamens; unilocular ovary with marginal placenta
done
clear
C)
united, valvate sepals; free, imbricate petals; free stamens; unilocular ovary with axile placenta
done
clear
D)
united, valvate sepals; free, twisted petals; free stamens; unilocular ovary with marginal placenta
done
clear
View Answer play_arrow
question_answer 156) Phycology is the study of:
A)
lichens
done
clear
B)
Ficus
done
clear
C)
fungi
done
clear
D)
algae
done
clear
View Answer play_arrow
question_answer 157) R.Q. of sprouting potato tubers will be:
A)
1
done
clear
B)
< 1
done
clear
C)
> 1
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 158) The pacemaker of the human heart is:
A)
AV node
done
clear
B)
SV node
done
clear
C)
SA node
done
clear
D)
tricuspid valve
done
clear
View Answer play_arrow
question_answer 159) Haversian systems are found in the bones of:
A)
Pigeon
done
clear
B)
Panther
done
clear
C)
Pipe fish
done
clear
D)
Python
done
clear
View Answer play_arrow
question_answer 160) Which type of the following ecological pyramids are never inverted?
A)
Pyramids of dry biomass
done
clear
B)
Pyramids of energy
done
clear
C)
Pyramids of biomass
done
clear
D)
Pyramids of number
done
clear
View Answer play_arrow
question_answer 161) Chlorophyll molecules are green in colour because they:
A)
transmit green light
done
clear
B)
transform green light
done
clear
C)
reflect green light
done
clear
D)
absorb green light
done
clear
View Answer play_arrow
question_answer 162) HIV decreases natural immunity of body by:
A)
attacking T-lymphocytes
done
clear
B)
attacking B-lymphocytes
done
clear
C)
destroying antibodies
done
clear
D)
destroying erythrocytes
done
clear
View Answer play_arrow
question_answer 163) The level of glucose in the blood is controlled by:
A)
liver
done
clear
B)
ileum
done
clear
C)
gall bladder
done
clear
D)
duodenum
done
clear
View Answer play_arrow
question_answer 164) Hardening of arteries due to deposition of cholesterol is called:
A)
rhinitis
done
clear
B)
stenosis
done
clear
C)
thrombosis
done
clear
D)
atherosclerosis
done
clear
View Answer play_arrow
question_answer 165) The probability of the male child of a haemophilic father and normal mother becoming haemophilic is:
A)
0%
done
clear
B)
25%
done
clear
C)
100%
done
clear
D)
50%
done
clear
View Answer play_arrow
question_answer 166) The cohesive force existing between molecules of water is contribution to:
A)
plasmolysis
done
clear
B)
translocation
done
clear
C)
ascent of sap
done
clear
D)
osmosis
done
clear
View Answer play_arrow
question_answer 167) Balbiani rings are the structural features of:
A)
allosomes
done
clear
B)
autosomes
done
clear
C)
lampbrush chromosomes
done
clear
D)
polytene chromosomes
done
clear
View Answer play_arrow
question_answer 168) In ATP, the high energy bond is the one which links:
A)
ribose with phosphate
done
clear
B)
phosphate to phosphate
done
clear
C)
adenine with phosphate
done
clear
D)
adenine with ribose
done
clear
View Answer play_arrow
question_answer 169) An example for in situ biological conservation method is:
A)
biosphere reserves
done
clear
B)
zoos
done
clear
C)
botanical gardens
done
clear
D)
seed banks
done
clear
View Answer play_arrow
question_answer 170) A common feature shared by guard cells and mesophyll cells is:
A)
presence of chloroplasts
done
clear
B)
dumb - bell shaped structure
done
clear
C)
differentially thick cell wall
done
clear
D)
uniformely thin cell wall
done
clear
View Answer play_arrow
question_answer 171) A polysome is formed by:
A)
a cluster of ribosomal subunits
done
clear
B)
many mRNAs being attached to a ribosome
done
clear
C)
a cluster of ribosomes
done
clear
D)
many ribosomes attached to Mrna
done
clear
View Answer play_arrow
question_answer 172) Construction of a recombinant DNA involves:
A)
cleaving and rejoining DNA segments with endonuclease alone
done
clear
B)
cleaving DNA segments with endonuclease and rejoining them with ligase
done
clear
C)
cleaving DNA segments with ligase and rejoining them with endonuclease
done
clear
D)
cleaving and rejoining DNA segements with ligase alone
done
clear
View Answer play_arrow
question_answer 173) In the production of test tube babies:
A)
fertilization and foetus formation is external
done
clear
B)
fertilization and foetus formation is internal
done
clear
C)
fertilization is internal and foetus formation is external
done
clear
D)
fertilization is external and foetus formation is internal
done
clear
View Answer play_arrow
question_answer 174) To promote the growth of lateral branches of a plant:
A)
axillary buds are removed
done
clear
B)
apical bud is removed
done
clear
C)
auxin is applied to the apical bud
done
clear
D)
auxin is applied to the decapitated shoot tip
done
clear
View Answer play_arrow
question_answer 175) Vital capacity of lungs is:
A)
IRV + ERV
done
clear
B)
IRV + ERV + TV - RV
done
clear
C)
inspiratory reserve volume (IRE) + expiratory reserve volume (ERV) + tidal volume (TV) + residual volume (RV)
done
clear
D)
IRV + ERV + TV
done
clear
View Answer play_arrow
question_answer 176) Choose the correct statement about neuro-hypophysis.
A)
it stores the hormones produced by adenohypophysis
done
clear
B)
it is poorly developed and functionless in humans
done
clear
C)
it stores and releases hormones secreted by hypothalamus
done
clear
D)
it secretes its own hormones
done
clear
View Answer play_arrow
question_answer 177) Sprems of an animal species A cannot normally fertilize the ovum of another species B because:
A)
fertilizin of A and B are not compatible
done
clear
B)
antifertilizin of A and fertilizin of B are not compatible
done
clear
C)
fertilizin of A and antifertilizin of B are not compatible
done
clear
D)
antifertilizin of A and B are not compatible
done
clear
View Answer play_arrow
question_answer 178) HCl of the gastric juice:
A)
activates both ptyalin and pepsin
done
clear
B)
inactivates both ptyalin and pepsin
done
clear
C)
activates ptyalin and inactivates pepsin
done
clear
D)
inactivats ptyalin and activates pepsin
done
clear
View Answer play_arrow
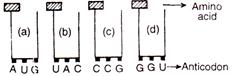
question_answer 179)
Find the sequence of binding of the following amino acyl - tRNA complexes during translation of a mRNA transcribed by a DNA segment having the base sequence 3 TACATGGGTCCG5. Choose the answer showing the correct order of alphabets:
A)
a, b, d, c
done
clear
B)
b, a, d, c
done
clear
C)
a, b, c, d
done
clear
D)
b, a, c, d
done
clear
View Answer play_arrow
question_answer 180)
Choose the correct combination of alphabets which matches the zoological names given under Column I with their common names given under Column II: Column I Column II A Labeo rohita E Jungle fowl B Gallus gallus F Carp C Bos indicas Q Tussar silkmoth D Antheraea mylitta H Cattle
A)
A = F, B = G, C = E, D = H
done
clear
B)
A = G, B = E, C = H, D = F
done
clear
C)
A = F, B = E, C = H, D = G
done
clear
D)
A = F, B = E, C = G, D = H
done
clear
View Answer play_arrow
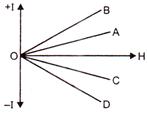
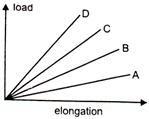 The thinnest Wire is elongation represented by the line:
The thinnest Wire is elongation represented by the line:
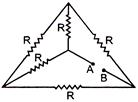
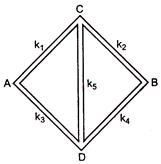 have thermal conductivities \[{{k}_{1}},\text{ }{{k}_{2}},\text{ }{{k}_{3}},\text{ }{{k}_{4}}\]and \[{{k}_{5}}\]when points A and B are maintained at different temperatures. No heat flows through the central rod if:
have thermal conductivities \[{{k}_{1}},\text{ }{{k}_{2}},\text{ }{{k}_{3}},\text{ }{{k}_{4}}\]and \[{{k}_{5}}\]when points A and B are maintained at different temperatures. No heat flows through the central rod if: