question_answer 1) All components of the electromagnetic spectrum in vacuum have the same
A)
energy
done
clear
B)
velocity
done
clear
C)
wavelength
done
clear
D)
frequency
done
clear
View Answer play_arrow
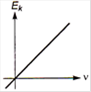
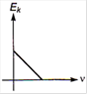
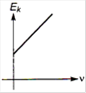
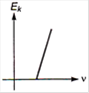
question_answer 2) Which one of the following graph represents the variation of maximum kinetic energy \[\left( {{E}_{k}} \right)\] of the emitted electrons with frequency v in photoelectric effect correctly?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 3) A and B are two metals with threshold frequencies \[1.8\times {{10}^{14}}Hz\]and\[2.2\times {{10}^{14}}Hz\]. Two identical photons of energy 0.825 eV each are incident on them. Then photoelectrons are emitted by (Take h\[=\text{ }6.6\times {{10}^{-34}}J-s\])
A)
B alone
done
clear
B)
A alone
done
clear
C)
neither A nor B
done
clear
D)
both A and B
done
clear
View Answer play_arrow
question_answer 4) The ionization energy of \[L{{i}^{2+}}\]is equal to
A)
9hcR
done
clear
B)
6hcR
done
clear
C)
2hcR
done
clear
D)
hcR
done
clear
View Answer play_arrow
question_answer 5) In the Wheatstone s network given, P = 10\[\Omega \], Q = 20\[\Omega \], R = 15\[\Omega \], S = 30\[\Omega \], the current passing through the battery (of negligible internal resistance) is
A)
0.36 A
done
clear
B)
zero
done
clear
C)
0.18 A
done
clear
D)
0.72 A
done
clear
View Answer play_arrow
question_answer 6) Electrons in a certain energy level\[n={{n}_{1}}\], can emit 3 spectral lines. When they are in another energy level, \[n={{n}_{2}}\], they can emit 6 spectral lines. The orbital speed of the electrons in the orbits are in the ratio
A)
4 : 3
done
clear
B)
3 : 4
done
clear
C)
2 : 1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 7) A circular coil carrying a certain current produces a magnetic Held Bp at its centre. The coil is now rewound so as to have 3 turns and the same current is passed through it. The new magnetic field at the centre is
A)
\[\frac{{{B}_{0}}}{9}\]
done
clear
B)
\[9{{B}_{0}}\]
done
clear
C)
\[\frac{{{B}_{0}}}{3}\]
done
clear
D)
\[3{{B}_{0}}\]
done
clear
View Answer play_arrow
question_answer 8) A proton and a deuteron with the same initial kinetic energy enter a magnetic field in a direction perpendicular to the direction of the field. The ratio of the radii of the circular trajectories described by them is
A)
1 : 4
done
clear
B)
1 :\[\sqrt{2}\]
done
clear
C)
1 : 1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 9) Two tangent galvanometers A and B have coils of radii 8 cm and 16 cm respectively and resistance 8\[\Omega \]. each. They are connected in parallel with a cell of emf 4 V and negligible internal resistance. The deflections produced in the tangent galvanometers A and B are \[30{}^\circ \] and \[60{}^\circ \] respectively. If A has 2 turns, then B must have
A)
18 turns
done
clear
B)
12 turns
done
clear
C)
6 turns
done
clear
D)
2 turns
done
clear
View Answer play_arrow
question_answer 10) A charged particle is moving in a magnetic field of strength B perpendicular to the direction of the field. If q and m denote the charge and mass of the particle respectively, then the frequency of rotation of the particle is
A)
\[f=\frac{qB}{2\pi m}\]
done
clear
B)
\[f=\frac{qB}{2\pi {{m}^{2}}}\]
done
clear
C)
\[f=\frac{2{{\pi }^{2}}m}{qB}\]
done
clear
D)
\[f=\frac{2\pi m}{qB}\]
done
clear
View Answer play_arrow
question_answer 11) Two identical capacitors each of capacitance 5 \[\mu \]F are charged to potentials 2 kV and 1 kV respectively. Their -ve ends are connected together. When the +ve ends are also connected together, the loss of energy of the system is
A)
160 J
done
clear
B)
zero
done
clear
C)
5 J
done
clear
D)
1.25 J
done
clear
View Answer play_arrow
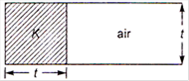
question_answer 12)
A parallel plate capacitor with air as the dielectric has capacitance C. A slab of dielectric constant K and having the same thickness as the separation between the plates is introduced so as to fill one-fourth of the capacitor as shown in the figure. The new capacitance will be
A)
\[\left( K+3 \right)\frac{C}{4}\]
done
clear
B)
\[\left( K+2 \right)\frac{C}{4}\]
done
clear
C)
\[\left( K+1 \right)\frac{C}{4}\]
done
clear
D)
\[\frac{KC}{4}\]
done
clear
View Answer play_arrow
question_answer 13) A current of 5 A is passing through a metallic wire of cross-sectional area\[4\times {{10}^{-6}}{{m}^{2}}\]. If the density of charge carriers of the wire is\[5\times {{10}^{26}}{{m}^{-3}}\], the drift velocity of the electrons will be
A)
\[1\times {{10}^{2}}{{m}^{-1}}\]
done
clear
B)
\[1.56\times {{10}^{-2}}m{{s}^{-1}}\]
done
clear
C)
\[1.56\times {{10}^{-3}}m{{s}^{-1}}\]
done
clear
D)
\[1\times {{10}^{-2}}m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 14) Two bulbs rated 25 W-220 V and 100 W-220 V are connected in series to a 440 V supply. The,
A)
100 W bulb fuses
done
clear
B)
25 W bulb fuses
done
clear
C)
both the bulbs fuse
done
clear
D)
neither of the bulb fuses
done
clear
View Answer play_arrow
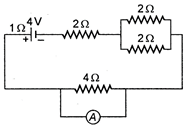
question_answer 15)
The current passing through the ideal ammeter in the circuit given below is
A)
1.25 A
done
clear
B)
1 A
done
clear
C)
0.75 A
done
clear
D)
0.5 A
done
clear
View Answer play_arrow
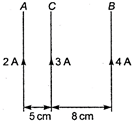
question_answer 16)
A and B are two infinitely long straight parallel conductors. C is another straight conductor of length 1m kept parallel to A and B as shown in the figure. Then the force experienced by C is
A)
towards A equal to \[0.6\times {{10}^{-5}}N\]
done
clear
B)
towards B equal to \[5.4\times {{10}^{-5}}N\]
done
clear
C)
towards A equal to \[5.4\times {{10}^{-5}}N\]
done
clear
D)
towards B equal to \[0.6\times {{10}^{-5}}N\]
done
clear
View Answer play_arrow
question_answer 17) An electric bulb has a rated power of 50 W at 100 V. If it is used on an AC source 200 V, 50 Hz, a choke has to be used in series with it. This choke should have an inductance of
A)
0.1 mH
done
clear
B)
1 mH
done
clear
C)
0.1 H
done
clear
D)
1.1 H
done
clear
View Answer play_arrow
question_answer 18) An inductance of \[\left( \frac{200}{\pi } \right)\] mH, a capacitance pf \[\left( \frac{{{10}^{-3}}}{\pi } \right)\]F and a resistance of 10\[\Omega \] are connected in series with an AC source 220 V, 50 Hz. The phase angle of the circuit is
A)
\[\frac{\pi }{6}\]
done
clear
B)
\[\frac{\pi }{4}\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
\[\frac{\pi }{3}\]
done
clear
View Answer play_arrow
question_answer 19) A step-down transformer reduces the voltage of a transmission line from 2200 V to 220 V. The power delivered by it is 880 W and its efficiency is 88%. The input current is
A)
4.65 mA
done
clear
B)
0.045 A
done
clear
C)
0.45 A
done
clear
D)
4.65 A
done
clear
View Answer play_arrow
question_answer 20) Current in a coil changes from 4 A to zero in 0.1 s and the emf induced is 100 V. The self inductance of the coil is
A)
0.25 H
done
clear
B)
0.4 H
done
clear
C)
2.5 H
done
clear
D)
4 H
done
clear
View Answer play_arrow
question_answer 21) The electromagnetic theory of light failed to explain
A)
photoelectric effect
done
clear
B)
polarisation
done
clear
C)
diffraction
done
clear
D)
interference
done
clear
View Answer play_arrow
question_answer 22) Light from two coherent sources of the same amplitude A and wavelength \[\lambda \] illuminates the screen. The intensity of the central maximum is\[{{I}_{0}}\]. If the sources were incoherent, the intensity at the same point will be
A)
\[4{{I}_{0}}~~~~~~~~~~\]
done
clear
B)
\[2{{I}_{0}}\]
done
clear
C)
\[{{I}_{0}}\]
done
clear
D)
\[\frac{{{I}_{0}}}{2}\]
done
clear
View Answer play_arrow
question_answer 23) In Youngs double slit experiment with sodium vapour lamp of wavelength 589 nm and the slits 0.589 mm apart, the half angular width of the central maximum is
A)
\[si{{n}^{-1}}\left( 0.01 \right)\]
done
clear
B)
\[si{{n}^{-1}}\left( 0.0001 \right)\]
done
clear
C)
\[si{{n}^{-1}}\left( 0.001 \right)~\]
done
clear
D)
\[si{{n}^{-1}}\left( 0.1 \right)\]
done
clear
View Answer play_arrow
question_answer 24) A single slit Fraunhofer diffraction pattern is formed with white light. For what wavelength of light the third secondary maximum in the diffraction pattern coincides with the second secondary maximum in the pattern for red light of wavelength\[\text{6500}\overset{\text{o}}{\mathop{\text{A}}}\,\]?
A)
\[4400\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[\text{4100}\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[4642.8\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[9100\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 25) The head lights of a jeep are 1.2 m apart. If the pupil of the eye of an observer has a diameter of mm and light of wavelength \[5896\overset{\text{o}}{\mathop{\text{A}}}\,\] is used, what should be the maximum distance of the jeep from the observer if the two head lights are just separated?
A)
33.9 km
done
clear
B)
33.9 m
done
clear
C)
3.34 m
done
clear
D)
3.39 m
done
clear
View Answer play_arrow
question_answer 26) The de-Broglie wavelength of a proton (charge\[=1.6\times {{10}^{-19}}C\],\[mass=1.6\times {{10}^{-27}}kg\]) accelerated through a potential difference of kV is
A)
\[\text{600}\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.9\times {{10}^{-12}}m\]
done
clear
C)
\[7{{A}^{o}}\]
done
clear
D)
\[0.9\text{ }nm\]
done
clear
View Answer play_arrow
question_answer 27) A radioactive element forms its own isotope after 3 consecutive disintegrations. The particles emitted are
A)
3 \[\beta \]-particles
done
clear
B)
2 \[\beta \]-particles and 1 \[\alpha \]-particle
done
clear
C)
2 \[\beta \]-particles and 1 \[\gamma \]-particle
done
clear
D)
2 \[\beta \]-particles and 1 \[\beta \]-particle
done
clear
View Answer play_arrow
question_answer 28) A radioactive substance contains 10000 nuclei and its half-life period is 20 days. The number of nuclei present at the end of 10 days is
A)
7070
done
clear
B)
9000
done
clear
C)
8000
done
clear
D)
7500
done
clear
View Answer play_arrow
question_answer 29) In Raman effect Stokes lines are spectral lines having
A)
frequency greater than that of the original line
done
clear
B)
wavelength equal to that of the original line
done
clear
C)
wavelength less than that of the original line
done
clear
D)
wavelength greater than that of the original line
done
clear
View Answer play_arrow
question_answer 30) The principle of LASER action involves
A)
amplification of particular frequency emitted by the system
done
clear
B)
population inversion
done
clear
C)
stimulated emission
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 31) A ray of light is travelling from glass to air. (refractive index of glass = 1.5). The angle of incidence is\[50{}^\circ \]. The deviation of the ray is
A)
\[0{}^\circ \]
done
clear
B)
\[80{}^\circ \]
done
clear
C)
\[{{50}^{0}}-{{\sin }^{-1}}\left[ \frac{\sin \,{{50}^{0}}}{1.5} \right]\]
done
clear
D)
\[{{50}^{0}}-{{\sin }^{-1}}\left[ \frac{\sin \,{{50}^{0}}}{1.5} \right]-{{50}^{0}}\]
done
clear
View Answer play_arrow
question_answer 32) A vessel of height 2d is half-filled with a liquid of refractive index \[\sqrt{2}\] and the other half with a liquid of refractive index n (the given liquids are immiscible). Then the apparent depth of the inner surface of the bottom- of the vessel (neglecting the thickness of the bottom of the vessel) will be
A)
\[\frac{n}{d\left( n+\sqrt{2} \right)}\]
done
clear
B)
\[\frac{d\left( n+\sqrt{2} \right)}{n\sqrt{2}}\]
done
clear
C)
\[\frac{\sqrt{2}n}{d\left( n+\sqrt{2} \right)}\]
done
clear
D)
\[\frac{nd}{d+\sqrt{2n}}\]
done
clear
View Answer play_arrow
question_answer 33) A ray of light is incident normally on one face of a right angled isosceles prism. It then grazes the hypotenuse. The refractive index of the material of the prism is
A)
1.33
done
clear
B)
1.414
done
clear
C)
1.5
done
clear
D)
1.732
done
clear
View Answer play_arrow
question_answer 34) Two thin equiconvex lenses each of focal length 0.2 m are placed coaxially with their optic centre 0.5 m apart. Then the focal length of the combination is
A)
- 0.4 m
done
clear
B)
0.4 m
done
clear
C)
- 0.1 m
done
clear
D)
0.1 m
done
clear
View Answer play_arrow
question_answer 35) A prism of a certain angle deviates the red and blue rays by \[8{}^\circ \] and \[12{}^\circ \]respectively. Another prism of the same angle deviates the red and blue rays by \[10{}^\circ \] and \[14{}^\circ \]respectively. The prisms are small angled and made of different materials. The dispersive powers of the materials of the prisms are in the ratio
A)
5 : 6
done
clear
B)
9 : 11
done
clear
C)
6 : 5
done
clear
D)
11 : 9
done
clear
View Answer play_arrow
question_answer 36) When the angle of incidence is \[60{}^\circ \] on the surface of a glass slab, it is found that the reflected ray is completely polarised. The velocity of light in glass is
A)
\[\sqrt{2}\times {{10}^{8}}m{{s}^{-1}}\]
done
clear
B)
\[\sqrt{3}\times {{10}^{8}}m{{s}^{-1}}\]
done
clear
C)
\[2\times {{10}^{8}}m{{s}^{-1}}\]
done
clear
D)
\[3\times {{10}^{8}}m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 37) A 20 cm length of a certain solution causes right handed rotation of\[38{}^\circ \]. A 30 cm length of another solution causes left handed rotation of\[24{}^\circ \]. The optical rotation caused by 30 cm length of a mixture of the above solutions in the volume ratio 1 : 2 is
A)
left handed rotation of \[14{}^\circ \]
done
clear
B)
right handed rotation of \[14{}^\circ \]
done
clear
C)
left handed rotation of \[3{}^\circ \]
done
clear
D)
right handed rotation of 3°
done
clear
View Answer play_arrow
question_answer 38) Two identical charges repel each other with a force equal to 10 mg wt when they are 0.6 m a part in air\[\left( g=10m{{s}^{-2}} \right)\]. The value of each charge is
A)
\[2\text{ }mC\]
done
clear
B)
\[2\times {{10}^{-7}}C\]
done
clear
C)
\[2\text{ }nC\]
done
clear
D)
\[2\mu C\]
done
clear
View Answer play_arrow
question_answer 39) The potential of the electric field produced by point charge at any point (x, y, z) is given by V = 3 x 2 + 5, where x, y are in metres and V is in volts. The intensity of the electric field at (-2,1,0) is
A)
\[+17\text{ }V{{m}^{-1}}\]
done
clear
B)
\[-17\text{ }V{{m}^{-1}}\]
done
clear
C)
\[+12\text{ }V{{m}^{-1}}\]
done
clear
D)
\[-12\text{ }V{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 40) The potential of a large liquid drop when eight liquid drops are combined is 20 V. Then the potential of each single drop was
A)
10 V
done
clear
B)
7.5 V
done
clear
C)
5 V
done
clear
D)
2.5 V
done
clear
View Answer play_arrow
question_answer 41) The dimensional formula for impulse is
A)
\[\left[ ML{{T}^{-1}} \right]\]
done
clear
B)
\[\left[ M{{L}^{-1}}T \right]\]
done
clear
C)
\[\left[ {{M}^{-1}}L{{T}^{-1}} \right]~\]
done
clear
D)
\[\left[ M{{L}^{-1}}{{T}^{-1}} \right]\]
done
clear
View Answer play_arrow
question_answer 42) The maximum height attained by a projectile when thrown at an angle 9 with the horizontal is found to be half the horizontal range. Then 0 is equal to
A)
\[{{\tan }^{-1}}\left( 2 \right)\]
done
clear
B)
\[\frac{\pi }{6}\]
done
clear
C)
\[\frac{\pi }{4}\]
done
clear
D)
\[{{\tan }^{-1}}\left( \frac{1}{2} \right)\]
done
clear
View Answer play_arrow
question_answer 43) A shell of mass 20 kg at rest explodes into two fragments whose masses are in the ratio 2:3. The smaller fragment moves with a velocity of 6ms-1. The kinetic energy of the larger fragment is
A)
96 J
done
clear
B)
216 J
done
clear
C)
144 J
done
clear
D)
360 J
done
clear
View Answer play_arrow
question_answer 44) Water rises in plant fibres due to
A)
capillarity
done
clear
B)
viscosity
done
clear
C)
fluid pressure
done
clear
D)
osmosis
done
clear
View Answer play_arrow
question_answer 45) The acceleration due to gravity becomes \[\left( \frac{g}{2} \right)\] (g = acceleration due to gravity on the surface of the earth) at a height equal to
A)
4 R
done
clear
B)
R
done
clear
C)
2 R
done
clear
D)
R
done
clear
View Answer play_arrow
question_answer 46) The cylindrical tube of a spray pump has a cross-section of\[8\text{ }c{{m}^{2}}\], one end of which has 40 fine holes each of area\[{{10}^{-8}}{{m}^{2}}\]. If the liquid flows inside the tube with a speed of 0.15 m\[mi{{n}^{-1}}\], the speed with which the liquid is ejected through the holes is
A)
\[50\text{ }m{{s}^{-1}}~\]
done
clear
B)
\[5\text{ }m{{s}^{-1}}\]
done
clear
C)
\[0.05\text{ }m{{s}^{-1}}\]
done
clear
D)
\[0.5\text{ }m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 47) During an adiabatic process, the cube of the pressure is found to be inversely proportional to the fourth power of the volume. Then the ratio of specific heats is
A)
1
done
clear
B)
1.33
done
clear
C)
1.67
done
clear
D)
1.4
done
clear
View Answer play_arrow
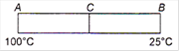
question_answer 48)
Two identical rods AC and CB made of two different metals having thermal conductivities in the ratio 2 : 3 are kept in contact with each other at the end C as shown in the figure. A is at \[100{}^\circ C\]and B is at\[25{}^\circ C\]. Then the junction C is at
A)
\[55{}^\circ C~\]
done
clear
B)
\[60{}^\circ C\]
done
clear
C)
\[75{}^\circ C\]
done
clear
D)
\[50{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 49) 310 J of heat is required to raise the temperature of 2 moles of an ideal gas at, constant pressure from \[25{}^\circ C\]to\[35{}^\circ C\]. The amount of heat required to raise the temperature of the gas through the same range at constant volume is
A)
384 J
done
clear
B)
144 J
done
clear
C)
276 J
done
clear
D)
452 J
done
clear
View Answer play_arrow
question_answer 50) A Carnots engine operates with source at \[{{127}^{0}}C\]and sink at\[27{}^\circ C\]. If the source supplies 40 kJ of heat energy, the work done by the engine is
A)
30 kJ
done
clear
B)
10 kJ
done
clear
C)
4 kJ
done
clear
D)
1 kJ
done
clear
View Answer play_arrow
question_answer 51) The maximum particle velocity in a wave motion is half the wave velocity. Then the amplitude of the wave is equal to
A)
\[\frac{\lambda }{4\pi }\]
done
clear
B)
\[\frac{2\lambda }{\pi }\]
done
clear
C)
\[\frac{\lambda }{2\pi }\]
done
clear
D)
\[\lambda \]
done
clear
View Answer play_arrow
question_answer 52) The ratio of the velocity of sound in hydrogen \[\left( \gamma =\frac{5}{3} \right)\] to that in helium y = - at the same temperature is
A)
\[\sqrt{\frac{5}{42}}\]
done
clear
B)
\[\sqrt{\frac{5}{21}}\]
done
clear
C)
\[\sqrt{\frac{42}{5}}\]
done
clear
D)
\[\sqrt{\frac{21}{5}}\]
done
clear
View Answer play_arrow
question_answer 53) An engine moving towards a wall with a velocity \[50\text{ }m{{s}^{-1}}\] emits a note of\[1.2\text{ }kHz\]. Speed of sound in air is\[350\text{ }m{{s}^{-1}}\]. The frequency of the note after reflection from the wall as heard by the driver of the engine is
A)
2.4 kHz
done
clear
B)
0.24 kHz
done
clear
C)
1.6 kHz
done
clear
D)
1.2 kHz
done
clear
View Answer play_arrow
question_answer 54) A glass tube is open at both the ends. A tuning fork of frequency f resonates with the air column inside the tube. Now the tube is placed vertically inside water so that half the length of the tube is filled with water. Now the air column inside the tube is in unison with another fork of frequency f.Then
A)
f = f
done
clear
B)
f = 4f
done
clear
C)
f = 2f
done
clear
D)
f = \[\frac{f}{2}\]
done
clear
View Answer play_arrow
question_answer 55) The surface temperature of the sun which has maximum energy emission at 500 nm is 6000 K. The temperature of a star which has maximum energy emission at 400 nm will be
A)
8500 K
done
clear
B)
4500 K
done
clear
C)
7500 K
done
clear
D)
6500 K
done
clear
View Answer play_arrow
question_answer 56) The volume of a nucleus is directly proportional
A)
A
done
clear
B)
\[{{A}^{3}}\]
done
clear
C)
\[\sqrt{A}\]
done
clear
D)
\[{{A}^{1/3}}\] (where A = mass number of the nucleus)
done
clear
View Answer play_arrow
question_answer 57) An electron is
A)
a hadron
done
clear
B)
a baryon
done
clear
C)
a nucleon
done
clear
D)
a lepton
done
clear
View Answer play_arrow
question_answer 58) Minority carriers in a p -type semiconductor are
A)
free electrons
done
clear
B)
holes
done
clear
C)
neither holes nor free electrons
done
clear
D)
both holes and free electrons
done
clear
View Answer play_arrow
question_answer 59) In a reverse biased diode when the applied voltage changes by 1V, the current is found to change by 0.5 \[\mu \]A. The reverse bias resistance of the diode is
A)
\[2\times {{10}^{5}}\Omega \]
done
clear
B)
\[2\times {{10}^{6}}\Omega \]
done
clear
C)
\[200\,\Omega \]
done
clear
D)
\[2\,\Omega \]
done
clear
View Answer play_arrow
question_answer 60)
The truth table given below is for (A and B are the inputs, Y is the output) A B Y 0 0 1 0 1 1 1 0 1 1 1 0
A)
NOR
done
clear
B)
AND
done
clear
C)
XOR
done
clear
D)
NAND
done
clear
View Answer play_arrow
question_answer 61) The number of unidentate ligands in the complex ion is called
A)
oxidation number
done
clear
B)
primary valency
done
clear
C)
coordination number
done
clear
D)
EAN
done
clear
View Answer play_arrow
question_answer 62) \[2S{{O}_{2}}(g)+{{O}_{2}}(g)\] is an example for
A)
neutralisation reaction
done
clear
B)
homogeneous catalysis
done
clear
C)
heterogeneous catalysis
done
clear
D)
irreversible reaction
done
clear
View Answer play_arrow
question_answer 63) The amino acid which is not optically active is
A)
lactic acid
done
clear
B)
serine
done
clear
C)
alanine
done
clear
D)
glycine
done
clear
View Answer play_arrow
question_answer 64) For a stable molecule, the value of bond order must be
A)
there is no relationship between stability and bond order
done
clear
B)
zero
done
clear
C)
positive
done
clear
D)
negative
done
clear
View Answer play_arrow
question_answer 65) Which one of the following is a second order reaction?
A)
\[{{H}_{2}}+B{{r}_{2}}\xrightarrow{{}}2HBr\]
done
clear
B)
\[N{{H}_{4}}N{{O}_{3}}\xrightarrow{{}}{{N}_{2}}+3{{H}_{2}}O\]
done
clear
C)
\[{{H}_{2}}+C{{l}_{2}}\xrightarrow{sunlight}2HCl\]
done
clear
D)
\[C{{H}_{3}}COOC{{H}_{3}}+NaOH\xrightarrow{{}}C{{H}_{3}}COONa+{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 66) Denatured alcohol is
A)
ethanol + methanol
done
clear
B)
rectified spirit + methanol + naphtha
done
clear
C)
undistilled ethanol
done
clear
D)
rectified spirit
done
clear
View Answer play_arrow
question_answer 67) During the formation of a chemical bond
A)
electron-electron repulsion becomes more than the nucleus-electron attraction
done
clear
B)
energy of the system does not change
done
clear
C)
enetgy/increases
done
clear
D)
energy decreases
done
clear
View Answer play_arrow
question_answer 68) One mole of oxygen at 273 K and one mole of sulphur dioxide at 546 K are taken in two separate containers, then,
A)
kinetic energy of \[{{O}_{2}}>\] kinetic energy of \[S{{O}_{2}}\]
done
clear
B)
kinetic energy of \[{{O}_{2}}<\] kinetic energy of \[S{{O}_{2}}\]
done
clear
C)
kinetic energy of both are equal
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 69) \[+I\] effect is shown by
A)
\[-C{{H}_{3}}\]
done
clear
B)
\[-Br\]
done
clear
C)
\[-Cl\]
done
clear
D)
\[-N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 70) Formation of coloured solution is possible when metal ion in the compound contains
A)
paired electrons
done
clear
B)
lone pair of electrons
done
clear
C)
unpaired electrons
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 71) Benzene reacts with chlorine in sunlight to give a final product
A)
\[CC{{l}_{4}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{6}}C{{l}_{6}}\]
done
clear
C)
\[{{C}_{6}}C{{l}_{6}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}Cl\]
done
clear
View Answer play_arrow
question_answer 72) In the periodic table metals usually used as catalysts belong to
A)
f-block
done
clear
B)
d-block
done
clear
C)
p-block
done
clear
D)
s-block
done
clear
View Answer play_arrow
question_answer 73) Daltons law of partial pressure is applicable to which one of the following systems?
A)
\[N{{H}_{3}}+HCl\]
done
clear
B)
\[NO+{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}+C{{l}_{2}}\]
done
clear
D)
\[CO+{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 74) The general formula of a cycloalkane is
A)
\[{{C}_{n}}{{H}_{n}}\]
done
clear
B)
\[{{C}_{n}}{{H}_{2n}}\]
done
clear
C)
\[{{C}_{n}}{{H}_{2n-2}}\]
done
clear
D)
\[{{C}_{n}}{{H}_{2n+2}}\]
done
clear
View Answer play_arrow
question_answer 75) In acetylene molecule, between the carbon atoms there are
A)
three pi bonds
done
clear
B)
one sigma and two pi bonds
done
clear
C)
two sigma and one pi bonds
done
clear
D)
three sigma bonds
done
clear
View Answer play_arrow
question_answer 76) Which of the following is an intensive property?
A)
temperature
done
clear
B)
viscosity
done
clear
C)
surface tension
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 77) Hofmanns bromamide reaction is to convert
A)
acid to alcohol
done
clear
B)
alcohol to acid
done
clear
C)
amide to amine
done
clear
D)
amine to amide
done
clear
View Answer play_arrow
question_answer 78) IUPAC name of \[N{{a}_{3}}[Co{{(N{{O}_{2}})}_{6}}]\] is
A)
sodium hexanitrito cobaltate (II)
done
clear
B)
sodium hexanitro cobaltate (III)
done
clear
C)
sodium hexanitrito cobaltate (III)
done
clear
D)
sodium cobaltinitrite
done
clear
View Answer play_arrow
question_answer 79) In equilibrium state the value of \[\Delta G\] is
A)
zero
done
clear
B)
negative
done
clear
C)
positive
done
clear
D)
may be negative or positive
done
clear
View Answer play_arrow
question_answer 80) How many chiral carbon atoms are present in 2,3. 4-trichloropentane?
A)
\[4\]
done
clear
B)
\[1\]
done
clear
C)
\[2\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 81) Which one of the following shows functional isomerism?
A)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
B)
\[{{C}_{3}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
D)
\[C{{H}_{2}}C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 82) In the ionic equation \[-Bio_{3}^{-}+6{{H}^{+}}+x{{e}^{-}}\xrightarrow{{}}\] \[B{{i}^{3+}}+3{{H}_{2}}O\], the values of x is:
A)
\[6\]
done
clear
B)
\[2\]
done
clear
C)
\[4\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 83) Molarity of a given orthophosphoric acid solution is 3 M. Its normality is
A)
\[9N\]
done
clear
B)
\[0.3N\]
done
clear
C)
\[3N\]
done
clear
D)
\[1N\]
done
clear
View Answer play_arrow
question_answer 84) Acidified sodium fusion extract on addition of ferric chloride solution gives blood red colouration which confirms the presence of
A)
\[S\] and \[Cl\]
done
clear
B)
\[N\] and \[S\]
done
clear
C)
\[N\]
done
clear
D)
\[S\]
done
clear
View Answer play_arrow
question_answer 85) A body of mass 10 mg is moving with a velocity of \[100\text{ }m{{s}^{-1}}\]. The wavelength of de-Broglie wave associated with it would be \[(h=6.63\times {{10}^{-34}}js)\]
A)
\[6.63\times {{10}^{-35}}m\]
done
clear
B)
\[6.63\times {{10}^{-34}}m\]
done
clear
C)
\[6.63\times {{10}^{-31}}m\]
done
clear
D)
\[6.63\times {{10}^{-37}}m\]
done
clear
View Answer play_arrow
question_answer 86) Angle strain in cyclopropane is
A)
\[{{24}^{o}}44\]
done
clear
B)
\[{{9}^{o}}44\]
done
clear
C)
\[44\]
done
clear
D)
\[-{{5}^{o}}16\]
done
clear
View Answer play_arrow
question_answer 87) The number of antibonding electron pairs in \[O_{2}^{2-}\]molecular ion on the basis of molecular orbital theory is (Atomic number of 0 is 18.)
A)
\[5\]
done
clear
B)
\[4\]
done
clear
C)
\[3\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 88) Hydroxyl ion concentration of \[{{10}^{-2}}M\,\,HCl\] is
A)
\[1\times {{10}^{1}}mol\text{ }d{{m}^{-3}}\]
done
clear
B)
\[1\times {{10}^{-12}}mol\text{ }d{{m}^{-3}}\]
done
clear
C)
\[1\times {{10}^{-1}}mol\text{ }d{{m}^{-3}}\]
done
clear
D)
\[1\times {{10}^{-14}}mol\text{ }d{{m}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 89) Geometrical isomerism is shown by
A)
\[-C-C-\]
done
clear
B)
done
clear
C)
\[-C=C-\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 90) The oxidation state of iron in \[{{K}_{4}}[Fe{{(CN)}_{6}}]\] is
A)
\[1\]
done
clear
B)
\[4\]
done
clear
C)
\[3\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 91) During the extraction of gold the following reactions take place \[Au+C{{N}^{-}}+{{H}_{2}}O\xrightarrow{{{O}_{2}}}[X]\] \[[X]+Zn\xrightarrow{{}}[Y]+Au\]- X and V are respectively
A)
\[{{[Au{{(CN)}_{2}}]}^{-}}\]and \[{{[Zn{{(CN)}_{6}}]}^{4-}}\]
done
clear
B)
\[{{[Au{{(CN)}_{4}}]}^{2-}}\]and \[{{[Zn{{(CN)}_{4}}]}^{2-}}\]
done
clear
C)
\[{{[Au{{(CN)}_{4}}]}^{3-}}\] and \[{{[Zn{{(CN)}_{4}}]}^{2-}}\]
done
clear
D)
\[{{[Au{{(CN)}_{2}}]}^{-}}\]and[ \[{{[Zn{{(CN)}_{4}}]}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 92) The number of gram molecules of chlorine in \[6.02\times {{10}^{25}}\] hydrogen chloride molecules is
A)
\[10\]
done
clear
B)
\[100\]
done
clear
C)
\[50\]
done
clear
D)
\[5\]
done
clear
View Answer play_arrow
question_answer 93) Graphite is a soft solid lubricant extremely difficult to melt. The reason for this anomalous behavior is that graphite
A)
is an allotropic form of carbon
done
clear
B)
is a non-crystalline substance
done
clear
C)
has carbon atoms arranged in large plates of rings of strongly bond carbon atoms with weak interplate bonds
done
clear
D)
has molecules of variable molecular masses like polymers
done
clear
View Answer play_arrow
question_answer 94) Paracetamol is a/an
A)
antipyretic
done
clear
B)
analgesic
done
clear
C)
both [a] and [b]
done
clear
D)
antimalarial
done
clear
View Answer play_arrow
question_answer 95) Which one of the following has maximum number of atoms of oxygen?
A)
2 g of carbon monoxide
done
clear
B)
2 g of carbon dioxide
done
clear
C)
2 g of sulphur dioxide
done
clear
D)
2 got water
done
clear
View Answer play_arrow
question_answer 96) \[M{{g}^{2+}}\]c is isoelectronic with
A)
\[C{{u}^{2+}}\]
done
clear
B)
\[Z{{n}^{2+}}\]
done
clear
C)
\[N{{a}^{+}}\]
done
clear
D)
\[C{{a}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 97) Gram molecular volume of oxygen at STP is
A)
\[3200\text{ }c{{m}^{3}}\]
done
clear
B)
\[5600c{{m}^{3}}\]
done
clear
C)
\[22400c{{m}^{3}}\]
done
clear
D)
\[11200c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 98) Presence of halogen in organic compounds can be detected using
A)
Leibigs test
done
clear
B)
Dumas test
done
clear
C)
Kjeldahltest
done
clear
D)
Beilstiens test
done
clear
View Answer play_arrow
question_answer 99) The electronic configuration of \[C{{r}^{3+}}\] is
A)
\[[Ar]3{{d}^{4}}\,4{{s}^{2}}\]
done
clear
B)
\[[Ar]3{{d}^{3}}\,4{{s}^{0}}\]
done
clear
C)
\[[Ar]3{{d}^{2}}\,4{{s}^{1}}\]
done
clear
D)
\[[Ar]3{{d}^{5}}\,4{{s}^{1}}\]
done
clear
View Answer play_arrow
question_answer 100) What is the equivalent weight of \[SnC{{l}_{2}}\] in the following reaction \[SnC{{l}_{2}}+C{{l}_{2}}\xrightarrow{{}}SnC{{l}_{4}}\]
A)
\[95\]
done
clear
B)
\[45\]
done
clear
C)
\[60\]
done
clear
D)
\[30\]
done
clear
View Answer play_arrow
question_answer 101) Which of the following forms a colourless solution in aqueous medium?
A)
\[C{{r}^{3+}}\]
done
clear
B)
\[{{V}^{3+}}\]
done
clear
C)
\[S{{c}^{3+}}\]
done
clear
D)
\[T{{i}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 102) When a sulphur sol is evaporated sulphur is Obtained. On mixing with water sulphur sol is not formed. The sol is
A)
lyophilic
done
clear
B)
reversible -
done
clear
C)
hydrophobic
done
clear
D)
hydrophilic
done
clear
View Answer play_arrow
question_answer 103) An alkyi halide reacts with alcoholic ammonia in a sealed tube/the product formed will be
A)
a primary amine
done
clear
B)
a secondary amine
done
clear
C)
a tertiary amine
done
clear
D)
a mixture of all the three
done
clear
View Answer play_arrow
question_answer 104) When cone.\[{{H}_{2}}S{{O}_{4}}\] is heated with \[{{P}_{2}}{{O}_{5}}\] the acid is converted into
A)
sulphur trioxide
done
clear
B)
sulphur dioxide
done
clear
C)
sulphur
done
clear
D)
a mixture of sulphur dioxide and sulphur trioxide
done
clear
View Answer play_arrow
question_answer 105) Entropy of the universe is
A)
constant
done
clear
B)
zero
done
clear
C)
continuously decreasing
done
clear
D)
continuously increasing
done
clear
View Answer play_arrow
question_answer 106) Which one of the following salts on being dissolved in water gives \[pH>7\]cat \[{{25}^{o}}C\]?
A)
\[KCN\]
done
clear
B)
\[KN{{O}_{3}}\]
done
clear
C)
\[N{{H}_{4}}Cl\]
done
clear
D)
\[N{{H}_{4}}CN\]
done
clear
View Answer play_arrow
question_answer 107) The reagent used in Clemmensens reduction is
A)
conc. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[Zn-Hg/conc.HCl\]
done
clear
C)
\[aqKOH\]
done
clear
D)
\[ale.\text{ }KOH\]
done
clear
View Answer play_arrow
question_answer 108) When \[KBr\] is dissolved in water, \[{{K}^{+}}\] ions are
A)
hydrated
done
clear
B)
hydrolysed
done
clear
C)
reduced
done
clear
D)
oxidized
done
clear
View Answer play_arrow
question_answer 109) The noble gas mixture is cooled in a coconut bulb at 173 K. The gases that are not adsorbed are
A)
\[Ne\] and \[Xe\]
done
clear
B)
\[He\] and \[Xe\]
done
clear
C)
\[Ar\] and \[Kr\]
done
clear
D)
\[He\] and \[Ne\]
done
clear
View Answer play_arrow
question_answer 110) The volume of \[10N\]and \[4N\text{ }HCl\]required to make 1 L of \[7N\text{ }HCl\]are
A)
\[0.50L\]of \[10N\text{ }HCl\]and \[0.50L\]of \[4N\text{ }HCl\]
done
clear
B)
\[0.60L\] of \[10N\text{ }HCl\] and \[0.40L\]of \[4N\text{ }HCl\]
done
clear
C)
\[0.80L\]of \[10N\text{ }HCl\]and \[0.20L\]of \[4N\text{ }HCl\]
done
clear
D)
\[0.75L\]of \[10N\text{ }HCl\] and \[0.25L\]of \[4N\text{ }HCl\]
done
clear
View Answer play_arrow
question_answer 111) A metal present in vitamin \[{{B}_{12}}\] is
A)
aluminium
done
clear
B)
zinc
done
clear
C)
iron
done
clear
D)
cobalt
done
clear
View Answer play_arrow
question_answer 112) An oxide of the element contains 20% 02 by weight. Calculate the equivalent weight of the element.
A)
\[8\]
done
clear
B)
\[16\]
done
clear
C)
\[32\]
done
clear
D)
\[12\]
done
clear
View Answer play_arrow
question_answer 113) Maximum number of molecules of \[C{{H}_{3}}I\] that can react with a molecule of \[C{{H}_{3}}N{{H}_{2}}\]are
A)
\[3\]
done
clear
B)
\[4\]
done
clear
C)
\[2\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 114) The relation between \[\Delta H\] and \[\Delta U\] is
A)
\[\Delta H=\Delta U+RT\]
done
clear
B)
\[\Delta H=\Delta U-nRT\]
done
clear
C)
\[\Delta H=\Delta U-\Delta nRT\]
done
clear
D)
\[\Delta U=\Delta H-\Delta nRT\]
done
clear
View Answer play_arrow
question_answer 115) Identify the ore not cotaining iron.
A)
limonite
done
clear
B)
siderite
done
clear
C)
carnallite
done
clear
D)
chalcopyrites
done
clear
View Answer play_arrow
question_answer 116) In which of the following process, maximum increase in entropy is observed?
A)
Melting of ice
done
clear
B)
Sublimation of naphthalene
done
clear
C)
Condensation of water
done
clear
D)
Dissolution of salt in water
done
clear
View Answer play_arrow
question_answer 117) Decomposition of benzene diazonium chloride by using \[C{{u}_{2}}C{{l}_{2}}/HCl\] to form chlorobenzene is
A)
Raschigs reaction
done
clear
B)
Sandmeyers reaction
done
clear
C)
Kolbes reaction
done
clear
D)
Cannizaros reaction
done
clear
View Answer play_arrow
question_answer 118) Which complex cannot ionise in solution?
A)
\[[CoC{{l}_{3}}{{(N{{H}_{3}})}_{3}}]\]
done
clear
B)
\[{{K}_{4}}[Fe{{(CN)}_{6}}]\]
done
clear
C)
\[{{K}_{2}}[Pt({{F}_{6}})]\]
done
clear
D)
\[[Pt{{(N{{H}_{3}})}_{6}}]C{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 119) Considering the reaction \[C(s)+{{O}_{2}}(g)\]\[\xrightarrow{{}}C{{O}_{2}}(g)+393.5kJ\] the sings of \[\Delta H,\,\,\Delta S\] and \[\Delta G\,\] respectively are
A)
\[+.-,-\]
done
clear
B)
\[-,+,+\]
done
clear
C)
\[-,-,-\]
done
clear
D)
\[-,+,-\]
done
clear
View Answer play_arrow
question_answer 120) The product formed when hydroxylamine condenses with a carbonyl compound is called
A)
hydrazide
done
clear
B)
oxime
done
clear
C)
hydrazine
done
clear
D)
hydrazo
done
clear
View Answer play_arrow
question_answer 121) In which of the following organisms, self-fertilization is seen?
A)
Fish
done
clear
B)
Roundworm
done
clear
C)
Earthworm
done
clear
D)
Liver fluke
done
clear
View Answer play_arrow
question_answer 122) Rauwolfia serpentina belongs to family
A)
Apocyanaceae
done
clear
B)
Solanaceae
done
clear
C)
Liliaceae
done
clear
D)
Fabaceae
done
clear
View Answer play_arrow
question_answer 123)
A)
C
done
clear
B)
D
done
clear
C)
A
done
clear
D)
B
done
clear
View Answer play_arrow
question_answer 124) In ABO blood groups, how many phenotypes are found?
A)
6
done
clear
B)
8
done
clear
C)
1
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 125) The tumor inducing capacity of Agrobacterium tumefadeiis is located in large extra chromosomal plasmids called
A)
Ri plasmid
done
clear
B)
lambda phage
done
clear
C)
pBR 322
done
clear
D)
Ti plasmid
done
clear
View Answer play_arrow
question_answer 126) If a length of DMA has 45,000 base pairs, how many complete turns will the DNA molecule takes?
A)
4,500
done
clear
B)
45,000
done
clear
C)
45
done
clear
D)
450
done
clear
View Answer play_arrow
question_answer 127) The process in which mature differentiated cells reverse to meristematic activity to form callus is called
A)
dedifferenriarion
done
clear
B)
differentiation
done
clear
C)
cyto - differentiation
done
clear
D)
redifferentiation
done
clear
View Answer play_arrow
question_answer 128) The lateral roots originate from
A)
endoderm cells
done
clear
B)
pericycle cells
done
clear
C)
epiblema
done
clear
D)
cortical cells below root hairs
done
clear
View Answer play_arrow
question_answer 129) Which accessory genital gland occurs only in mammalian male?
A)
Prostate gland
done
clear
B)
Perineal gland
done
clear
C)
Cowpers gland
done
clear
D)
Bartholian gland
done
clear
View Answer play_arrow
question_answer 130) When the concentration of the soil solutes is low, the absorption of water
A)
remains normal
done
clear
B)
is stopped
done
clear
C)
is increased
done
clear
D)
is decreased
done
clear
View Answer play_arrow
question_answer 131)
In the diagram of lenticel identify the parts as A, B, C, D
A)
A - phellem, B - periderm, C - phellogen, D -phelloderm
done
clear
B)
A - phellem, B - complementary cells, C -phelloderm, D - periderm
done
clear
C)
A - complementary cells, B - phellogen, C -phelloderm, D - periderm
done
clear
D)
A - complementary - cells, B - phellem, C -periderm, D - phelloderm
done
clear
View Answer play_arrow
question_answer 132) Sterilization of tissue culture medium is done by
A)
autoclaving of medium at \[120{}^\circ C\]for 15 minutes
done
clear
B)
Filtering the medium through Fine sieve
done
clear
C)
mixing the medium with antifungal agents
done
clear
D)
keeping the medium at \[-\text{ }20{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 133)
Match the following: A. Leishmania donovani 1. Malaria B. Wuchereria bancrofti 2. Amoebiasis C. Trypanosoma gambiense 3. Kala - azar D. Entamoeba histolytica 4. Sleeping sickness 5. Filariasis
A)
A- 4 B- 3 C- 2 D-1
done
clear
B)
A-3 B-4 C-5 D-1
done
clear
C)
A-3 B-5 C-4 D-2
done
clear
D)
A-3 B-5 C-2 D-1
done
clear
View Answer play_arrow
question_answer 134) The idea of Natural Selection as the fundamental process of evolutionary changes was reached
A)
By Alfred Russel Wallace in 1901
done
clear
B)
Independently by Charles Darwin and Alfred Russel Wallace in 1859
done
clear
C)
Independently by Charles Darwin and Alfred Russel Wallace in 1900
done
clear
D)
By Charles Darwin in 1866
done
clear
View Answer play_arrow
question_answer 135) Auxins originates at the tip of the stem and controls growth elsewhere. The movement of auxin is largely
A)
basipetal
done
clear
B)
acropetal
done
clear
C)
both [a] and [b]
done
clear
D)
centripetal
done
clear
View Answer play_arrow
question_answer 136) Name the class of the Mycota, which is commonly called- fungi imperfecti?
A)
Deuteromycota
done
clear
B)
Ascomycota
done
clear
C)
Zygomycota
done
clear
D)
Basidiomycota
done
clear
View Answer play_arrow
question_answer 137) Which one is not correct about Krebs cycle?
A)
It is also called citric acid cycle
done
clear
B)
The intermediate compound which links glycolysis with Krebs cycle is malic acid
done
clear
C)
It occurs in mitochondria
done
clear
D)
It starts with six carbon compound
done
clear
View Answer play_arrow
question_answer 138) Which would do maximum harm to a tree?
A)
loss of half of its branches
done
clear
B)
loss of all its bark
done
clear
C)
loss of all its leaves
done
clear
D)
loss of half of its leaves
done
clear
View Answer play_arrow
question_answer 139)
A)
C
done
clear
B)
D
done
clear
C)
A
done
clear
D)
B
done
clear
View Answer play_arrow
question_answer 140) Rh- person donated blood to Rh+ person for the second time. Then,
A)
\[R{{h}^{-}}\]person will die
done
clear
B)
Nothing happens to \[R{{h}^{+}}\]person
done
clear
C)
\[R{{h}^{+}}\]blood starts reacting to \[R{{h}^{+}}\]blood
done
clear
D)
\[R{{h}^{+}}\]person will die
done
clear
View Answer play_arrow
question_answer 141) Edaphology is
A)
study of elephants
done
clear
B)
study of snakes
done
clear
C)
study of amphibians
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 142) Pineal gland of human brain secretes melatonin concerned with
A)
anger
done
clear
B)
body temperature
done
clear
C)
colouration of skin
done
clear
D)
sleep
done
clear
View Answer play_arrow
question_answer 143) When a tall plant with round seeds (TIRR) crossed with a dwarf plant with wrinkle seeds (ttrr), the F; generation consists of tall plants with round seeds. What would be the proportion of dwarf plant with wrinkle seeds in F1 generation?
A)
\[\frac{1}{4}\]
done
clear
B)
\[\frac{1}{16}\]
done
clear
C)
\[0\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 144) Cell wall consists of
A)
lignin, hemicellulose, protein and lipid
done
clear
B)
hemicellulose, cellulose, tubulin and lignin
done
clear
C)
lignin, hemicellulose, pectin and lipid
done
clear
D)
Lignin, hemicellulose, pectin and cellulose
done
clear
View Answer play_arrow
question_answer 145) The post anal tail is present in
A)
chordates
done
clear
B)
vertebrates
done
clear
C)
invertebrates
done
clear
D)
in all of them
done
clear
View Answer play_arrow
question_answer 146) The term cytoplasm and nucleoplasm were given by
A)
Purkinje
done
clear
B)
Strasburger
done
clear
C)
Brown
done
clear
D)
Flemming
done
clear
View Answer play_arrow
question_answer 147) Which of the following experiment is called physiological demonstration of osmosis?
A)
Thistle funnel-whose mouth is tied with egg membrane
done
clear
B)
Thistle funnel - whose mouth is tied with parchment paper
done
clear
C)
Potometer
done
clear
D)
Bell jar experiment
done
clear
View Answer play_arrow
question_answer 148) The net gain of ATP during glycolysis is
A)
six
done
clear
B)
eight
done
clear
C)
two
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 149) Coronary heart disease is due to
A)
Streptococci bacteria
done
clear
B)
inflammation of pericardium
done
clear
C)
weakening of the heart valves
done
clear
D)
insufficient blood supply to the heart muscles
done
clear
View Answer play_arrow
question_answer 150) Manas sanctuary is located at
A)
Rajasthan
done
clear
B)
Assam
done
clear
C)
Bihar
done
clear
D)
Gujarat
done
clear
View Answer play_arrow
question_answer 151) Checking of reradiating heat by atmospheric dust\[{{O}_{3}}\], \[C{{O}_{2}}\]and water vapours is
A)
green house effect
done
clear
B)
solar effect
done
clear
C)
ozone layer effect
done
clear
D)
radioactive effect
done
clear
View Answer play_arrow
question_answer 152) Mutation cannot change
A)
RNA
done
clear
B)
environment
done
clear
C)
enzyme
done
clear
D)
DNA
done
clear
View Answer play_arrow
question_answer 153) Liberation of \[{{O}_{2}}\] when green cells in water are exposed to sunlight in the presence of suitable acceptor is called
A)
Arnons reaction
done
clear
B)
Emersons enhance effect
done
clear
C)
Blackmanns reaction
done
clear
D)
Hills reaction
done
clear
View Answer play_arrow
question_answer 154) Guttation is mainly due to
A)
root pressure
done
clear
B)
imbibition
done
clear
C)
osmosis
done
clear
D)
transpiration
done
clear
View Answer play_arrow
question_answer 155) Statement A : All metatherians are placental mammals. Statement B : All placental mammals have menstrual cycle.
A)
Statement A is true and statement B is false
done
clear
B)
Statement B is true and statement A is false
done
clear
C)
Both the statements A and B are true
done
clear
D)
Both the statements A and B are false
done
clear
View Answer play_arrow
question_answer 156) Population density of terrestrial organisms is measured in terms of individual per
A)
\[mete{{r}^{3}}\]
done
clear
B)
\[mete{{r}^{4}}\]
done
clear
C)
\[meter\]
done
clear
D)
\[mete{{r}^{2}}\]
done
clear
View Answer play_arrow
question_answer 157) Nitrogenous waste products are eliminated mainly as
A)
urea in tadpole and uric acid in adult frog
done
clear
B)
urea in adult frog and ammonia in tadpole
done
clear
C)
urea in tadpole as well as in adult frog
done
clear
D)
urea in tadpole and ammonia in adult frog
done
clear
View Answer play_arrow
question_answer 158) In man, the blue eye colour is recessive to the brown eye colour. If the boy has brown eye and his mother is blue eyed, what would be the phenorype of his father?
A)
Black eye
done
clear
B)
Brown eye
done
clear
C)
Green eye
done
clear
D)
Blue eye
done
clear
View Answer play_arrow
question_answer 159) Munch hypothesis is based on
A)
translocation of food due to TP gradient and imbibition force
done
clear
B)
translocation of food due to Turgor Pressure (TP) gradient
done
clear
C)
translocation of food due to imbibition force
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 160) Interferons are
A)
anti - bacterial protein
done
clear
B)
anti - viral protein
done
clear
C)
complex protein
done
clear
D)
anti - clotting protein
done
clear
View Answer play_arrow
question_answer 161) The first process by which water enters into the seed coat when a seed is placed in suitable environment for germination is
A)
osmosis
done
clear
B)
active transport
done
clear
C)
absorption
done
clear
D)
imbibitions
done
clear
View Answer play_arrow
question_answer 162) .......... is the taxon, which is likely to move into endangered category in near future, if conditions prevail as it is
A)
vulnerable
done
clear
B)
endanger
done
clear
C)
rare
done
clear
D)
extinct
done
clear
View Answer play_arrow
question_answer 163) A localised inflammatory response appears at the site of infection causes redness, swelling, pain and heat due to certain chemical, they are
A)
histamine and prostaglandins
done
clear
B)
cerumen and mucus
done
clear
C)
histamine and cerumen
done
clear
D)
prostaglandins and cerumen
done
clear
View Answer play_arrow
question_answer 164) Non - keratinised stratified epithelium occurs in
A)
vagina, cervix and buccal cavity
done
clear
B)
vagina, cervix, buccal cavity and anus
done
clear
C)
vagina and cervix
done
clear
D)
buccal cavity and anus
done
clear
View Answer play_arrow
question_answer 165) Saccus entericus is secreted by
A)
crypts of Leiberkuhn
done
clear
B)
Brunners gland
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 166) Synthesis of food in \[{{C}_{4}}\]pathway occurs in chlorophyll of
A)
guard cells
done
clear
B)
bundle sheath
done
clear
C)
spongy mesophyll
done
clear
D)
palisade cells
done
clear
View Answer play_arrow
question_answer 167) The sequence of structural gene in lac operon concept is
A)
lac A, lac Y, lac Z
done
clear
B)
lac A, lac Z, lac Y
done
clear
C)
lac Y, lac Z, lac A
done
clear
D)
lac Z, lac Y, lac A
done
clear
View Answer play_arrow
question_answer 168) Pericarp and placentae are edible part of simple fleshy berry fruit
A)
jack fruit
done
clear
B)
banana
done
clear
C)
tomato
done
clear
D)
date palm
done
clear
View Answer play_arrow
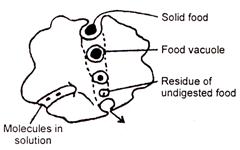
question_answer 169)
In the diagram, which of the following processes are shown in Amoeba?
A)
Exocytosis
done
clear
B)
Phagocyiosis
done
clear
C)
Pinocytosis
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 170) An essential element is that which
A)
improve health of the plant
done
clear
B)
is irreplaceable and, indispensable for growth of plants
done
clear
C)
is found in plant ash
done
clear
D)
is available in the soil
done
clear
View Answer play_arrow
question_answer 171) Residual volume is
A)
lesser than tidal volume
done
clear
B)
greater than inspiratory volume
done
clear
C)
greater than vital capacity
done
clear
D)
greater than tidal volume
done
clear
View Answer play_arrow
question_answer 172) Find the odd example
A)
sea lily
done
clear
B)
sea fan
done
clear
C)
sea cucumber
done
clear
D)
sea urchin
done
clear
View Answer play_arrow
question_answer 173) Which one is correct?
A)
Blood = plasma + RBC + WBC + blood platelets
done
clear
B)
Plasma = blood - lymphocytes
done
clear
C)
Neuron = cyton + dendrite + axon + synapse
done
clear
D)
Lymph = plasma + RBC + WBC
done
clear
View Answer play_arrow
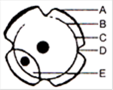
question_answer 174)
In the given diagram name the parts A, B, C, D and E
A)
A - germ pore, B-generative cell, C - intine, D -exine, E - vegetative cell
done
clear
B)
A - germ pore, B - generative cell, C - exine, D -intine, E - vegetative cell
done
clear
C)
A - intine, B - exine, C - germ pore, D -generative cell, E - vegetative cell
done
clear
D)
A - exine, B - intine, C - vegetative cell, D -germ pore, E - generative cell
done
clear
View Answer play_arrow
question_answer 175) The largest RBCs have been seen in
A)
elephant
done
clear
B)
whale
done
clear
C)
amphibia
done
clear
D)
man
done
clear
View Answer play_arrow
question_answer 176) Nucleic acid occurs in
A)
golgi body
done
clear
B)
lysosmoes
done
clear
C)
cytoplasm
done
clear
D)
mitochondria and chloroplast
done
clear
View Answer play_arrow
question_answer 177) The number of mitotic cell division required to produce 256 cells from single cell would be
A)
10
done
clear
B)
12
done
clear
C)
6
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 178) The central dogma of protein synthesis in teminious is
A)
g-RNA \[\to \]DNA \[\to \]m - RNA \[\to \] Protein
done
clear
B)
DNA \[\to \] g-RNA \[\to \] m - RNA \[\to \] Protein
done
clear
C)
DNA \[\to \] DNA \[\to \] m - RNA \[\to \] Protein
done
clear
D)
m - RNA \[\to \] g - RNA \[\to \] DNA \[\to \] Protein
done
clear
View Answer play_arrow
question_answer 179) In tissue culture roots can be induced by
A)
lower concentration of cytokinin and higher concentration of auxins
done
clear
B)
only cytokinin and no auxins
done
clear
C)
no cytokinin and only auxins
done
clear
D)
higher concentration of cytokinin and lower concentration of auxins
done
clear
View Answer play_arrow
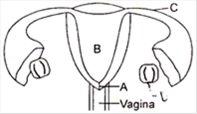
question_answer 180)
A)
A - oviduct, B - uterus, C - outduct, D - ovary
done
clear
B)
A - cervix, B - uterus, C - ovary, D - tumor
done
clear
C)
A - uterus, B - uterine cavity, C - oviducal funnel, D - ovary
done
clear
D)
A - cervix, B - uterine cavity, C - fallopian tube, D - ovary
done
clear
View Answer play_arrow
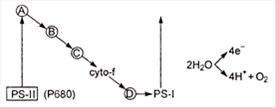 In the above schematic diagram, which is plastocyanin?
In the above schematic diagram, which is plastocyanin?
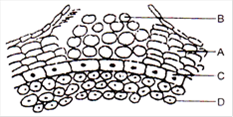
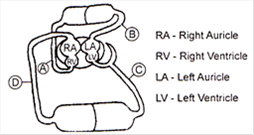 In the above given diagram which blood vessel represents vena cava?
In the above given diagram which blood vessel represents vena cava?