question_answer 1) When an additional charge of 2C is given to a capacitor, energy stored in it is increased by 21%. The original charge of the capacitor is
A)
\[30C\]
done
clear
B)
\[40C\]
done
clear
C)
\[10C\]
done
clear
D)
\[20C\]
done
clear
View Answer play_arrow
question_answer 2)
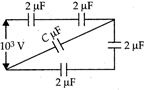
When a potential difference of \[{{10}^{3}}V\]is applied between A and B, a charge of 0.75 mC is stored in the system of capacitors as shown. The value of C is ( in \[\mu F\])
A)
\[\frac{1}{2}\]
done
clear
B)
\[2\]
done
clear
C)
\[2.5\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 3)
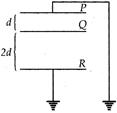
See the diagram. Area of each plate is \[2.0{{m}^{2}}\]and \[d=2\times {{10}^{-3}}m\]. A charge of \[8.85\times {{10}^{-8}}C\]is given to Q. Then the potential 2d of Q becomes
A)
\[13V\]
done
clear
B)
\[10V\]
done
clear
C)
\[6.67V\]
done
clear
D)
\[8.825V\]
done
clear
View Answer play_arrow
question_answer 4) Three conductors draw currents of 1 A, 2 A and 3 A respectively, when connected in turn across a battery. If they are connected in series and the combination is connected across the same battery, the current drawn will be
A)
\[\frac{2}{7}A\]
done
clear
B)
\[\frac{3}{7}A\]
done
clear
C)
\[\frac{4}{7}A\]
done
clear
D)
\[\frac{5}{7}A\]
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
question_answer 5)
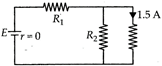
In the circuit, \[{{R}_{1}}={{R}_{2}}\]. The value of E and \[{{R}_{1}}\] are ____ (E - EMF, \[{{R}_{1}}\]- resistance)
A)
\[180V,60\Omega ~\]
done
clear
B)
\[120V,60\Omega ~\]
done
clear
C)
\[180V,10\Omega ~\]
done
clear
D)
\[120V,10\Omega ~\].
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
question_answer 6) Masses of three wires of copper are in the ratio of \[1:3:5\]and their lengths are in the ratio of \[5:3:1\]. The ratio of their electrical resistances is
A)
\[1:3:5\]
done
clear
B)
\[5:3:1\]
done
clear
C)
\[1:15:125\]
done
clear
D)
\[125:15:1\]
done
clear
View Answer play_arrow
question_answer 7) For a transformer, the turns ratio is 3 and its efficiency is 0.75. The current flowing in the primary coil is 2 A and the voltage applied to it is 100 V. Then the voltage and the current flowing in the secondary coil are _____ respectively.
A)
\[150V,1.5A\]
done
clear
B)
\[300V,0.5A\]
done
clear
C)
\[300V,1.5A\]
done
clear
D)
\[150V,0.5\text{ }A\]
done
clear
View Answer play_arrow
question_answer 8) A proton and helium nucleus are shot into a magnetic field at right angles to the field with same kinetic energy. Then the ratio of their radii is
A)
\[1:1\]
done
clear
B)
\[1:2\]
done
clear
C)
\[2:1\]
done
clear
D)
\[1:4\]
done
clear
View Answer play_arrow
question_answer 9) Two identical circular coils A and B are kept on a horizontal tube side by side without touching each other. If the current in the coil A increases with time, in response, the coil B
A)
is attracted by A
done
clear
B)
remains stationary
done
clear
C)
is repelled
done
clear
D)
rotates
done
clear
View Answer play_arrow
question_answer 10)
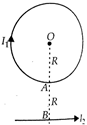
In the diagram, are the strength of the currents in the loop and straight conductors respectively. OA=OB=R. The net magnetic field at the canter O is zero. Then the ratio of the currents in the loop and the straight conductors is
A)
\[\pi \]
done
clear
B)
\[2\pi \]
done
clear
C)
\[\frac{1}{\pi }\]
done
clear
D)
\[\frac{1}{2\pi }\]
done
clear
View Answer play_arrow
question_answer 11) Two tangent galvanometers, which are identical except in their number of turns are connected in parallel. he ratio of their resistances of the coils is \[1:3\]. If the deflections in the two tangent galvanometers are \[{{30}^{o}}\]and \[{{60}^{o}}\] respectively, then the ratio of their number of turns is
A)
\[1:1\]
done
clear
B)
\[3:1\]
done
clear
C)
\[1:2\]
done
clear
D)
\[1:6\]
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
question_answer 12) A charged particle with a velocity \[2\times {{10}^{3}}m{{s}^{-1}}\] passes unelected through electric field and magnetic fields in mutually perpendicular directions. The magnetic field is 1.5 T. The magnitude of electric field will be
A)
\[1.5\times {{10}^{3}}N{{C}^{-1}}\]
done
clear
B)
\[2\times {{10}^{3}}N{{C}^{-1}}\]
done
clear
C)
\[8\times {{10}^{3}}N{{C}^{-1}}\]
done
clear
D)
\[1.33\times {{10}^{3}}N{{C}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 13) In R-L-C series circuit, the potential differences across each element is 20 V. Now the value of the resistance alone is doubled, then P.D. across R, L and C respectively
A)
\[20V,10V,10V\]
done
clear
B)
\[20V,20V,20V\]
done
clear
C)
\[20V,40V,40V\]
done
clear
D)
\[10V,20V,20V\]
done
clear
View Answer play_arrow
question_answer 14) A rectangular coil of 100 turns and size\[0.1m\times 0.05m\] is placed perpendicular to a magnetic field of 0.1 T. If the field drops to 0.05 T in 0.05 second, the magnitude of the e.m.f. induced in the coil is
A)
\[\sqrt{2}\]
done
clear
B)
\[\sqrt{3}\]
done
clear
C)
\[\sqrt{0.6}\]
done
clear
D)
\[\sqrt{6}\]
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
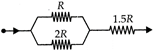
question_answer 15)
In the circuit diagram, heat produces in R, 2R and 1.5R are in the ratio of
A)
\[4:2:3\]
done
clear
B)
\[8:4:27\]
done
clear
C)
\[2:4:3\]
done
clear
D)
\[27:8:4\]
done
clear
View Answer play_arrow
question_answer 16) A series combination of resistor (R), capacitor is connected to an A.C. source of angular frequency W. Keeping the voltage same, if the frequency is changed to\[\frac{\omega }{3}\], the current becomes half of the ; original current. Then the ratio of the capacitive reactance and resistance at the former frequency is
A)
\[\sqrt{0.6}\]
done
clear
B)
\[\sqrt{3}\]
done
clear
C)
\[\sqrt{2}\]
done
clear
D)
\[\sqrt{6}\]
done
clear
View Answer play_arrow
question_answer 17) Pick out the correct statement from the following:
A)
Mercury vapour lamp produces line emission spectrum.
done
clear
B)
Oil flame produces line emission spectrum.
done
clear
C)
Band spectrum helps us to study molecular structure.
done
clear
D)
Sunlight spectrum is an example for line absorption spectrum.
done
clear
View Answer play_arrow
question_answer 18) Light emitted during the de excitation of electron from \[n=3\] to \[n=2\], when incident on a metal, photoelectrons are just emitted from that metal. In which of the following dexcitations photoelectric effect is not possible?
A)
From \[n=2\] to \[n=1\]
done
clear
B)
From \[n=3\] to \[n=1\]
done
clear
C)
From \[n=5\] to \[n=2\]
done
clear
D)
From \[n=4\] to \[n=3\]
done
clear
View Answer play_arrow
question_answer 19) The additional energy that should be given to an electron to reduce its de-Broglie wavelength from nm to 0.5 nm is
A)
2 times the initial kinetic energy
done
clear
B)
3 times the initial kinetic energy
done
clear
C)
0.5 times the initial kinetic energy
done
clear
D)
4 times the initial kinetic energy
done
clear
View Answer play_arrow
question_answer 20) The ionisation energy of an electron in the ground state of helium atom is 24.6 eV. The energy required to remove both the electron is
A)
\[51.8eV\]
done
clear
B)
\[79\text{ }eV\]
done
clear
C)
\[38.2eV\]
done
clear
D)
\[49.2\text{ }eV\]
done
clear
View Answer play_arrow
question_answer 21)
____________ \[3E\] ____________ \[5E/3\] ____________ \[E\]
The figure shows the energy level of certain atom. When the electron deexcites from \[3E\] to\[E\], an electromagnetic wave of wavelength \[\lambda \] is emitted. What is the wavelength of the electromagnetic wave emitted when the electron de excites from \[\frac{5E}{3}\]to E?
A)
\[3\lambda \]
done
clear
B)
\[2\lambda \]
done
clear
C)
\[5\lambda \]
done
clear
D)
\[\frac{3\lambda }{5}\]
done
clear
View Answer play_arrow
question_answer 22) Maximum velocity of the photoelectron emitted by a metal is \[1.8\times {{10}^{6}}m{{s}^{-1}}\]. Take the value of specific charge of the electron is \[1.8\times {{10}^{11}}C\,k{{g}^{-1}}\]. Then the stopping potential in volt is
A)
\[1\]
done
clear
B)
\[3\]
done
clear
C)
\[9\]
done
clear
D)
\[6\]
done
clear
View Answer play_arrow
question_answer 23) \[{{\lambda }_{1}}\] and \[{{\lambda }_{2}}\] are used to illuminate the slits. \[{{\beta }_{1}}\] and \[{{\beta }_{2}}\] are the corresponding fringe widths. The wavelength \[{{\lambda }_{1}}\] can produce photoelectric effect when incident on a metal. But the wavelength \[{{\lambda }_{2}}\] cannot produce photoelectric effect. The correct relation between \[{{\beta }_{1}}\] and \[{{\beta }_{2}}\] is
A)
\[{{\beta }_{1}}<{{\beta }_{2}}\]
done
clear
B)
\[{{\beta }_{1}}={{\beta }_{2}}\]
done
clear
C)
\[{{\beta }_{1}}>{{\beta }_{2}}\]
done
clear
D)
\[{{\beta }_{1}}\ge {{\beta }_{2}}\]
done
clear
View Answer play_arrow
question_answer 24) Pick out the correct statement/s from the following. (1) Electron emission during p-decay is always accompanied by neutrino. (2) Nuclear force is charge independent. (3) Fusion is the chief source of stellar energy.
A)
(1), (2) are correct
done
clear
B)
(1), (3) are correct
done
clear
C)
Only (1) is correct
done
clear
D)
(2), (3) are correct
done
clear
View Answer play_arrow
question_answer 25) A nucleus \[_{Z}{{X}^{A}}\] emits an a-particle with velocity v. The recoil speed of the daughter nucleus is
A)
\[\frac{A-4}{4v}\]
done
clear
B)
\[\frac{4v}{A-4}\]
done
clear
C)
\[v\]
done
clear
D)
\[\frac{v}{4}\]
done
clear
View Answer play_arrow
question_answer 26) A radioactive substance emits 100 beta particles in the first 2 seconds and 50 beta particles in the next 2 seconds. The mean life of the sample is
A)
4 seconds
done
clear
B)
2 seconds
done
clear
C)
\[\frac{2}{0.693}\]seconds
done
clear
D)
\[2\times 0.693\] seconds
done
clear
View Answer play_arrow
question_answer 27) In which of the following statements, the obtained impure semiconductor is of\[p-type\]?
A)
Germanium is doped with bismuth
done
clear
B)
Silicon is doped with antimony
done
clear
C)
Germanium is doped with gallium
done
clear
D)
Silicon is doped with phosphorus
done
clear
View Answer play_arrow
question_answer 28) The width of the depletion region in a P-N junction diode is
A)
increased by reverse bias
done
clear
B)
increased by forward bias
done
clear
C)
decreased by reverse bias
done
clear
D)
independent of the bias voltage -
done
clear
View Answer play_arrow
question_answer 29) When the transistor is used as an amplifier
A)
Emitter-base junction must be reverse biased, Collector-base junction must be forward biased.
done
clear
B)
Emitter-base junction must be forward biased, Collector-base junction must be forward biased.
done
clear
C)
Emitter-base junction must be reverse biased, Collector-base junction must be reverse biased.
done
clear
D)
Emitter-base junction must be forward biased, Collector-base junction must be reverse biased.
done
clear
View Answer play_arrow
question_answer 30) Which of the following is not made by quarks?
A)
Neutron
done
clear
B)
Positron
done
clear
C)
Proton
done
clear
D)
\[\pi \]-meson
done
clear
View Answer play_arrow
question_answer 31) Which one of the following is NOT correct?
A)
In forward biased condition diode conducts.
done
clear
B)
If the packing fraction is negative, the element is stable.
done
clear
C)
Binding energy is the energy equivalent to mass defect.
done
clear
D)
Radioactive element can undergo spontaneous fission.
done
clear
View Answer play_arrow
question_answer 32) The output of an OR gate is connected to both the inputs of a NAND gate. The combination will serve as
A)
AND gate
done
clear
B)
NOT gate
done
clear
C)
NAND gate
done
clear
D)
NOR gate
done
clear
View Answer play_arrow
question_answer 33) A and B are the two radioactive elements. The mixture of these elements show a total activity of 1200 disintegrations/minute. The half life of A is 1 day and that of B is 2 days. What will be the total activity after 4 days? Given: The initial number of atoms in A and B are equal.
A)
200dis/min
done
clear
B)
250 dis/min
done
clear
C)
500 dis/min
done
clear
D)
150 dis/min
done
clear
View Answer play_arrow
question_answer 34) The binding energy/nucleon of deuteron \[{{(}_{1}}{{H}^{2}})\]and the helium atom \[{{(}_{2}}H{{e}^{4}})\] are \[1.1MeV\]and \[7MeV\]respectively. If the two deuteron atoms fuse to form a single helium atom, then the energy released is
A)
\[26.9\text{ }MeV\]
done
clear
B)
\[25.8\text{ }MeV\]
done
clear
C)
\[23.6\text{ }MeV\]
done
clear
D)
\[12.9\text{ }MeV\]
done
clear
View Answer play_arrow
question_answer 35) Which one of the following is NOT correct?
A)
Dimensional formula of thermal conductivity \[\left( K \right)\] is \[{{M}^{1}}{{L}^{1}}{{T}^{-3}}{{K}^{-1}}\].
done
clear
B)
Dimensional formula of potential \[\left( V \right)\], \[{{M}^{1}}{{L}^{1}}{{T}^{-2}}{{A}^{-1}}\].
done
clear
C)
Dimensional formula of permeability of free space \[({{\mu }_{0}})\] is \[{{M}^{1}}{{L}^{1}}{{T}^{-2}}{{A}^{-2}}\]
done
clear
D)
Dimensional formula of \[RC\]is \[{{M}^{0}}{{L}^{0}}{{T}^{-1}}\].
done
clear
View Answer play_arrow
question_answer 36) In a lift moving up with an acceleration of \[5m{{s}^{-2}}\], a ball is dropped from a height of 1.25 m. The time taken by the ball to reach the floor of the lift is ......(nearly) (g \[=10m{{s}^{-2}}\])
A)
0.3 second
done
clear
B)
0.2 second
done
clear
C)
0.16 second
done
clear
D)
0.4 second
done
clear
View Answer play_arrow
question_answer 37) A gun fires a small bullet with kinetic energy K. Then kinetic energy of the gun while recoiling is
A)
K
done
clear
B)
more than K
done
clear
C)
less than K
done
clear
D)
\[\sqrt{K}\]
done
clear
View Answer play_arrow
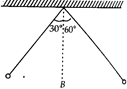
question_answer 38)
From a fixed support, two small identical spheres arc suspended by means of strings of length 1 m each. They are pulled aside as shown and then released. B is the mean position. Then the two spheres collide,
A)
at B after 0.25 second
done
clear
B)
at B after 0.5 second
done
clear
C)
on the right side of B after some time
done
clear
D)
on the right side of B when the strings are inclined at \[{{15}^{o}}\] with B
done
clear
View Answer play_arrow
question_answer 39) A truck accelerates from speed v to 2v. Work done in during this is
A)
three times as the work done in accelerating it from rest to v.
done
clear
B)
. same as the work done in accelerating it from rest to v.
done
clear
C)
four times as the work done in accelerating it from rest to v.
done
clear
D)
less than the work done in accelerating it from rest to v.
done
clear
View Answer play_arrow
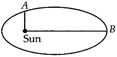
question_answer 40)
Earth is moving around the Sun in elliptical orbit as shown. The ratio of OB and OA is R. Then ratio of Earth at A and B is
A)
\[{{R}^{-1}}\]
done
clear
B)
\[\sqrt{R}\]
done
clear
C)
\[R\]
done
clear
D)
\[{{R}^{2/3}}\]
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
question_answer 41) A projectile is projected at \[10\text{ }m{{s}^{-1}}\]cby making at an angle \[{{60}^{o}}\] to the horizontal. After some time its velocity makes an angle of \[{{30}^{o}}\] to the horizontal. Its speed at this instant is
A)
\[\frac{10}{\sqrt{3}}\]
done
clear
B)
\[10\sqrt{3}\]
done
clear
C)
\[\frac{5}{\sqrt{3}}\]
done
clear
D)
\[5\sqrt{3}\]
done
clear
View Answer play_arrow
question_answer 42) For which combination of working temperatures of source and sink, the efficiency of Carnots heat engine is maximum?
A)
\[600K,400K\]
done
clear
B)
\[400K,200K\]
done
clear
C)
\[500K,300K\]
done
clear
D)
\[300K,100K\]
done
clear
View Answer play_arrow
question_answer 43) A solid cylinder of radius R made of a material of thermal conductivity \[{{K}_{1}}\] is surrounded by a cylindrical shell of inner radius R and outer radius 1R made of a material of thermal conductivity\[{{K}_{2}}\]. The two ends of the combined system are maintained at two different temperatures. Then there is no loss of heat across the cylindrical surface and the system is in steady state. The effective thermal conductivity of the system is
A)
\[{{K}_{1}}+{{K}_{2}}\]
done
clear
B)
\[\frac{{{K}_{1}}{{K}_{2}}}{{{K}_{1}}+{{K}_{2}}}\]
done
clear
C)
\[\frac{3{{K}_{1}}+{{K}_{2}}}{4}\]
done
clear
D)
\[\frac{{{K}_{1}}+3{{K}_{2}}}{4}\]
done
clear
View Answer play_arrow
question_answer 44) Two stars A and B radiate maximum energy at the wavelengths of 360 nm and 480 nm respectively. Then the ratio of the surface temperatures of A and B is
A)
\[3:4\]
done
clear
B)
\[81:256\]
done
clear
C)
\[4:3\]
done
clear
D)
\[256:81\]
done
clear
View Answer play_arrow
question_answer 45) Two solids P and Q float in water. It is observed that P floats with half of its volume immersed and Q floats with \[\frac{{{2}^{rd}}}{3}\] of its volume is immersed. The ratio of densities of P and Q is
A)
\[\frac{4}{3}\]
done
clear
B)
\[\frac{3}{4}\]
done
clear
C)
\[\frac{2}{3}\]
done
clear
D)
\[\frac{3}{2}\]
done
clear
View Answer play_arrow
question_answer 46) The equation of a transverse wave is given by \[y=0.05\sin \pi (2t-0.02x)\], where x, y are in metre and t is in second. The minimum distance of separation between two particles which are in phase and the wave velocity are respectively ____
A)
\[50m,50m{{s}^{-1}}\]
done
clear
B)
\[100m,100m{{s}^{-1}}\]
done
clear
C)
\[50m,100m{{s}^{-1}}\]
done
clear
D)
\[100m,50m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 47) The frequency of the second overtone of the open pipe is equal to the frequency of first overtone of the closed pipe. The ratio of the lengths of the open pipe and the closed pipe is
A)
\[2:1\]
done
clear
B)
\[1:2\]
done
clear
C)
\[1:3\]
done
clear
D)
\[3:1\]
done
clear
View Answer play_arrow
question_answer 48) A person with vibrating tuning fork of frequency 338 Hz is moving towards a vertical wall with a speed of \[2\text{ }m{{s}^{-1}}\]. Velocity of sound in air is \[340m{{s}^{-1}}\]. The number of beats heard by that person per second is
A)
\[2\]
done
clear
B)
\[4\]
done
clear
C)
\[6\]
done
clear
D)
\[8\]
done
clear
View Answer play_arrow
question_answer 49) Pick out the WRONG statement from the following.
A)
Lateral shift increases as the angle of incidence increases.
done
clear
B)
Lateral shift increases as the value of refractive index increases.
done
clear
C)
Normal shift decreases as the value of refractive index increases.
done
clear
D)
Both normal shift and lateral shift are directly proportional to the thickness of the medium.
done
clear
View Answer play_arrow
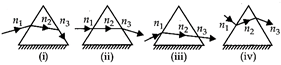
question_answer 50)
The refraction through the prisms are as shown. Pick out the WRONG statement from the following. Path of the light ray in
A)
(i) is correct if \[{{n}_{2}}>{{n}_{1}}\] and \[{{n}_{2}}>{{n}_{3}}\]
done
clear
B)
(ii) is correct if \[{{n}_{1}}={{n}_{2}}\] and \[{{n}_{2}}>{{n}_{3}}\]
done
clear
C)
(iii) is correct if \[{{n}_{2}}>{{n}_{1}}\] and \[{{n}_{2}}={{n}_{3}}\]
done
clear
D)
(iv) is correct if \[{{n}_{1}}>{{n}_{2}}\] and \[{{n}_{2}}<{{n}_{3}}\]
done
clear
View Answer play_arrow
question_answer 51) The distance between an object and its real image produced by a converging lens is 0.72 m. The magnification is 2. What will be the magnification when the object is moved by 0.04 m towards the lens?
A)
2
done
clear
B)
4
done
clear
C)
3
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 52) The speed of light in media \[{{M}_{1}}\]and \[{{M}_{2}}\] are \[1.5\times {{10}^{8}}m{{s}^{-1}}\] and \[2\times {{10}^{8}}m{{s}^{-1}}\]respectively. A ray travels from medium \[{{M}_{1}}\] to the medium \[{{M}_{2}}\] with an angle of incidence \[\theta \]. The ray suffers total internal reflection. Then the value of the .angle of incidence \[\theta \] is
A)
\[>{{\sin }^{-1}}\left( \frac{3}{4} \right)\]
done
clear
B)
\[<{{\sin }^{-1}}\left( \frac{3}{4} \right)\]
done
clear
C)
\[={{\sin }^{-1}}\left( \frac{4}{3} \right)\]
done
clear
D)
\[\le {{\sin }^{-1}}\left( \frac{4}{3} \right)\]
done
clear
View Answer play_arrow
question_answer 53)
Which of the following phenomena support the wave theory of light? 1. Scattering 2. Interference 3.Diffraction 4. Velocity of light in a denser medium is less than the velocity of light in the rarer medium
A)
\[1,2,3\]
done
clear
B)
\[1,2,4\]
done
clear
C)
\[2,3,4\]
done
clear
D)
\[1,3,4\]
done
clear
View Answer play_arrow
question_answer 54) White light reflected from a soap film (Refractive index = 1.5) has a maxima at 600 nm and a minima at 450 nm with no minimum in between. Then the thickness of the film is ____\[\times {{10}^{-7}}m\].
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 55) A cylindrical tube of length 0.2 m and radius R with sugar solution of concentration C produce a rotation of \[\theta \] in the plane of vibration of a plane polarized light. The same sugar solution is transferred to another tube of length 0.3 m of same radius. The remaining gap is filled by distilled water. Now the optical rotation produced is
A)
\[\theta \]
done
clear
B)
\[2\frac{\theta }{3}\]
done
clear
C)
\[3\frac{\theta }{2}\]
done
clear
D)
\[9\frac{\theta }{4}\]
done
clear
View Answer play_arrow
question_answer 56) Radii of curvature of a converging lens are in the ratio \[1:2\]. Its focal length is 6 cm and refractive index is 1.5. Then its radii of curvature are ____respectively.
A)
9 cm and 18 cm
done
clear
B)
6 cm and 12 cm
done
clear
C)
3 cm and 6 cm
done
clear
D)
4.5 cm and 9 cm
done
clear
View Answer play_arrow
question_answer 57) A small oil drop of mass \[{{10}^{-6}}kg\] is hanging in at rest between two plates separated by 1 mm having a potential difference of 500 V. The charge on the drop is ____ \[(g=10m{{s}^{-2}})\]
A)
\[2\times {{10}^{-9}}C\]
done
clear
B)
\[2\times {{10}^{-11}}C\]
done
clear
C)
\[2\times {{10}^{-6}}C\]
done
clear
D)
\[2\times {{10}^{-8}}C\]
done
clear
View Answer play_arrow
question_answer 58)
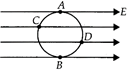
A uniform electric field in the plane of the paper as shown. Here A, B, C, D are the points on the circle. \[{{V}_{1}},{{V}_{2}},{{V}_{3}},{{V}_{4}}\] are the potentials at those points respectively. The
A)
\[{{V}_{A}}={{V}_{C}},{{V}_{B}}={{V}_{D}}\]
done
clear
B)
\[{{V}_{A}}={{V}_{C}},{{V}_{B}}>{{V}_{D}}\]
done
clear
C)
\[{{V}_{A}}>{{V}_{C}},{{V}_{B}}>{{V}_{D}}\]
done
clear
D)
\[{{V}_{A}}={{V}_{B}},{{V}_{C}}={{V}_{D}}\]
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
question_answer 59) Two metal spheres of radii 0.01 m and 0.02 m are given a charge of \[15mC\]and \[45mC\]respectively. They are then connected by a wire. The final charge on the first sphere is ____\[\times {{10}^{-3}}C\]
A)
\[40\]
done
clear
B)
\[30\]
done
clear
C)
\[20\]
done
clear
D)
\[10\]
done
clear
View Answer play_arrow
question_answer 60) Two concentric spheres of radii R and r have positive charges \[{{q}_{1}}\]and \[{{q}_{2}}\] with equal surface charge densities. What is the electric potential at their common center?
A)
\[\frac{\sigma }{{{\varepsilon }_{0}}}(R+r)\]
done
clear
B)
\[\frac{\sigma }{{{\varepsilon }_{0}}}(R-r)\]
done
clear
C)
\[\frac{\sigma }{{{\varepsilon }_{0}}}\left( \frac{1}{R}+\frac{1}{r} \right)\]
done
clear
D)
\[\frac{\sigma }{{{\varepsilon }_{0}}}\left( \frac{R}{r} \right)\]
done
clear
View Answer play_arrow
question_answer 61) Methane can be converted into ethane by the Reactions
A)
chlorination followed by the reaction with alcoholic \[KOH\]
done
clear
B)
chlorination followed by the reaction with aqueous \[KOH\]
done
clear
C)
Chlorination followed by Wurtz reaction
done
clear
D)
Chlorination followed by decarboxylation.
done
clear
View Answer play_arrow
question_answer 62) Intramolecular hydrogen bonding is formed in
A)
\[{{H}_{2}}O\]
done
clear
B)
salicylaldehyde
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
benzophenone.
done
clear
View Answer play_arrow
question_answer 63) If 50% of the reactant is converted into a product in a first order reaction in 25 minutes, how much of it would react in 100 minutes?
A)
\[93.75%\]
done
clear
B)
\[87.5%\]
done
clear
C)
\[75%\]
done
clear
D)
\[100%\]
done
clear
View Answer play_arrow
question_answer 64) The number of optical isomers of the compound, \[C{{H}_{3}}-CHBr-CHBr-COOH\] is
A)
\[0\]
done
clear
B)
\[1\]
done
clear
C)
\[3\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 65) When limestone is heated, \[C{{O}_{2}}\] is given off. The metallurgical operation is
A)
smelting
done
clear
B)
reduction
done
clear
C)
calcination
done
clear
D)
roasting.
done
clear
View Answer play_arrow
question_answer 66) The rate of reaction increases with rise in temperature because of
A)
increase in number of activated molecules
done
clear
B)
increase in energy of activation
done
clear
C)
decrease in energy of activation
done
clear
D)
increase in the number of effective collisions.
done
clear
View Answer play_arrow
question_answer 67) Meso compounds do not show optical activity because
A)
they do not contain chiral carbon atoms
done
clear
B)
they have non-superimposable mirror images
done
clear
C)
they contain plane of symmetry
done
clear
D)
they do not contain plane of symmetry.
done
clear
View Answer play_arrow
question_answer 68) When formic acid is heated with concentrated \[{{H}_{2}}S{{O}_{4}}\], the gas evolved is
A)
only \[C{{O}_{2}}\]
done
clear
B)
only \[CO\]
done
clear
C)
a mixture of \[CO\] and \[C{{O}_{2}}\]
done
clear
D)
a mixture of \[S{{O}_{2}}\]and \[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 69) Temperature coefficient of a reaction is 2. When temperature is increased from \[{{30}^{o}}C\]to \[{{90}^{o}}C\], the rate of reaction is increased by
A)
60 times
done
clear
B)
64 times
done
clear
C)
150 times
done
clear
D)
400 times
done
clear
View Answer play_arrow
question_answer 70) Conversion of benzene to acetophenone can e brought by
A)
Wurtz reaction
done
clear
B)
Wurtz-Fittigs reaction
done
clear
C)
Friedel Crafts alkylation
done
clear
D)
Friedel Crafts acylation.
done
clear
View Answer play_arrow
question_answer 71) Excess of \[PC{{l}_{5}}\] reacts with concentrated \[{{H}_{2}}S{{O}_{4}}\] giving
A)
chlorosulphuric acid
done
clear
B)
sulphurous acid
done
clear
C)
sulphuryl chloride
done
clear
D)
thionyl chloride.
done
clear
View Answer play_arrow
question_answer 72) An example for a neutral buffer is
A)
ammonium hydroxide and ammonium chloride
done
clear
B)
acetic acid and sodium acetate
done
clear
C)
acetic acid and ammonium hydroxide
done
clear
D)
citric acid and sodium citrate.
done
clear
View Answer play_arrow
question_answer 73) Least energetic conformation of cyclohexane is
A)
chair conformation
done
clear
B)
boat conformation
done
clear
C)
cis conformation
done
clear
D)
E-Z form.
done
clear
View Answer play_arrow
question_answer 74) Which of the following is employed in flash tubes in photography?
A)
\[Ar\]
done
clear
B)
\[Ne\]
done
clear
C)
\[Kr\]
done
clear
D)
\[Xe\]
done
clear
View Answer play_arrow
question_answer 75) Conjugate base of H2P04- is
A)
\[HPO_{4}^{-}\]
done
clear
B)
\[HPO_{4}^{2-}\]
done
clear
C)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
D)
\[PO_{4}^{3-}\]
done
clear
View Answer play_arrow
question_answer 76) An alkyi bromide (X) reacts with sodium in ether to form 4, 5-diethyloctane, the compound X is
A)
\[C{{H}_{3}}{{(C{{H}_{2}})}_{3}}Br\]
done
clear
B)
\[C{{H}_{3}}{{(C{{H}_{2}})}_{5}}Br\]
done
clear
C)
\[C{{H}_{3}}{{(C{{H}_{2}})}_{3}}CH(Br)C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-{{(C{{H}_{2}})}_{2}}-CH(Br)-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 77) Which one of the following shows highest magnetic moment?
A)
\[F{{e}^{2+}}\]
done
clear
B)
\[C{{o}^{2+}}\]
done
clear
C)
\[C{{r}^{3+}}\]
done
clear
D)
\[N{{i}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 78) The emf of a galvanic cell constituted with the electrodes \[Z{{n}^{2+}}|Zn(-0.76V)\] and \[F{{e}^{2+}}|Fe\] \[(-0.41V)\] is
A)
\[-0.35V\]
done
clear
B)
\[+1.17V\]
done
clear
C)
\[+0.35V\]
done
clear
D)
\[-1.17V\]
done
clear
View Answer play_arrow
question_answer 79)
which of the following pairs are correctly hatched? Reactants Products I. \[RX+Ag{{(OH)}_{(eq)}}\] \[RH\] II. \[RX+AgC{{N}_{(alc)}}\] \[RNC\] III. \[RX+KC{{N}_{(alc)}}\] \[RNC\] IV. \[RX+N{{a}_{(ether)}}\] \[R-R\]
A)
I alone
done
clear
B)
I and II
done
clear
C)
II and III
done
clear
D)
II and IV
done
clear
View Answer play_arrow
question_answer 80) In a transition series, with the increase in atomic number, the paramagnetism
A)
increases gradually
done
clear
B)
decreases gradually
done
clear
C)
first increases to a maximum and then decreases
done
clear
D)
first decreases to a minimum and then increases
done
clear
View Answer play_arrow
question_answer 81) Identify a species which is NOT a Bronsted acid but a Lewis acid.
A)
\[B{{F}_{3}}\]
done
clear
B)
\[H_{3}^{+}O\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[HCl\]
done
clear
View Answer play_arrow
question_answer 82) The compound formed when calcium acetate and calcium formate is dry distilled.
A)
Acetone
done
clear
B)
Acetaldehyde
done
clear
C)
Benz aldehyde
done
clear
D)
Acetophenone
done
clear
View Answer play_arrow
question_answer 83) \[{{d}^{2}}-s{{p}^{3}}\] hybridisation of the atomic orbitals gives
A)
square planar structure
done
clear
B)
triangular structure
done
clear
C)
tetrahedral structure
done
clear
D)
octahedral structure.
done
clear
View Answer play_arrow
question_answer 84) The pH of \[{{10}^{-8}}HCl\]solution is
A)
8
done
clear
B)
6.9586
done
clear
C)
more than 8
done
clear
D)
slightly more than 7
done
clear
View Answer play_arrow
question_answer 85) Which of the following is strongly acidic?
A)
Phenol
done
clear
B)
o-cresol
done
clear
C)
p-nitrophenol
done
clear
D)
p-cresol
done
clear
View Answer play_arrow
question_answer 86) A group of atoms can function as a ligand only when
A)
it is a small molecule
done
clear
B)
it has an unshared electron pair
done
clear
C)
it is a negatively charged ion
done
clear
D)
it is a positively charged ion.
done
clear
View Answer play_arrow
question_answer 87) Which of the following is NOT a colligative property?
A)
Elevation in boiling point
done
clear
B)
Depression in freezing point
done
clear
C)
Osmotic pressure
done
clear
D)
Lowering of vapour pressure
done
clear
View Answer play_arrow
question_answer 88) Acetone and propanal are
A)
functional isomers
done
clear
B)
position isomers
done
clear
C)
geometrical isomers
done
clear
D)
optical isomers.
done
clear
View Answer play_arrow
question_answer 89) Which of the following is diamagnetic?
A)
\[{{H}^{2+}}\]
done
clear
B)
\[H{{e}^{2+}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 90) \[3g\]of urea is dissolved in \[45g\]of\[{{H}_{2}}O\]. The relative lowering in vapour pressure is
A)
\[0.05\]
done
clear
B)
\[0.04\]
done
clear
C)
\[0.02\]
done
clear
D)
\[0.01\]
done
clear
View Answer play_arrow
question_answer 91) The reagent used to distinguish between acetaldehyde and Benz aldehyde is
A)
Tollens reagent
done
clear
B)
Fehlings solution
done
clear
C)
2, 4-dinitrophenylhydrazine
done
clear
D)
semicarbazide
done
clear
View Answer play_arrow
question_answer 92) Metallic lustre is due to
A)
high density of metals
done
clear
B)
high polish on the surface of metals
done
clear
C)
reflection of light by mobile electrons
done
clear
D)
chemical inertness of metals.
done
clear
View Answer play_arrow
question_answer 93) Which of the following aqueous solution will exhibit highest boiling point?
A)
\[0.01M\]urea
done
clear
B)
\[0.01M\] \[KN{{O}_{3}}\]
done
clear
C)
\[0.01M\]\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
D)
\[0.015M\]\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\]
done
clear
View Answer play_arrow
question_answer 94) Which one of the following gives amine on heating with amide?
A)
\[B{{r}_{2}}\] in aqueous KOH
done
clear
B)
\[B{{r}_{2}}\] in alcoholic KOH
done
clear
C)
\[C{{l}_{2}}\] in sodium
done
clear
D)
Sodium in ether
done
clear
View Answer play_arrow
question_answer 95) The number of antibonding electrons present in \[{{O}_{2}}\] molecular ion is
A)
\[8\]
done
clear
B)
\[6\]
done
clear
C)
\[5\]
done
clear
D)
\[4\]
done
clear
E)
None of the Above
done
clear
View Answer play_arrow
question_answer 96) The process is spontaneous at the given temperature, if
A)
\[\Delta H\] is \[+ve\] and \[\Delta S\] is -\[-ve\]
done
clear
B)
\[\Delta H\] is \[-ve\] and \[\Delta S\] is \[+ve\]
done
clear
C)
\[\Delta H\] is \[+ve\] and \[\Delta S\] is +\[+ve\]
done
clear
D)
\[\Delta H\] is \[+ve\] and \[\Delta S\] is equal to zero.
done
clear
View Answer play_arrow
question_answer 97) Glucose when reduced with HI and red phosphorus gives
A)
n-hexane
done
clear
B)
n-heptane
done
clear
C)
n-pentane
done
clear
D)
n-octane.
done
clear
View Answer play_arrow
question_answer 98) The stability of a lyophobic colloid is due to
A)
adsorption of covalent molecules on the colloid
done
clear
B)
the size of the particles
done
clear
C)
the charge on the particles
done
clear
D)
Tyndall effect.
done
clear
View Answer play_arrow
question_answer 99) Oils are liquids at room temperature since they contain higher percentage of
A)
oleates
done
clear
B)
palmitates
done
clear
C)
stearates
done
clear
D)
myristates
done
clear
View Answer play_arrow
question_answer 100) Which of the following cations will have minimum flocculation value for arsenic sulphide sol?
A)
\[N{{a}^{+}}\]
done
clear
B)
\[M{{g}^{2+}}\]
done
clear
C)
\[C{{a}^{2+}}\]
done
clear
D)
\[A{{l}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 101) The value of entropy of solar system is
A)
increasing
done
clear
B)
decreasing
done
clear
C)
constant
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 102) In face-centred cubic lattice, a unit cell is shared equally by how many unit cells?
A)
\[6\]
done
clear
B)
\[4\]
done
clear
C)
\[2\]
done
clear
D)
\[8\]
done
clear
View Answer play_arrow
question_answer 103) The number of disulphide linkages present in insulin are
A)
\[4\]
done
clear
B)
\[3\]
done
clear
C)
\[2\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 104) The process of zone refining is used in the purification of
A)
\[Al\]
done
clear
B)
\[Ge\]
done
clear
C)
\[Cu\]
done
clear
D)
\[Ag\]
done
clear
View Answer play_arrow
question_answer 105) The number of water molecules present in a drop of water weighing 0.018 g is
A)
\[6.022\times {{10}^{26}}\]
done
clear
B)
\[6.022\times {{10}^{23}}\]
done
clear
C)
\[6.022\times {{10}^{19}}\]
done
clear
D)
\[6.022\text{ }\times {{10}^{20}}\]
done
clear
View Answer play_arrow
question_answer 106) Empirical formula of a compound is \[C{{H}_{2}}O\] and its molecular mass is 90, the molecular formula of the compound is
A)
\[{{C}_{3}}{{H}_{6}}{{O}_{3}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{4}}{{O}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\]
done
clear
D)
\[C{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 107) Hybridised states of carbon in graphite and diamond are respectively
A)
\[s{{p}^{3}},s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{3}},s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{2}},s{{p}^{2}}\]
done
clear
D)
\[s{{p}^{2}},s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 108) The mass of \[112c{{m}^{3}}\]of \[N{{H}_{3}}\]gas at STP is
A)
\[0.085g\]
done
clear
B)
\[0.850g\]
done
clear
C)
\[8.500g\]
done
clear
D)
\[80.500g\]
done
clear
View Answer play_arrow
question_answer 109) IUPAC name of \[C{{H}_{3}}-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-C{{H}_{2}}-\underset{COOH}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-C{{H}_{3}}\] is
A)
4-hydroxy-l-methyl pentanoic acid
done
clear
B)
4-hydroxy-2-methyl pentanoic acid
done
clear
C)
2-hydroxy-4-methyl pentanoic acid
done
clear
D)
2-hydroxy-2-methyl pentanoic acid
done
clear
View Answer play_arrow
question_answer 110) Alkali metals have negative reduction potential and hence they behave as
A)
oxidising agents
done
clear
B)
Lewis bases
done
clear
C)
reducing agents
done
clear
D)
electrolytes
done
clear
View Answer play_arrow
question_answer 111) Which of the following gases has the highest value of RMS velocity at 298 K?
A)
\[C{{H}_{4}}\]
done
clear
B)
\[CO\]
done
clear
C)
\[C{{l}_{2}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 112) Cycloalkane formed when 1,4-dibroinopentane is heated with sodium is
A)
methyl cyclobutane
done
clear
B)
cyclopentane
done
clear
C)
cyclobutane
done
clear
D)
methyl cyclopentane.
done
clear
View Answer play_arrow
question_answer 113) In the reaction, \[2FeS{{O}_{4}}+{{H}_{2}}S{{O}_{4}}+{{H}_{2}}{{O}_{2}}\to F{{e}_{2}}{{(S{{O}_{4}})}_{3}}+2{{H}_{2}}O\] the oxidizing agent is
A)
\[FeS{{O}_{4}}\]
done
clear
B)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
D)
both \[{{H}_{2}}S{{O}_{4}}\]and \[{{H}_{2}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 114) For the thermochemical equation, \[2{{H}_{2(g)}}+{{O}_{2(g)}}\to 2{{H}_{2}}{{O}_{(l)}};\,\Delta H=-571.6kJ\] Heat of decomposition of water is
A)
\[-571.6\text{ }kJ\]
done
clear
B)
\[+571.6\text{ }kJ\]
done
clear
C)
\[-1143.2\text{ }kJ\]
done
clear
D)
\[+285.8kJ\]
done
clear
View Answer play_arrow
question_answer 115) In Buna-S, the symbol Bu stands for
A)
1-butene
done
clear
B)
n-butene
done
clear
C)
2-butene
done
clear
D)
butadiene.
done
clear
View Answer play_arrow
question_answer 116) The electronic configuration of \[C{{u}^{2+}}\] ion is
A)
\[[Ar]\,3{{d}^{8}}\,4{{s}^{1}}\]
done
clear
B)
\[[Ar]\,3{{d}^{9}}\,4{{s}^{0}}\]
done
clear
C)
\[[Ar]\,3{{d}^{7}}\,4{{s}^{2}}\]
done
clear
D)
\[[Ar]\,3{{d}^{8}}\,4{{s}^{0}}\]
done
clear
View Answer play_arrow
question_answer 117) The yield of the products in the reaction, \[{{A}_{2(g)}}+2{{B}_{(g)}}\rightleftharpoons {{C}_{(g)}}+Q\,kJ\] would be higher at
A)
high temperature and high pressure
done
clear
B)
high temperature and low pressure
done
clear
C)
low temperature and high pressure
done
clear
D)
low temperature and low pressure.
done
clear
View Answer play_arrow
question_answer 118) Mesomeric effect involves
A)
delocalisation of \[\pi \]-electrons
done
clear
B)
delocalisation of \[\sigma \]-electrons
done
clear
C)
partial displacement of electrons
done
clear
D)
delocalisation of\[\pi \] and \[\sigma \]-electrons.
done
clear
View Answer play_arrow
question_answer 119) Which one of the following sets of ions represents the collection of isoelectronic species?
A)
\[{{K}^{+}},C{{l}^{-}},M{{g}^{2+}},S{{c}^{3+}}\]
done
clear
B)
\[N{{a}^{+}},C{{a}^{2+}},S{{c}^{3+}},{{F}^{-}}\]
done
clear
C)
\[{{K}^{+}},C{{a}^{2+}},S{{c}^{3+}},C{{l}^{-}}\]
done
clear
D)
\[N{{a}^{+}},M{{g}^{2+}},A{{l}^{3+}},C{{l}^{-}}\]
done
clear
View Answer play_arrow
question_answer 120) Adsorption theory is applicable for
A)
homogeneous catalysis
done
clear
B)
heterogeneous catalysis
done
clear
C)
autocatalysis
done
clear
D)
induced catalysis.
done
clear
View Answer play_arrow
question_answer 121)
Match the following list of animals with their level of organization and choose the correct sequence. Column I Column II A. Organ level (p) Pheretima B. Cellular aggregate level (q) Fasciola C. Tissue level (r) Spongilla D. Organ system level (s) Obelia
A)
\[A-(s),B-(r),C-(p),D-(q)\]
done
clear
B)
\[A-(s),B-(q),C-(r),D-(p)\]
done
clear
C)
\[A-(q),B-(s),C-(r),D-(p)\]
done
clear
D)
\[A-(q),B-(r),C-(s),D-(p)\]
done
clear
View Answer play_arrow
question_answer 122) Oxidative decarboxylation occurs during the formation of
A)
cirtic acid and succinic acid
done
clear
B)
citric acid and oxaloacetic acid
done
clear
C)
acetyl CoA and succinyl CoA
done
clear
D)
oxaloacetic acid and oxalosuccinic acid.
done
clear
View Answer play_arrow
question_answer 123) The edible part of the fruit of apple is
A)
endocarp
done
clear
B)
thalamus
done
clear
C)
pericarp
done
clear
D)
perianth.
done
clear
View Answer play_arrow
question_answer 124) Given below is an electron acceptor. Mention its status, which is labelled as A. \[Cy{{t}^{++}}\xrightarrow{2{{e}^{-}}}Cy{{t}^{+++}}\bigcirc A\]
A)
Oxidized
done
clear
B)
Reduced
done
clear
C)
Phosphorylated
done
clear
D)
Hydrated
done
clear
View Answer play_arrow
question_answer 125)
The floral formula
A)
Hibiscus
done
clear
B)
Banana
done
clear
C)
Tulip
done
clear
D)
Vinca
done
clear
View Answer play_arrow
question_answer 126) Interferoris are the protein molecules produced from the
A)
normal cells
done
clear
B)
infected host cells
done
clear
C)
macrophages
done
clear
D)
B lymphocytes.
done
clear
View Answer play_arrow
question_answer 127) Tikka is a
A)
fungal disease
done
clear
B)
viral disease
done
clear
C)
bacterial disease
done
clear
D)
protozoan disease.
done
clear
View Answer play_arrow
question_answer 128) Which of the following statements is correct?
A)
Each back cross is a test cross.
done
clear
B)
Each test cross is a back cross.
done
clear
C)
Crossing \[{{F}_{2}}\] with \[{{F}_{1}}\] is a test cross.
done
clear
D)
Crossing \[{{F}_{2}}\] with \[{{P}_{1}}\] is called a test cross.
done
clear
View Answer play_arrow
question_answer 129) Amrithmahal is a/an
A)
dual purpose breed
done
clear
B)
exotic breed
done
clear
C)
crossbreed
done
clear
D)
draught breed.
done
clear
View Answer play_arrow
question_answer 130) Gynaecomastia is the symptom of
A)
Klinefelters syndrome
done
clear
B)
Downs syndrome
done
clear
C)
Turners syndrome
done
clear
D)
cri-du-chat syndrome.
done
clear
View Answer play_arrow
question_answer 131) The branch of biology that deals with study of fossil animals is known as
A)
parabiology
done
clear
B)
phylogeny
done
clear
C)
paleontology
done
clear
D)
parazoology.
done
clear
View Answer play_arrow
question_answer 132) A colourblind man marries the daughter of another colourblind man whose wife has a normal genotype for colour vision. In their progeny
A)
all the children would be colourblind
done
clear
B)
all their sons are colourblind
done
clear
C)
none of the daughters would be colourblind
done
clear
D)
half of their sons and half of their daughters would be colourblind.
done
clear
View Answer play_arrow
question_answer 133) The plant which has antidiabetic properties is
A)
Ocimum sanctum
done
clear
B)
Gymnema sylvestre
done
clear
C)
Adhatoda vasica
done
clear
D)
Phyllanthus emblica
done
clear
View Answer play_arrow
question_answer 134) Deforestation means
A)
growing plants and trees in an area where there is no forest
done
clear
B)
growing plants and trees in an area where the forest is removed
done
clear
C)
growing plants and trees in a pond
done
clear
D)
removal of plants and trees.
done
clear
View Answer play_arrow
question_answer 135) Lysosomes are produced by
A)
Golgi complex
done
clear
B)
mitochondria
done
clear
C)
endoplasmic reticulum
done
clear
D)
leucoplasts.
done
clear
View Answer play_arrow
question_answer 136) Kokkarebellur Bird Sanctuary is located in
A)
Mandya
done
clear
B)
Mysore
done
clear
C)
Chamarajnagar
done
clear
D)
Hassan.
done
clear
View Answer play_arrow
question_answer 137) Which one of the following is also called Sewall Wright effect?
A)
Isolation
done
clear
B)
Gene pool
done
clear
C)
Genetic drift
done
clear
D)
Gene flow
done
clear
View Answer play_arrow
question_answer 138) Oran is a
A)
sacred grove
done
clear
B)
sacred landscape
done
clear
C)
sacred animal
done
clear
D)
endangered animal.
done
clear
View Answer play_arrow
question_answer 139) Put the following parts of a reflex arc in the correct order beginning with the sensory receptor. A. Motor neuron B. Interneuron C. Effector D. Sensory neuron E. Sensory receptor.
A)
\[E,D,B,A,C\]
done
clear
B)
\[E,D,A,B,C\]
done
clear
C)
\[A,B,C,D,E\]
done
clear
D)
\[A,E,D,B,C\]
done
clear
View Answer play_arrow
question_answer 140) The trachea terminates into
A)
bronchial tree
done
clear
B)
atrium
done
clear
C)
bronchi
done
clear
D)
alveoli.
done
clear
View Answer play_arrow
question_answer 141)
Match the entries in Column - I with those of Column - II and choose the correct answer given below. Column - I Column - II A. FSH p. Normal growth B. GH q. Ovulation C. Prolactin r. Parturition D. Oxytocin s. Water diuresis t. Milk secretion
A)
\[A-(q),B-(p),C-(t),D-(r)\]
done
clear
B)
\[A-(q),B-(p),C-(t),D-(s)\]
done
clear
C)
\[A-(p),B-(t),C-(r),D-(q)\]
done
clear
D)
\[A-(q),B-(t),C-(s),D-(r)\]
done
clear
View Answer play_arrow
question_answer 142) Formation of activation calyx in the egg takes place
A)
before fertilization
done
clear
B)
after fertilization
done
clear
C)
at the time of cleavage
done
clear
D)
at the time of amphimixis.
done
clear
View Answer play_arrow
question_answer 143) Which of the following part of cockroach leg is attached to thorax ventrally?
A)
Trochanter
done
clear
B)
Claw
done
clear
C)
Femur
done
clear
D)
Coxa
done
clear
View Answer play_arrow
question_answer 144)
Match the entries in Column - I with those of Column - II and choose the correct answer given below. Column - I Column - II [A] Cytokinins (p) Stress hormone [B] Auxins . (q) Ripening of fruits [C] Abscisic acid (r) Apical dominance Ethylene (s) Bolting (t) Richmond Lang effect
A)
\[A-(t),B-(r),C-(p),D-(q)\]
done
clear
B)
\[A-(t),B-(r),C-(t),D-(s)\]
done
clear
C)
\[A-(r),B-(s),C-(q),D-(p)\]
done
clear
D)
\[A-(q),B-(q),C-(t),D-(r)\]
done
clear
View Answer play_arrow
question_answer 145) Left auricle receives pure blood from the
A)
pulmonary veins
done
clear
B)
pulmonary artery
done
clear
C)
superior venacava
done
clear
D)
Inferior venacava.
done
clear
View Answer play_arrow
question_answer 146) The semi-digested food that moves down the oesophagus is known as
A)
bolus
done
clear
B)
chyme
done
clear
C)
rugae
done
clear
D)
protein .
done
clear
View Answer play_arrow
question_answer 147) During the transportation of gases, to maintain the ionic balance, chloride ions shift from
A)
RBCs to plasma
done
clear
B)
plasma to RBCs
done
clear
C)
lungs to blood
done
clear
D)
blood to lungs.
done
clear
View Answer play_arrow
question_answer 148) Read the statements A and B and select the correct option Statement A: Atherosclerosis is a disease characterized by the thickening of arterial walls Statement B : Deposition of cholesterol and triglycerides in the arterial walls causes atherosclerosis.
A)
Statement A is correct, B is incorrect.
done
clear
B)
Both the statements are correct but not related to each other.
done
clear
C)
Both the statements are correct and B is the reason for A.
done
clear
D)
Both the statements are incorrect.
done
clear
View Answer play_arrow
question_answer 149) Juxtaglomerular cells secrete A when there is a fall in \[\underline{B}\] ion concentration. Choose the correct pair labelled as A and B.
A)
A: Renin B : Chloride
done
clear
B)
A: Carbonic anhydrase B : Sodium
done
clear
C)
A: ATPase B : Potassium
done
clear
D)
A: Renin B : Sodium
done
clear
View Answer play_arrow
question_answer 150) Ileocaecal valve is present in between
A)
colon and large intestine
done
clear
B)
colon and small intestine
done
clear
C)
stomach and small intestine
done
clear
D)
cardiac, stomach and fundus.
done
clear
View Answer play_arrow
question_answer 151)
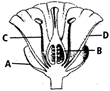
The diagram given below denotes the various parts of a typical flower. Identify the labelled parts A, B, C and D and choose the correct option.
A)
A - Petals, B - Sepals, C - Stamens, D - Pistil
done
clear
B)
a - Sepals, B - Pistil, C - Petals, D - Stamens
done
clear
C)
A - Sepals, B - Pistil, C - Stamens, D - Petals
done
clear
D)
A - Sepals, B - Petals, C - Pistil, D - Stamens
done
clear
View Answer play_arrow
question_answer 152)
Read the statements A and B and identify the correct choice from those given below. Statement A: The egg of frog is moderately telolecithal. Statement B: Sooner or later the cleavage pattern becomes irregular.
A)
Statement A is correct, B is incorrect.
done
clear
B)
Statement B is correct, A is incorrect.
done
clear
C)
Both the statements A and B are correct.
done
clear
D)
Statement A is the reason-for statement B.
done
clear
View Answer play_arrow
question_answer 153) The most unstable RNA is
A)
messenger RNA
done
clear
B)
soluble RNA
done
clear
C)
ribosomal RNA
done
clear
D)
heterogeneous nuclear RNA.
done
clear
View Answer play_arrow
question_answer 154) Choose the right one which denotes genetic diversity.
A)
Chromosomes\[\to \] Nucleotides \[\to \] Genes \[\to \] Individuals \[\to \] Populations
done
clear
B)
Populations \[\to \] Individuals \[\to \] Chromosomes\[\to \]Nucleotides \[\to \] Genes
done
clear
C)
Genes \[\to \] Nucleotides \[\to \]Chromosomes \[\to \]Individuals \[\to \]Populations
done
clear
D)
Nucleotides \[\to \] Genes \[\to \] Chromosome\[\to \] Individuals \[\to \]Populations
done
clear
View Answer play_arrow
question_answer 155) The portion of an eukaryotic gene which is transcribed but not translated is
A)
exon
done
clear
B)
intron
done
clear
C)
cistron
done
clear
D)
codon.
done
clear
View Answer play_arrow
question_answer 156) The appearance of chancre/rashes all over the body are the symptoms of
A)
gonorrhoea
done
clear
B)
AIDS
done
clear
C)
syphilis
done
clear
D)
fever.
done
clear
View Answer play_arrow
question_answer 157) Read the statements A and B and select the correct option. Statement A: Synthesis of mRNA takes place in 5- 3 direction. Statement B: Reading of mRNA is always in 3 - 5 direction.
A)
Both the statements are incorrect.
done
clear
B)
Statement A is incorrect, B is correct.
done
clear
C)
Statement B is incorrect, A is correct.
done
clear
D)
Both the statements A and B are correct.
done
clear
View Answer play_arrow
question_answer 158) Assimilatory power is
A)
\[NADP{{H}_{2}}\]
done
clear
B)
\[ATP\]
done
clear
C)
\[ATP\] and \[NADP{{H}_{2}}\]
done
clear
D)
\[NAD{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 159) Eco RI cleaves the DNA strands to produce
A)
blunt ends
done
clear
B)
sticky ends
done
clear
C)
satellite ends
done
clear
D)
ori replication end.
done
clear
View Answer play_arrow
question_answer 160) Read the statements A and B and identify the correct choice from those given. Statement A: Women are at the peak of conception on the 14th day of menstrual cycle. Statement B: Vasectomy is the method normally employed to avoid conception in females.
A)
Statement A is incorrect, B is correct.
done
clear
B)
Statement A is correct, B is incorrect.
done
clear
C)
Both the statements are correct.
done
clear
D)
Both the statements are incorrect.
done
clear
View Answer play_arrow
question_answer 161) The sequence of nitrogenous bases in one strand of DNA are \[3TAC\text{ }GCG\text{ }ACG\text{ }5.\]The complementary DNA strand should have
A)
\[5\,AUG\,\,CGC\,\,\,TGC\,3\]
done
clear
B)
\[3ATG\,\,CGC\,\,TGC\,5~\] .
done
clear
C)
5UAC GCG ACG 3
done
clear
D)
\[5ATG\text{ }CGC\text{ }TGC\text{ }3\]
done
clear
View Answer play_arrow
question_answer 162) Which one of the following statements is correct regarding spinal cord?
A)
It is composed of outer grey matter and inner white matter.
done
clear
B)
It is composed of outer white matter and inner grey matter.
done
clear
C)
It is composed of outer grey matter and inner colorless matter.
done
clear
D)
It is composed of grey matter only.
done
clear
View Answer play_arrow
question_answer 163)
Match the entries in Column - I with those of Column - II and choose the correct answer. Column -1 Column - II [A] Restriction endonucteases (p) Kohler and Milstein [B] Poly merase chain (q) Alee Jeffreys Reaction [C] DNA fingerprinting (r) Arber [D] Monoclonal antibodies (s) Karry Mullis
A)
\[A-(r),B-(s),C-(q),D-(p)\]
done
clear
B)
\[A-(r),B-(q),C-(s),D-(p)\]
done
clear
C)
\[A-(q),B-(r),C-(s),D-(p)\]
done
clear
D)
\[A-(q),B-(s),C-(r),D-(q)\]
done
clear
View Answer play_arrow
question_answer 164) Which taxonomic term may be suggested for any rank in the classification?
A)
Class
done
clear
B)
Order
done
clear
C)
Species
done
clear
D)
Taxon
done
clear
View Answer play_arrow
question_answer 165) In one of the techniques of recombinant insulin production the genes for a and P polypeptides were inserted into the plasmid by the side of
A)
antibiotic resistance gene
done
clear
B)
lac z promoter gene
done
clear
C)
\[\beta \]- galactosidase gene
done
clear
D)
ori site
done
clear
View Answer play_arrow
question_answer 166) Which one site of the following does not belong to Kingdom Monera?
A)
Slime moulds
done
clear
B)
Mycoplasma
done
clear
C)
Eubacteria
done
clear
D)
Archaebacteria
done
clear
View Answer play_arrow
question_answer 167)
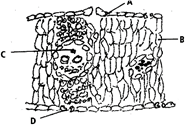
The diagram given below represents the T.S. of a monocot leaf. Identify the parts labelled as A, B, C and D, identify their functions and choose the correct option from those given below.
A)
A: Motor action B: Photosynthesis C: Conduction D: Transpiration
done
clear
B)
A: Motor action B: Conduction C: Photosynthesis D: Transpiration
done
clear
C)
A: Transpiration B: Photosynthesis C: Conduction D: Transpiration
done
clear
D)
A: Transpiration B: Conduction C: Photosynthesis D: Motor action
done
clear
View Answer play_arrow
question_answer 168) Which of the following tissues is not a component of any complex tissue?
A)
Parenchyma
done
clear
B)
Collenchyma
done
clear
C)
Sclerenchyma
done
clear
D)
Tracheids
done
clear
View Answer play_arrow
question_answer 169) Mosses and ferns are
A)
Thallophytes of plant kingdom
done
clear
B)
Angiosperms of plant kingdom
done
clear
C)
Gymnosperms of plant kingdom
done
clear
D)
Amphibians of plant kingdom
done
clear
View Answer play_arrow
question_answer 170) Plasmodesmata are usually observed between
A)
sieve tubes and bast fibres
done
clear
B)
trachea and phloem fibres
done
clear
C)
xylem parenchyma and xylem fibres
done
clear
D)
sieve tubes and companion cells.
done
clear
View Answer play_arrow
question_answer 171) The embryo sac of an angiosperm is made up of
A)
8 cells
done
clear
B)
7 cells and 8 nuclei
done
clear
C)
8 nuclei
done
clear
D)
8 cells and 7 nuclei.
done
clear
View Answer play_arrow
question_answer 172) Cork cambium of dicot stem originates from
A)
dedifferentiated parenchyma cells of cortex
done
clear
B)
dedifferentiated collenchyma cells of cortex
done
clear
C)
parenchyma cells of medullary ray
done
clear
D)
parenchyma cells of pericycle.
done
clear
View Answer play_arrow
question_answer 173)
Match the Column -1 with Column - II and choose the correct answer from those given below. Column - I Column - II [A] Algae (p) Gymnosperms [B] Riccia (q) Pond scum [C] Spirogym (r) Autotrophic [D] Gnetum (s) Liverwort
ln the original paper, the word monocot is misprinted as dicot
A)
\[A-(r),B-(s),C-(q),D-(p)\]
done
clear
B)
\[A-(p),B-(s),C-(q),D-(r)\]
done
clear
C)
\[A-(s),B-(p),C-(r),D-(q)\]
done
clear
D)
\[A-(r),B-(q),C-(s),D-(p)\]
done
clear
View Answer play_arrow
question_answer 174) The opening and closing of stomata is controlled by the activity of
A)
guard cells
done
clear
B)
epidermal cells
done
clear
C)
mesophyll cells
done
clear
D)
lenticels.
done
clear
View Answer play_arrow
question_answer 175) In which of the following phyla the adult shows radial symmetry, while the larva shows bilateral symmetry?
A)
Annelids
done
clear
B)
Arthropods
done
clear
C)
Molluscs
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow
question_answer 176) A thin film of water covering the soil particles and held strongly by attractive forces is called
A)
run away
done
clear
B)
hygroscopic
done
clear
C)
gravitational
done
clear
D)
capillary.
done
clear
View Answer play_arrow
question_answer 177) Which one of the following groups of 3 animals each is correctly matched with their one characteristic morphological features?
A)
Animals Morphological features Centipede, Prawn, Sea urchin - Jointed appendages
done
clear
B)
Animals Morphological features Cockroach, Locust Taenia Metameric segmentation
done
clear
C)
Animals Morphological features Scorpion, Spider Cockroach Ventral solid nerve cord
done
clear
D)
Animals Morphological features Liver fluke, Sea anemone. Sea cucumber Bilateral symmetry
done
clear
View Answer play_arrow
question_answer 178)
Consider the following statements and select the correct option. Statement A: Pure water has maximum water Potential Statement B: The osmotic potential is zero in pure water.
A)
Both statements are correct and B is not the reason for A.
done
clear
B)
Both statements are incorrect.
done
clear
C)
Both statements are correct and B is the reason for A.
done
clear
D)
Both statements are correct.
done
clear
View Answer play_arrow
question_answer 179) A bivalent of meiosis I consists of
A)
four chromatids and two centromeres
done
clear
B)
two chromatids and one centromere
done
clear
C)
two chromatids and two centromeres
done
clear
D)
four chromatids and four centromeres.
done
clear
View Answer play_arrow
question_answer 180) Electrons from excited chlorophyll molecules photosystem II are .accepted first by
A)
ferredoxin
done
clear
B)
pheophytin
done
clear
C)
cytochrome b
done
clear
D)
cytochrome f.
done
clear
View Answer play_arrow