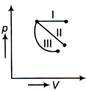
question_answer 1) The equation of a wave is given by \[y=\]A\[\sin \,\omega \left( \frac{x}{v}=k \right),\]where \[\omega \] is angular velocity, v be linear velocity. Then dimension off c is
A)
\[\left[ LT \right]\]
done
clear
B)
\[\left[ T \right]\]
done
clear
C)
\[\left[ {{T}^{1}} \right]\]
done
clear
D)
\[\left[ {{T}^{2}} \right]\]
done
clear
View Answer play_arrow
question_answer 2) The units of modulus of rigidity are
A)
N-m
done
clear
B)
N/m
done
clear
C)
N-m2
done
clear
D)
N/m2
done
clear
View Answer play_arrow
question_answer 3) One car moving on a straight road covers one-third of the distance with 20 km/h and the rest with 60 km/h. The average speed is
A)
40 km/h
done
clear
B)
80 km/h
done
clear
C)
46 2 km/h
done
clear
D)
36 km/h
done
clear
View Answer play_arrow
question_answer 4) The velocity at the maximum height of a projectile is half its initial velocity of projection \[u\] Its range on the horizontal plane is
A)
\[\frac{\sqrt{3}{{u}^{2}}}{2g}\]
done
clear
B)
\[\frac{{{u}^{2}}}{3g}\]
done
clear
C)
\[\frac{3{{u}^{2}}}{2g}\]
done
clear
D)
\[\frac{3{{u}^{2}}}{g}\]
done
clear
View Answer play_arrow
question_answer 5)
Three masses\[{{m}_{1}},{{m}_{2}},{{m}_{3}}\] are joined from weightless string and are kept on frictionless table. They are pulled by a force \[{{T}_{3}}\] of 40 N. If \[{{\text{m}}_{\text{1}}}\text{=10}\,\text{kg,}\]\[{{\text{m}}_{2}}\text{=6}\,\text{kg,}\,\]\[{{\text{m}}_{3}}\text{=}\,\text{4}\,\text{kg,}\,\] Then, value of \[{{T}_{2}}\] will be
A)
20 N
done
clear
B)
40 N
done
clear
C)
10N
done
clear
D)
32 N
done
clear
View Answer play_arrow
question_answer 6) A ship of mass \[3\times {{10}^{7}}kg\] initially at rest is pulled by a force of \[5\times {{10}^{4}}N\] through a distance of 3 m. Assuming that the resistance due to water is negligible. The speed of the ship will be
A)
0.1 m/s
done
clear
B)
1.5 m/s
done
clear
C)
5 m/s
done
clear
D)
60 m/s
done
clear
View Answer play_arrow
question_answer 7) A body of mass m moving with velocity v makes a head on collision with another body of mass 2m which is initially at rest. The loss of kinetic energy of the colliding body (mass m) is
A)
\[\frac{1}{2}\]of its initial kinetic energy
done
clear
B)
\[\frac{1}{9}\]of its initial kinetic energy
done
clear
C)
\[\frac{8}{9}\]of its initial kinetic energy
done
clear
D)
\[\frac{1}{4}\]of its initial kinetic energy
done
clear
View Answer play_arrow
question_answer 8) If momentum is increased by 20%, then the kinetic energy will increase by
A)
40%
done
clear
B)
44%
done
clear
C)
66%
done
clear
D)
50%
done
clear
View Answer play_arrow
question_answer 9) The moment of inertia of uniform thin rod of length L and mass M about an axis passing through a point at a distance \[\frac{L}{3}\] from one of its ends and perpendicular to the rod is
A)
\[\frac{7M{{L}^{2}}}{48}\]
done
clear
B)
\[\frac{M{{L}^{2}}}{9}\]
done
clear
C)
\[\frac{M{{L}^{2}}}{12}\]
done
clear
D)
\[\frac{M{{L}^{2}}}{3}\]
done
clear
View Answer play_arrow
question_answer 10) Ratio of moment of inertia of two rings is 2 : 1 and ratio of their diameters is 2 : 1. Then, the ratio of their mass will be
A)
2 : 1
done
clear
B)
1 : 2
done
clear
C)
1 : 4
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 11) A solid sphere is rotating about a diameter at an angular velocity \[\omega \] If it cools so that its radius reduces two\[\frac{1}{n}\] of its original value, its angular velocity becomes
A)
\[\frac{\omega }{n}\]
done
clear
B)
\[\frac{\omega }{{{n}^{2}}}\]
done
clear
C)
\[n\omega \]
done
clear
D)
\[{{n}^{2}}\omega \]
done
clear
View Answer play_arrow
question_answer 12) The escape velocity of an object from the earth depends upon the mass of the earth \[\text{(}{{\text{M}}_{\text{e}}}\text{),}\] its mean density \[\text{( }\!\!\rho\!\!\text{ ),}\] its radius \[\text{(}{{\text{R}}_{e}}\text{),}\] and the gravitational constant \[\text{(G),}\] Thus, the formula for escape velocity is
A)
\[{{v}_{es}}={{R}_{e}}\sqrt{\frac{8\pi }{3}\text{G }\!\!\rho\!\!\text{ }}\]
done
clear
B)
\[{{v}_{es}}=M\sqrt{\frac{8\pi }{3}\text{G}{{\text{R}}_{e}}}\]
done
clear
C)
\[{{v}_{es}}=\sqrt{2GM{{R}_{e}}}\]
done
clear
D)
\[{{v}_{es}}=\sqrt{\frac{2GM}{R_{e}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 13) Orbital velocity of an artificial satellite does not depend upon
A)
mass of the earth
done
clear
B)
mass of the satellite
done
clear
C)
radius of the earth
done
clear
D)
acceleration due to gravity
done
clear
View Answer play_arrow
question_answer 14) The cyclotron frequency of an electron gyrating in a magnetic field of 1 T is approximately
A)
28MHz
done
clear
B)
280MHz
done
clear
C)
2.8 GHz
done
clear
D)
28 GHz
done
clear
View Answer play_arrow
question_answer 15) When the potential energy of a particle executing SHM is one-fourth of its maximum value during the oscillation, the displacement of the particle from the equilibrium position in terms of its amplitude a is
A)
\[\frac{a}{4}\]
done
clear
B)
\[\frac{a}{3}\]
done
clear
C)
\[\frac{a}{2}\]
done
clear
D)
\[\frac{2a}{3}\]
done
clear
View Answer play_arrow
question_answer 16) For a particle-executing SHM. Which of the following statements is not correct?
A)
The total energy of the particle always remains the same
done
clear
B)
The restoring force is always directed towards a fixed point
done
clear
C)
The restoring force is maximum at the extreme positions
done
clear
D)
The acceleration of the particle is maximum in the equilibrium position
done
clear
View Answer play_arrow
question_answer 17) Period of oscillation of mass attached to a spring and performing SHM is T. The spring is now cut into four equal pieces and the same mass attached to one piece. Now, the period of its simple harmonic oscillations is
A)
\[2T\]
done
clear
B)
\[\frac{T}{4}\]
done
clear
C)
\[\frac{T}{2}\]
done
clear
D)
\[T\]
done
clear
View Answer play_arrow
question_answer 18) Two wires made of same material having lengths \[L\]and \[2L\] and radii \[r\] and \[2r\] respectively are clamped rigidly at their one end and are stretched by applying forces equal to F and 2F respectively. If extension in length of first wire is I, the extension in second wire is
A)
\[l\]
done
clear
B)
\[2l\]
done
clear
C)
\[\frac{1}{2}l\]
done
clear
D)
\[4l\]
done
clear
View Answer play_arrow
question_answer 19) A metal bar of length L and area of cross-section A is clamped between two rigid supports. Its Young's modulus for the material of the rod is Y and coefficient of linear expansion is a. If the temperature of the rod is increased by \[\Delta t{}^\circ C\] the force exerted by the rod on the supports is
A)
\[YAL\Delta t\]
done
clear
B)
\[YA\alpha \Delta t\]
done
clear
C)
\[\frac{YA\alpha \Delta t}{A}\]
done
clear
D)
\[Y\alpha A\Delta t\]
done
clear
View Answer play_arrow
question_answer 20) The temperature of the hydrogen at which the average rms speed of its molecules is equal to that of oxygen molecules at a temperature of \[31{}^\circ C\] is
A)
\[-216{}^\circ C\]
done
clear
B)
\[-235{}^\circ C\]
done
clear
C)
\[-254{}^\circ C\]
done
clear
D)
\[-264{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 21) At \[27{}^\circ C\], the average kinetic energy of gas is \[6.25\times {{10}^{-21}}J\] At \[227{}^\circ C\] its average kinetic energy will be
A)
\[52.2\times {{10}^{-21}}\text{J}\]
done
clear
B)
\[5.22\times {{10}^{-21}}\text{J}\]
done
clear
C)
\[10.42\times {{10}^{-21}}\text{J}\]
done
clear
D)
\[11.35\times {{10}^{-21}}\text{J}\]
done
clear
View Answer play_arrow
question_answer 22) . An ideal gas is expanded adiabatically at an initial temperature of 300 K. So, that its volume is doubled. The final temperature of the hydrogen gas is\[\text{( }\!\!\gamma\!\!\text{ }\,\text{=1}\text{.40)}\]
A)
227.36 K
done
clear
B)
500.30 K
done
clear
C)
454.76 K
done
clear
D)
\[-47{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 23) For a diatomic gas, change in internal energy for unit change in temperature at constant pressure and constant volume is \[{{U}_{1}}\] and \[{{U}_{2}}\] respectively. \[{{U}_{1}}:{{U}_{2}}\] is
A)
5 : 3
done
clear
B)
3 : 5
done
clear
C)
1:1
done
clear
D)
7 : 5
done
clear
View Answer play_arrow
question_answer 24)
Figure shows three pV diagrams. In which case work done is minimum?
A)
\[\text{I}\]
done
clear
B)
\[\text{II}\]
done
clear
C)
\[\text{III}\]
done
clear
D)
Cannot say
done
clear
View Answer play_arrow
question_answer 25) Maximum wavelength of emitted radiation is \[4\mu m\] at 2000 K. Then, maximum wavelength at 2400 K is
A)
\[3.33\mu m\]
done
clear
B)
\[0.66\mu m\]
done
clear
C)
\[1\mu m\]
done
clear
D)
\[1m\]
done
clear
View Answer play_arrow
question_answer 26) A solid cube and a solid sphere have equal surface areas. Both are at the same temperature of \[120{}^\circ C\]. Then
A)
both of them will cool down at the same rate
done
clear
B)
the cube will cool down faster than the sphere
done
clear
C)
the sphere will cool down faster than the cube
done
clear
D)
whichever of the two is heavier will cool down faster
done
clear
View Answer play_arrow
question_answer 27) The time period of rotation of the sun is 25 days and its radius \[7\times {{10}^{8}}m.\] The Doppler shift for the light of wavelength \[6000\,\overset{\text{o}}{\mathop{\text{A}}}\,\] emitted from the surface of the sun will be
A)
\[0.04\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.40\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[4.00\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[40.0\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 28) A transverse wave is described by the equation \[y=a\,\sin \,2\pi \left( nt-\frac{x}{\lambda } \right)\]The maximum particle velocity will be equal to four times the wave velocity, if
A)
\[\lambda =\frac{\pi a}{4}\]
done
clear
B)
\[\lambda =\frac{\pi a}{2}\]
done
clear
C)
\[\lambda =\pi a\]
done
clear
D)
\[\lambda =2\pi a\]
done
clear
View Answer play_arrow
question_answer 29) In Young's double slit experiment, when wavelength used is \[6000\,\overset{\text{o}}{\mathop{\text{A}}}\,\] and the screen is 40 cm from the slits, the fringes are 0.012 cm apart. What is the distance between the slits?
A)
0.024cm
done
clear
B)
2.4cm
done
clear
C)
0.24cm
done
clear
D)
0.2cm
done
clear
View Answer play_arrow
question_answer 30) Two coherent sources must have the same
A)
amplitude
done
clear
B)
phase difference
done
clear
C)
frequency
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 31) On a glass plate a light wave is incident at an angle of \[60{}^\circ C\]. If the reflected and the refracted waves are mutually perpendicular, the refractive index of material is
A)
\[\frac{\sqrt{3}}{2}\]
done
clear
B)
\[\sqrt{3}\]
done
clear
C)
\[\frac{3}{2}\]
done
clear
D)
\[\frac{1}{\sqrt{3}}\]
done
clear
View Answer play_arrow
question_answer 32) An object placed 10 cm in front of lens has an image 20 cm behind the lens. What is the power of the lens (in diopter)?
A)
1.5 D
done
clear
B)
3.0 D
done
clear
C)
-15 D
done
clear
D)
+15D
done
clear
View Answer play_arrow
question_answer 33) A ray passes through a prism of angle \[60{}^\circ C\] in minimum deviation position and suffers a deviation of \[30{}^\circ \]. What is the angle of incidence on the prism?
A)
\[30{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
\[90{}^\circ \]
done
clear
View Answer play_arrow
question_answer 34) A total charge +Q is located at the centre of a cubical box. The total flux going out through one wall of the box is
A)
\[Q/{{\varepsilon }_{0}}\]
done
clear
B)
\[Q/3{{\varepsilon }_{0}}\]
done
clear
C)
\[Q/4{{\varepsilon }_{0}}\]
done
clear
D)
\[Q/6{{\varepsilon }_{0}}\]
done
clear
View Answer play_arrow
question_answer 35) The capacitance of an air capacitor is \[15\mu F\] and the separation between the parallel plates is 6 mm. A copper plate of 3 mm thickness is introduced symmetrically between the plates. The capacitance now becomes
A)
\[5\text{ }\mu F\]
done
clear
B)
\[7.5\text{ }\mu F\]
done
clear
C)
\[22.5\mu F\]
done
clear
D)
\[30\text{ }\mu F\]
done
clear
View Answer play_arrow
question_answer 36) Kirchhoffs law regarding the algebraic sum of currents at any junction in a circuit is based on the law of conservation of
A)
charge
done
clear
B)
energy
done
clear
C)
momentum
done
clear
D)
mass
done
clear
View Answer play_arrow
question_answer 37) The material of wire of potentiometer is made of
A)
copper
done
clear
B)
steel
done
clear
C)
manganin
done
clear
D)
aluminium
done
clear
View Answer play_arrow
question_answer 38) A current of 1.6 A is flowing through a copper voltameter. The number of \[C{{u}^{2+}}ions\] deposited per minute on the cathode is (Given, \[e=1.6\times {{10}^{-19}}C\])
A)
\[3.0\times {{10}^{20}}\]
done
clear
B)
\[1.5\times {{10}^{20}}\]
done
clear
C)
\[1.6\times {{10}^{19}}\]
done
clear
D)
\[3.2\times {{10}^{19}}\]
done
clear
View Answer play_arrow
question_answer 39) For electroplating of a spoon it is kept in voltameter at
A)
place of anode
done
clear
B)
place of cathode
done
clear
C)
between anode and cathode
done
clear
D)
at anyplace in electrolyte solution
done
clear
View Answer play_arrow
question_answer 40) Field inside a solenoid is
A)
directly proportional to its length
done
clear
B)
directly proportional to the current
done
clear
C)
inversely proportional to total number of turns
done
clear
D)
inversely proportional to current
done
clear
View Answer play_arrow
question_answer 41) Two parallel wires carrying currents in the same direction attract each other because of
A)
potential difference between them
done
clear
B)
mutual inductance between them
done
clear
C)
electric force between them
done
clear
D)
magnetic force between them
done
clear
View Answer play_arrow
question_answer 42) Two lines of force due to a bar magnet
A)
intersect at the neutral point
done
clear
B)
intersect near the poles of the magnet
done
clear
C)
intersect on the equatorial axis of the magnet
done
clear
D)
do not intersect at all
done
clear
View Answer play_arrow
question_answer 43) A transformer is an appliance for converting
A)
high current at low voltage to small current at high voltage
done
clear
B)
large current at low voltage to high current at high voltage
done
clear
C)
small current at high voltage to small current at low voltage
done
clear
D)
large current at high voltage to small current at low voltage
done
clear
View Answer play_arrow
question_answer 44) The power factor of a good choke coil is
A)
nearly zero
done
clear
B)
exactly zero
done
clear
C)
nearly one
done
clear
D)
exactly one
done
clear
View Answer play_arrow
question_answer 45) Penetrating power of X-rays depends on
A)
current flowing in the filament
done
clear
B)
applied potential difference
done
clear
C)
nature of the target
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 46) A proton moving with velocity v is acted upon by electric field E and magnetic field B. The proton will move undetected, if
A)
E is perpendicular to B
done
clear
B)
E is parallel to v and perpendicular to B
done
clear
C)
E, B and v are mutually perpendicular and \[v=\frac{E}{B}\]
done
clear
D)
E and B both are parallel to v
done
clear
View Answer play_arrow
question_answer 47) In terms of Rydberg's constant R, the wave number of the first Balmer line is
A)
\[R\]
done
clear
B)
\[\frac{3R}{4}\]
done
clear
C)
\[\frac{5R}{36}\]
done
clear
D)
\[\frac{8R}{9}\]
done
clear
View Answer play_arrow
question_answer 48) Ratio of the wavelength of first line Lyman series and first line of Balmer series is
A)
1:3
done
clear
B)
27 : 5
done
clear
C)
5 : 27
done
clear
D)
4:9
done
clear
View Answer play_arrow
question_answer 49) The mass number of an atomic nucleus
A)
is always greater than the atomic number
done
clear
B)
is always less than the atomic number
done
clear
C)
is always equal to the atomic number
done
clear
D)
can be sometimes equal to and sometimes greater than the atomic number
done
clear
View Answer play_arrow
question_answer 50) The rate of disintegration of fixed quantity of a radioactive element can be increased by
A)
increasing temperature
done
clear
B)
increasing the pressure
done
clear
C)
chemical reaction
done
clear
D)
it is not possible
done
clear
View Answer play_arrow
question_answer 51) When chlorine reacts with dil. \[NaOH\]under cold conditions, the oxidation state of chlorine changes from zero to
A)
\[-1\] and \[+5\]
done
clear
B)
\[+1\] and \[+4\]
done
clear
C)
\[+5\] and \[+3\]
done
clear
D)
\[-1\] and \[+1\]
done
clear
View Answer play_arrow
question_answer 52) The catalyst used in the manufacture of ammonia is
A)
\[{{V}_{2}}{{O}_{5}}\]
done
clear
B)
\[Pt\]
done
clear
C)
\[Fe\]
done
clear
D)
\[Ni{{(CO)}_{4}}\]
done
clear
View Answer play_arrow
question_answer 53) The unit is of the rate of a second order reaction is
A)
\[tim{{e}^{-1}}\]
done
clear
B)
\[mol\text{ }{{L}^{-1}}\text{ }tim{{e}^{-1}}\]
done
clear
C)
\[L\text{ }mo{{l}^{-1}}\text{ }tim{{e}^{-1}}\]
done
clear
D)
\[{{L}^{2}}\text{ }mo{{l}^{-2}}\text{ }tim{{e}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 54) Maximum lowering of vapour pressure is observed in the case of
A)
\[0.1\text{ }M\text{ }glucose\]
done
clear
B)
\[0.1\text{ }M\text{ }BaC{{l}_{2}}\]
done
clear
C)
\[0.1\text{ }M\text{ }MgS{{O}_{4}}\]
done
clear
D)
\[0.1\text{ }NaCl\]
done
clear
View Answer play_arrow
question_answer 55) A solution containing 4g of polyvinyl chloride polymer in 1 L of dioxane was found to have an osmotic pressure of \[4.1\times {{10}^{-4}}\] atm at\[{{27}^{o}}C\] The approximate molecular weight of the polymer is
A)
\[1500\]
done
clear
B)
\[10000\]
done
clear
C)
\[2.4\times {{10}^{5}}\]
done
clear
D)
\[2\times {{10}^{12}}\]
done
clear
View Answer play_arrow
question_answer 56) The molarity of pure water is
A)
\[100\]
done
clear
B)
\[50\]
done
clear
C)
\[18\]
done
clear
D)
\[55.55\]
done
clear
View Answer play_arrow
question_answer 57) The scientist who proposed the atomic model based on the quantization of energy for the first time is
A)
Max Planck
done
clear
B)
Niels Bohr
done
clear
C)
de-Broglie
done
clear
D)
Heisenberg
done
clear
View Answer play_arrow
question_answer 58) Dichloroacetic acid is a stronger acid than acetic acid. This is due to the occurrence of
A)
mesomeric effect
done
clear
B)
hyper conjugation
done
clear
C)
inductive effect
done
clear
D)
steric effect
done
clear
View Answer play_arrow
question_answer 59) Which of the following species does not exert a resonance effect?
A)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}\overset{+}{\mathop{N}}\,{{H}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}OH\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}Cl\]
done
clear
View Answer play_arrow
question_answer 60) Which one of the following monoenes does not exhibit geometric isomerism?
A)
\[{{C}_{4}}{{H}_{8}}\]
done
clear
B)
\[{{C}_{3}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{5}}{{H}_{10}}\]
done
clear
D)
\[{{C}_{8}}{{H}_{16}}\]
done
clear
View Answer play_arrow
question_answer 61) Which of the following diacid readily gives anhydride on heating?
A)
Fumaric
done
clear
B)
Maleic acid
done
clear
C)
Malic acid
done
clear
D)
Terephthalic acid
done
clear
View Answer play_arrow
question_answer 62) Decomposition of benzene diazonium chloride by using \[C{{u}_{2}}C{{l}_{2}}/HCl\]to form chlorobenzene is
A)
Raschig's reaction
done
clear
B)
Sandmeyer's reaction
done
clear
C)
Kolbe's reaction
done
clear
D)
Cannizzaro's reaction
done
clear
View Answer play_arrow
question_answer 63) The latent heat of fusion of ice at \[{{0}^{o}}C\]is \[80~\,cal/g\]. Entropy change \[(\Delta S)\]accompanying the melting of \[1\text{ }g\]of ice at \[{{0}^{o}}C\]would be (units: \[cal/g/K\])
A)
\[273\]
done
clear
B)
\[8.0\]
done
clear
C)
\[0.0\]
done
clear
D)
\[0.293\]
done
clear
View Answer play_arrow
question_answer 64) Which one of the following is a non-polar molecule?
A)
\[CC{{l}_{4}}\]
done
clear
B)
\[CHC{{l}_{3}}\]
done
clear
C)
\[C{{H}_{2}}C{{l}_{2}}\]
done
clear
D)
\[C{{H}_{3}}Cl\]
done
clear
View Answer play_arrow
question_answer 65) The stoichiometry of the following reaction is \[{{K}_{2}}{{S}_{2}}{{O}_{8}}(aq)+2KI(aq)\to \]\[2{{K}_{2}}S{{O}_{4}}(aq)+{{I}_{2}}(aq)\]
A)
\[2:2\]
done
clear
B)
\[1:1\]
done
clear
C)
\[1:2\]
done
clear
D)
\[2:1\]
done
clear
View Answer play_arrow
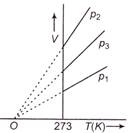
question_answer 66)
The volume-temperature graphs of a given mass of an ideal gas at constant pressures are shown below. What is the correct order of pressures?
A)
\[{{P}_{1}}>{{P}_{3}}>{{P}_{2}}\]
done
clear
B)
\[{{P}_{1}}>{{P}_{2}}>{{P}_{3}}\]
done
clear
C)
\[{{P}_{2}}>{{P}_{3}}>{{P}_{1}}\]
done
clear
D)
\[{{P}_{2}}>{{P}_{1}}>{{P}_{3}}\]
done
clear
View Answer play_arrow
question_answer 67) 1-butyne on hydration gives
A)
butan-1, 2-diol
done
clear
B)
butan-1-ol
done
clear
C)
butan-2-ol
done
clear
D)
butan-2-one
done
clear
View Answer play_arrow
question_answer 68) Conversion of chlorobenzene to phenol involves
A)
electrophilic substitution
done
clear
B)
nucleophilic substitution
done
clear
C)
free radical substitution
done
clear
D)
electrophilic addition
done
clear
View Answer play_arrow
question_answer 69) An alkyl halide reacts with alcoholic ammonia in a sealed tube, the product formed will be
A)
a primary amine
done
clear
B)
a secondary amine
done
clear
C)
a tertiary amine
done
clear
D)
a mixture of all the three
done
clear
View Answer play_arrow
question_answer 70) Which one of the following is the set of correct quantum numbers of an electron in 3d-orbital?
A)
\[n=3,l=0,m=0,\,s=-1/2\]
done
clear
B)
\[n=2,l=3,m=0,\,s=+1/2\]
done
clear
C)
\[n=3,l=1,m=0,\,s=-1/2\]
done
clear
D)
\[n=3,l=2,m=1,\,s=+1/2\]
done
clear
View Answer play_arrow
question_answer 71) The number of beta particles emitted in the radioactive decay series from \[^{238}{{U}_{92}}\] to \[^{206}{{U}_{82}}\] is
A)
\[10\]
done
clear
B)
\[8\]
done
clear
C)
6
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 72) What happens to the yield on application of high pressure in the Haber's synthesis of ammonia?
A)
Increases
done
clear
B)
Decreases
done
clear
C)
Unaffected
done
clear
D)
Reaction stops
done
clear
View Answer play_arrow
question_answer 73) Hydroxyl ion concentration of \[{{10}^{-2}}\text{ }M\]\[HCl\] is
A)
\[1\times {{10}^{1}}\text{ }mol\text{ }d{{m}^{-3}}\]
done
clear
B)
\[1\times {{10}^{-12}}\text{ }mol\text{ }d{{m}^{-3}}\]
done
clear
C)
\[1\times {{10}^{-1}}\text{ }mol\text{ }d{{m}^{-3}}\]
done
clear
D)
\[1\times {{10}^{-14}}\text{ }mol\text{ }d{{m}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 74) In the ionic equation;\[BiO_{3}^{-}+6{{H}^{+}}+x{{e}^{-}}\xrightarrow{{}}B{{i}^{3+}}+3{{H}_{2}}O,\] the values of x is
A)
\[6\]
done
clear
B)
\[2\]
done
clear
C)
\[4\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 75) Which of the following metal oxides is most basic?
A)
\[ZnO\]
done
clear
B)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
C)
\[A{{s}_{2}}{{O}_{3}}\]
done
clear
D)
\[{{K}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 76) The number of unpaired electrons in the square planar \[{{[Pt{{(CN)}_{4}}]}^{2-}}\] ion is
A)
\[2\]
done
clear
B)
\[1\]
done
clear
C)
\[0\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 77) In case of condensation of polymers
A)
high molecular weight polymers are formed all at once
done
clear
B)
lower molecular weight polymers are formed all at once
done
clear
C)
molecular weight of polymer rises throughout the reaction
done
clear
D)
have no specific relation to their molecular weight
done
clear
View Answer play_arrow
question_answer 78) The \[\alpha \]-amino acid which does not give purple colour in the ninhydrin test is
A)
proline
done
clear
B)
glycine
done
clear
C)
lysine
done
clear
D)
aspartic acid
done
clear
View Answer play_arrow
question_answer 79) Amylopectin is the polymer of
A)
\[\beta -D-glucose\]
done
clear
B)
\[\alpha -D-glucose\]
done
clear
C)
\[\beta -D-fructose\]
done
clear
D)
\[\alpha -D-fructose\]
done
clear
View Answer play_arrow
question_answer 80) Which of the products is formed when acetone is reacted with barium hydroxide solution?
A)
\[C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{2}}-\underset{OH}{\mathop{\underset{|}{\mathop{\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C}}\,}}\,}}\,}}\,-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{\overset{OH}{\mathop{\overset{|}{\mathop{C}}\,}}\,}}\,}}\,-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{\overset{OH}{\mathop{\overset{|}{\mathop{C}}\,}}\,}}\,}}\,-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 81) In metal carbonyl (organometallic) complexes, the \[M-C\]bond is
A)
ionic
done
clear
B)
covalent with ionic character
done
clear
C)
covalent
done
clear
D)
coordinate covalent
done
clear
View Answer play_arrow
question_answer 82) In the brown ring test, the brown colour of the ring is due to
A)
ferrous nitrate
done
clear
B)
ferric nitrate
done
clear
C)
a mixture of \[NO\]and \[N{{O}_{2}}\]
done
clear
D)
nitrosoferrous\[-I\] sulphate
done
clear
View Answer play_arrow
question_answer 83) If one mole of ammonia and one mole of hydrogen chloride are mixed in a closed container to form ammonium chloride gas, then
A)
\[\Delta H>\Delta U\]
done
clear
B)
\[\Delta H=\Delta U\]
done
clear
C)
\[\Delta H<\Delta U\]
done
clear
D)
there is no relationship
done
clear
View Answer play_arrow
question_answer 84) The volume of 10 N and 4 N \[HCl\]required to make \[1\text{ }L\]of \[7\text{ }N\]\[HCl\] are
A)
\[0.50\text{ }L\]of \[10\text{ }N\text{ }HCl\] and \[0.50\text{ }L\]of \[4\text{ }N\text{ }HCl\]
done
clear
B)
\[0.60\text{ }L\]of \[10\text{ }N\]\[HCl\] and \[0.40\text{ }L\]of \[4\text{ }N\text{ }HCl\]
done
clear
C)
\[0.80\text{ }L\]\[10\text{ }N\] \[HCl\]of and \[0.20\text{ }L\]of \[4\text{ }N\text{ }HCl\]
done
clear
D)
\[0.75\text{ }L\]of \[10\text{ }N\] \[HCl\] and \[0.25\text{ }L\]of \[4\text{ }N\text{ }HCl\]
done
clear
View Answer play_arrow
question_answer 85) If to a solution of acetic acid, sodium acetate is added, then
A)
\[[{{H}^{+}}]\] increases
done
clear
B)
\[[C{{H}_{3}}CO{{O}^{-}}]\]in decreases
done
clear
C)
Ionisation of \[C{{H}_{3}}COOH\]is suppressed
done
clear
D)
Ionisation constant of \[C{{H}_{3}}COOH\] decreases
done
clear
View Answer play_arrow
question_answer 86) Which of the following is not correct?
A)
Nuclei of atoms participate in nuclear reactions
done
clear
B)
\[_{20}C{{a}^{40}}\]and \[_{18}A{{r}^{40}}\] are isotones
done
clear
C)
1 amu of mass defect is approximately equal to \[931.5\text{ }MeV\]
done
clear
D)
Uranium \[({{U}^{238}})\] series is known as \[(4n+2)\] series
done
clear
View Answer play_arrow
question_answer 87) The order of electron affinity of halogens is
A)
\[F>Cl>Br>I\]
done
clear
B)
\[Cl>F>Br>I\]
done
clear
C)
\[Cl>F>I>Br\]
done
clear
D)
\[Br>Cl>F>I\]
done
clear
View Answer play_arrow
question_answer 88) The metallurgical process in which a metal is obtained in a fused state is called
A)
smelting
done
clear
B)
roasting
done
clear
C)
calcination
done
clear
D)
froth floatation
done
clear
View Answer play_arrow
question_answer 89) Identify the reaction that does not take place in a blast furnace.
A)
\[CaC{{O}_{3}}\xrightarrow{{}}CaO+C{{O}_{2}}\]
done
clear
B)
\[CaO+Si{{O}_{2}}\xrightarrow{{}}CaSi{{O}_{3}}\]
done
clear
C)
\[2F{{e}_{2}}{{O}_{3}}+3C\xrightarrow{{}}4Fe+3C{{O}_{2}}\]
done
clear
D)
\[C{{O}_{2}}+C\xrightarrow{{}}2CO\]
done
clear
View Answer play_arrow
question_answer 90) The product formed when hydroxylamine condenses with a carbonyl compound is called
A)
hydrazide
done
clear
B)
oxime
done
clear
C)
hydrazine
done
clear
D)
hydrazine
done
clear
View Answer play_arrow
question_answer 91) \[C{{H}_{3}}C{{H}_{2}}OH\xrightarrow[step\,I]{C{{l}_{2}}}C{{H}_{3}}CHO\xrightarrow[step\,II]{3C{{l}_{2}}}C{{l}_{3}}CCHO\] In above reactions, the role of \[C{{l}_{2}}\]in step I and step II respectively is
A)
oxidation, chlorination
done
clear
B)
reduction, chlorination
done
clear
C)
oxidation, addition
done
clear
D)
reduction, substitution
done
clear
View Answer play_arrow
question_answer 92) During a redox titration involving a solution containing \[F{{e}^{2+}}\] ions against \[MnO_{4}^{-}\] in the presence of excess of \[{{H}^{+}}\] ions, the number of electrons that gets transferred is
A)
\[6\]
done
clear
B)
\[5\]
done
clear
C)
\[4\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 93) The number of unidentate ligands in the complex ion is called
A)
oxidation number
done
clear
B)
primary valency
done
clear
C)
coordination number
done
clear
D)
RAN
done
clear
View Answer play_arrow
question_answer 94) An element with atomic number 21 is a
A)
halogen
done
clear
B)
representative element
done
clear
C)
transition element
done
clear
D)
alkali metal
done
clear
View Answer play_arrow
question_answer 95) \[2S{{O}_{2}}(g)+{{O}_{2}}(g)\] is an example for
A)
neutralisation reaction
done
clear
B)
homogeneous catalysis
done
clear
C)
heterogeneous catalysis
done
clear
D)
irreversible reaction
done
clear
View Answer play_arrow
question_answer 96) The relationship between Gibb's free energy change \[(\Delta G)\] and emf (E) of a reversible electrochemical cell is given by
A)
\[\Delta G=nFE\]
done
clear
B)
\[\Delta G=nF/E\]
done
clear
C)
\[\Delta G=-nFE\]
done
clear
D)
\[\Delta G=E/nF\]
done
clear
View Answer play_arrow
question_answer 97) Normal aluminium electrode coupled with normal hydrogen electrode gives an emf of \[1.66\text{ }V,\] so the standard potential of \[Al\] is
A)
\[-1.66V\]
done
clear
B)
\[+1.66V\]
done
clear
C)
\[-0.83\text{ }V\]
done
clear
D)
\[+0.83\text{ }V\]
done
clear
View Answer play_arrow
question_answer 98) Which one of the following chlorohydrocarbons readily undergoes solvolysis?
A)
\[C{{H}_{2}}=CHCl\]
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 99) Which of the following reacts with benzene in presence of anhydrous aluminium chloride and forms acetophenone?
A)
\[C{{H}_{3}}Cl\]
done
clear
B)
\[~C{{H}_{3}}COOH\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[C{{H}_{3}}COCl\]
done
clear
View Answer play_arrow
question_answer 100) Hydrolysis of \[NC{{l}_{3}}\]gives \[N{{H}_{3}}\]and X. Which of the following is X?
A)
\[HCl{{O}_{4}}\]
done
clear
B)
\[HCl{{O}_{3}}\]
done
clear
C)
\[HOCl\]
done
clear
D)
\[HCl{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 101) Which of the following is a spedes?
A)
Dipnoi
done
clear
B)
Mammaha
done
clear
C)
Canis familiaris
done
clear
D)
Carnivora
done
clear
View Answer play_arrow
question_answer 102) Locomotion does not occur in
A)
Amoeba
done
clear
B)
Pheretima
done
clear
C)
Leucosolenia
done
clear
D)
Maggot of Musca
done
clear
View Answer play_arrow
question_answer 103) On how many criteria living organisms have been classified into five kingdoms?
A)
Two
done
clear
B)
Four
done
clear
C)
Five
done
clear
D)
Three
done
clear
View Answer play_arrow
question_answer 104) Which of the following have more characters in common?
A)
Order
done
clear
B)
Family
done
clear
C)
Class
done
clear
D)
Phylum
done
clear
View Answer play_arrow
question_answer 105) The endoskeleton in the head of cockroach is known as
A)
notum
done
clear
B)
pleura
done
clear
C)
apodeme
done
clear
D)
tentorium
done
clear
View Answer play_arrow
question_answer 106) Which of the following eats its prey?
A)
Leech
done
clear
B)
Starfish
done
clear
C)
Sepia
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 107) Presence of claspers is an important character in
A)
Sphyma
done
clear
B)
Echeneis
done
clear
C)
Hippocampus
done
clear
D)
Exocoetus
done
clear
View Answer play_arrow
question_answer 108) The mammals evolved from the reptile in the
A)
Cretaceous
done
clear
B)
Triassic
done
clear
C)
Devonian
done
clear
D)
Carboniferous
done
clear
View Answer play_arrow
question_answer 109) Palaeolithic age relates to
A)
subman
done
clear
B)
modem man
done
clear
C)
backward man
done
clear
D)
prehistoric man
done
clear
View Answer play_arrow
question_answer 110) For natural selection, the important factor is
A)
disuse
done
clear
B)
variation
done
clear
C)
catastrophic
done
clear
D)
special creation
done
clear
View Answer play_arrow
question_answer 111) Schwann cells are present on which part of a neuron?
A)
Axon
done
clear
B)
Soma/body
done
clear
C)
Dendrites
done
clear
D)
Axon hillock
done
clear
View Answer play_arrow
question_answer 112) A dental disease characterized by mottling of teeth is due to presence of a certain chemical element in drinking water. Which is that element?
A)
Boron
done
clear
B)
Chlorine
done
clear
C)
Fluorine
done
clear
D)
Mercury
done
clear
View Answer play_arrow
question_answer 113) After the expiration of a normal tidal volume, a -person breathes in as much as air possible. The volume of air inspired is the
A)
vital capacity
done
clear
B)
in spiratory capacity
done
clear
C)
in spiratory reserve volume
done
clear
D)
functional residual capacity
done
clear
View Answer play_arrow
question_answer 114) Blood of which vessel in mammals carries least percentage of urea?
A)
Renal vein
done
clear
B)
Dorsal aorta
done
clear
C)
Renal artery
done
clear
D)
Posterior vena cava
done
clear
View Answer play_arrow
question_answer 115) One of the following is not found in mammalian urine.
A)
water
done
clear
B)
sucrose
done
clear
C)
ammonium salt
done
clear
D)
sodium chloride
done
clear
View Answer play_arrow
question_answer 116) Bone dissolving cells are
A)
osteoplasts
done
clear
B)
osteoclasts
done
clear
C)
chondroclasts
done
clear
D)
chondroblasts
done
clear
View Answer play_arrow
question_answer 117) Which one is odd?
A)
Ligament
done
clear
B)
Pronator
done
clear
C)
Adductor
done
clear
D)
Supinator
done
clear
View Answer play_arrow
question_answer 118) Orchidectomy is the removal of
A)
testes
done
clear
B)
spleen
done
clear
C)
ovaries
done
clear
D)
liver
done
clear
View Answer play_arrow
question_answer 119) Acrosome of sperm is formed from
A)
nucleus of spermatid
done
clear
B)
centrosome of spermatid
done
clear
C)
Golgi complex
done
clear
D)
mitochondria of spermatid
done
clear
View Answer play_arrow
question_answer 120) Atretic follicles are found in the
A)
liver
done
clear
B)
ovary
done
clear
C)
testis
done
clear
D)
thymus
done
clear
View Answer play_arrow
question_answer 121) If cells destined to form endoderm in a gastrula are removed, the new individual will be devoid of
A)
heart
done
clear
B)
brain
done
clear
C)
kidney
done
clear
D)
visceral organs
done
clear
View Answer play_arrow
question_answer 122) Which of these promotes growth?
A)
Pineal gland
done
clear
B)
Anterior pituitary
done
clear
C)
Adrenal gland
done
clear
D)
Posterior pituitary
done
clear
View Answer play_arrow
question_answer 123) Which one is the real product of honey bee?
A)
Pollen
done
clear
B)
Honey
done
clear
C)
Propolis
done
clear
D)
Beeswax
done
clear
View Answer play_arrow
question_answer 124) Which one is not transmitted by houseflies?
A)
Cholera
done
clear
B)
Typhoid
done
clear
C)
Dysentery
done
clear
D)
Yellow fever
done
clear
View Answer play_arrow
question_answer 125) The drug 'belladonna' is obtained from
A)
Atropa
done
clear
B)
Capsicum
done
clear
C)
Solanum
done
clear
D)
Rauwolffia
done
clear
View Answer play_arrow
question_answer 126) Sporeine was first developed by
A)
USA
done
clear
B)
Italy
done
clear
C)
Germany
done
clear
D)
France
done
clear
View Answer play_arrow
question_answer 127) The ovum of human female has auto somes
A)
22 pairs
done
clear
B)
21 pairs
done
clear
C)
23 pairs
done
clear
D)
44 pairs
done
clear
View Answer play_arrow
question_answer 128) The central dogma is not applicable in the case of
A)
retroviruses
done
clear
B)
all prokaryotes
done
clear
C)
transcription
done
clear
D)
reverse transcription
done
clear
View Answer play_arrow
question_answer 129) Antiserum is a serum containing
A)
antigens
done
clear
B)
leucocytes
done
clear
C)
antibodies
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 130) Chromosomal imbalance is most frequent during which of the following stages of human development?
A)
Foetal
done
clear
B)
Embryonic
done
clear
C)
Adult
done
clear
D)
Childhood
done
clear
View Answer play_arrow
question_answer 131) Genes not located within the nucleus are almost always found in
A)
cytosol
done
clear
B)
ribosome
done
clear
C)
cytoskeleton
done
clear
D)
cell membrane
done
clear
View Answer play_arrow
question_answer 132) A promoter site on DNA
A)
transcribes represser
done
clear
B)
regulates termination
done
clear
C)
initiates transcription
done
clear
D)
code for RNA polymerase
done
clear
View Answer play_arrow
question_answer 133) The chromosome cannot be stained with
A)
eosin
done
clear
B)
feulgen stain
done
clear
C)
haematoxylin
done
clear
D)
acetocarmine
done
clear
View Answer play_arrow
question_answer 134) Synthesized vaccines are also called
A)
first generation vaccines
done
clear
B)
second and third generation vaccines
done
clear
C)
monoclonal vaccines
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 135) PCR technique is applied for
A)
DNA amplification
done
clear
B)
DNA synthesis
done
clear
C)
reverse transcription
done
clear
D)
gene cloning
done
clear
View Answer play_arrow
question_answer 136) Fusion of lymphocytes with myeloma in hybridoma technology is a classical example of
A)
somatic hybridization
done
clear
B)
normal hybridization
done
clear
C)
micro propagation
done
clear
D)
cryopreservation
done
clear
View Answer play_arrow
question_answer 137) Allergic reactions develop in response to
A)
hirudin
done
clear
B)
serotonin
done
clear
C)
heparin
done
clear
D)
histamine
done
clear
View Answer play_arrow
question_answer 138) Natural silk contains
A)
nitrogen
done
clear
B)
potassium
done
clear
C)
magnesium
done
clear
D)
phosphorus
done
clear
View Answer play_arrow
question_answer 139) Earthworms are not found in
A)
mor humus
done
clear
B)
mull humus
done
clear
C)
loams
done
clear
D)
clay
done
clear
View Answer play_arrow
question_answer 140) The usual shape of growth curve is
A)
linear
done
clear
B)
zig-zag
done
clear
C)
sigmoid
done
clear
D)
bell-shaped
done
clear
View Answer play_arrow
question_answer 141) The bones of old person become brittle due to accumulation of
A)
sodium
done
clear
B)
calcium
done
clear
C)
phosphorus
done
clear
D)
magnesium
done
clear
View Answer play_arrow
question_answer 142) Which can be regenerated in mammals?
A)
Liver
done
clear
B)
Arms
done
clear
C)
Teeth
done
clear
D)
Eye
done
clear
View Answer play_arrow
question_answer 143) In embryo, cleavage brings about
A)
increased size
done
clear
B)
increased DNA content
done
clear
C)
change in shape and size
done
clear
D)
increased mass of protoplasm
done
clear
View Answer play_arrow
question_answer 144) Placental barriers are maximum in
A)
cat
done
clear
B)
pig
done
clear
C)
rat
done
clear
D)
man
done
clear
View Answer play_arrow
question_answer 145) A freshly laid unfertilized egg of hen contains
A)
100 cells
done
clear
B)
1 cell
done
clear
C)
109 cells
done
clear
D)
about a million cells
done
clear
View Answer play_arrow
question_answer 146) Structure of which remain unchanged during metamorphosis in frog's tadpole?
A)
Lungs
done
clear
B)
Brain
done
clear
C)
Heart
done
clear
D)
Intestine
done
clear
View Answer play_arrow
question_answer 147) The presence of RBCs in urine is known as
A)
uraemia
done
clear
B)
haematuria
done
clear
C)
alkaptonuria
done
clear
D)
poly cythaemia
done
clear
View Answer play_arrow
question_answer 148) Which of these genes are mutated frequently in cancer?
A)
Oncogenes
done
clear
B)
Tumour suppressor genes
done
clear
C)
Mutator genes
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 149) Demography involves
A)
changes in population size
done
clear
B)
composition of population
done
clear
C)
distribution of population
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 150) Birth rate is the simplest indicator of
A)
mortality
done
clear
B)
narality
done
clear
C)
fertility
done
clear
D)
growth
done
clear
View Answer play_arrow
question_answer 151) Pyrenoids are centre of
A)
fat storage
done
clear
B)
starch storage
done
clear
C)
protein formation
done
clear
D)
enzyme formation
done
clear
View Answer play_arrow
question_answer 152) A component not associated with Golgi bodies is
A)
cisternae
done
clear
B)
zone of exclusion
done
clear
C)
acid phosphatase
done
clear
D)
protein glycosylation
done
clear
View Answer play_arrow
question_answer 153) Which is more important for photosynthesis?
A)
Chloroplast
done
clear
B)
Chlorophyll
done
clear
C)
Plastid
done
clear
D)
Radiant energy
done
clear
View Answer play_arrow
question_answer 154) A plant is kept in dark for few days. The cells of leaves in this plant will show
A)
amyloplast
done
clear
B)
chromoplast
done
clear
C)
anthocyanin
done
clear
D)
etioplast
done
clear
View Answer play_arrow
question_answer 155) In plant cell wall
A)
secondary wall is always present
done
clear
B)
may be absent
done
clear
C)
there cannot be intercellular spaces
done
clear
D)
bear pores through which cytoplasm can pass
done
clear
View Answer play_arrow
question_answer 156) When a plant cell is placed in pure water, it will
A)
become rigid
done
clear
B)
smell
done
clear
C)
become flaccid
done
clear
D)
no change
done
clear
View Answer play_arrow
question_answer 157) How many times does the cytokinesis occur during entire process of meiosis
A)
1
done
clear
B)
3
done
clear
C)
2
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 158) Germ cells in gonads are produced by
A)
only mitosis
done
clear
B)
only meiosis
done
clear
C)
maturation without cell division
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 159) Where does meiosis occur in blue green algae/bacteria?
A)
Zygote
done
clear
B)
Zygospore
done
clear
C)
Akinete
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 160) In Pinus, number of chromosomes in its leaf cells is 24. What will be the number of chromosome in its endosperm?
A)
24
done
clear
B)
36
done
clear
C)
12
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 161) Imbibition is always accompanied by swelling or increase in the volume of the imbibant. However, the increase in the volume of the imbibant is
A)
more than die volume of water imbibed
done
clear
B)
some as the volume of the water imbibed
done
clear
C)
less than the volume of the water imbibed
done
clear
D)
it depends upon the type of imbibant
done
clear
View Answer play_arrow
question_answer 162) With the increase in temperature, the process of imbibitions
A)
decreases
done
clear
B)
increases
done
clear
C)
remain the same
done
clear
D)
no effect
done
clear
View Answer play_arrow
question_answer 163) Which of the following has higher water potential?
A)
1 M salt solution
done
clear
B)
1 M sugar solution
done
clear
C)
Distilled water
done
clear
D)
1 M sugar solution with 2-3 bars pressure applied to it
done
clear
View Answer play_arrow
question_answer 164) Cell A has\[{{\text{ }\!\!\Psi\!\!\text{ }}_{\text{W}}}\text{-3}\] bars and cell B has\[{{\text{ }\!\!\Psi\!\!\text{ }}_{\text{W}}}\text{-8}\] bars. The movement of water will be from
A)
cell A to cell B
done
clear
B)
cell B to cell A
done
clear
C)
data insufficient
done
clear
D)
water cannot move in negative value of\[{{\text{ }\!\!\Psi\!\!\text{ }}_{\text{W}}}\]
done
clear
View Answer play_arrow
question_answer 165) The term apoplast signifies
A)
cell wall, intercellular space and water-filled channels
done
clear
B)
protoplasts interconnected by plasmodesmata
done
clear
C)
cell wall, cytoplasm and central vacuole
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 166) Which factor is most important in regularity transpiration?
A)
Temperature
done
clear
B)
Humidity
done
clear
C)
Light
done
clear
D)
Wind
done
clear
View Answer play_arrow
question_answer 167) If concentration of \[C{{O}_{3}}\] in atmosphere is increased, the stomata are closed in
A)
dark
done
clear
B)
light
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 168) Tea yellow is a disease of tea plants produced due to the deficiency of
A)
phosphorus
done
clear
B)
sulphur
done
clear
C)
potassium
done
clear
D)
nitrogen
done
clear
View Answer play_arrow
question_answer 169) White-bud condition in maize is produced due to the deficiency of
A)
iron
done
clear
B)
molybdenum
done
clear
C)
zinc
done
clear
D)
boron
done
clear
View Answer play_arrow
question_answer 170) Root of which plant contains a red pigment that has affinity for oxygen?
A)
Carrot
done
clear
B)
Soybean
done
clear
C)
Mustard
done
clear
D)
Radish
done
clear
View Answer play_arrow
question_answer 171) Who proved that oxygen evolved in photosynthesis comes from water?
A)
Mayer
done
clear
B)
Calvin
done
clear
C)
Ruben
done
clear
D)
Hill
done
clear
View Answer play_arrow
question_answer 172) If an angiosperm is kept at compensation point
A)
it will die after sometime
done
clear
B)
its growth will be normal
done
clear
C)
its growth will be faster
done
clear
D)
its growth will stop but it will not die
done
clear
View Answer play_arrow
question_answer 173) Crassulacean acid metabolism operates in
A)
succulents
done
clear
B)
non-succulents
done
clear
C)
mesophytes
done
clear
D)
hydrophytes
done
clear
View Answer play_arrow
question_answer 174) When a tall plant with rounded seeds (TTRR) is crossed with a dwarf plant with wrinkled seeds (ttrr), the \[{{F}_{1}}\] generation consists of tall plants with rounded seeds? How many types of gametes an \[{{F}_{1}}\]plant would produce.
A)
One
done
clear
B)
Three
done
clear
C)
Four
done
clear
D)
Eight
done
clear
View Answer play_arrow
question_answer 175) The Nobel Prize for the discovery of TCA cycle and ATP biosynthesis was awarded to
A)
Hans Krebs
done
clear
B)
Lipman
done
clear
C)
Krebs and Lipman jointly
done
clear
D)
Vishniae and Ochoa
done
clear
View Answer play_arrow
question_answer 176) The process of deriving glucose from fats and proteins is called
A)
glycogenolysis
done
clear
B)
gluconeogenesis
done
clear
C)
glycogenesis
done
clear
D)
glycolysis
done
clear
View Answer play_arrow
question_answer 177) One turn of Krebs' cycle for the oxidation of 1 mole of sucrose produce. How many ATP molecules.
A)
12
done
clear
B)
24
done
clear
C)
22
done
clear
D)
11
done
clear
View Answer play_arrow
question_answer 178) Respiratory substrate fielding maximum number of ATP molecules is
A)
ketogenic amino acid
done
clear
B)
glucose
done
clear
C)
amylase
done
clear
D)
glycogen
done
clear
View Answer play_arrow
question_answer 179) Fermentation is
A)
anaerobic respiration
done
clear
B)
aerobic respiration
done
clear
C)
incomplete oxidation of carbohydrates
done
clear
D)
anaerobic complete oxidation of carbohydrates
done
clear
View Answer play_arrow
question_answer 180) Cyanide resistant respiration is found in
A)
plants
done
clear
B)
bacteria
done
clear
C)
viruses
done
clear
D)
animals
done
clear
View Answer play_arrow
question_answer 181) Which of the following chemical is used in Ganong's respire meter to determine RQ?
A)
10%KOH
done
clear
B)
10%NaOH
done
clear
C)
1% \[N{{H}_{4}}OH\]
done
clear
D)
1% \[Ca{{(OH)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 182) Two seeds are germinated, A in light and B in dark. Which grows taller in initial stage?
A)
A > B
done
clear
B)
B > A
done
clear
C)
A = B
done
clear
D)
No growth in either case
done
clear
View Answer play_arrow
question_answer 183) Excess amount of \[C{{O}_{2}}\]
A)
retards growth
done
clear
B)
accelerates growth
done
clear
C)
affects growth shortly
done
clear
D)
does not effect growth
done
clear
View Answer play_arrow
question_answer 184) Legume seeds exhibit dormancy due to
A)
undeveloped embryo
done
clear
B)
absence of cytokinins
done
clear
C)
hard seed coat
done
clear
D)
absence of \[G{{A}_{3}}\]
done
clear
View Answer play_arrow
question_answer 185) Morphogenesis is controlled by an interaction between
A)
auxins and gibberellins
done
clear
B)
auxins and cytokinins
done
clear
C)
gibberellins and cytokinins
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 186) Rhizophore of Selaginella is
A)
root
done
clear
B)
stem
done
clear
C)
leaf
done
clear
D)
organ sui generis
done
clear
View Answer play_arrow
question_answer 187) Endosperm in gymnosperms is
A)
haploid
done
clear
B)
triploid
done
clear
C)
diploid
done
clear
D)
tetraploid
done
clear
View Answer play_arrow
question_answer 188) Coralloid roots in Cycas have a symbiotic blue green alga that forms an algal zone in such roots. This alga is
A)
Aulosira
done
clear
B)
Anabaena
done
clear
C)
Oscillatoria
done
clear
D)
Chlorella
done
clear
View Answer play_arrow
question_answer 189) If Gnetum is not in flowering it may be mistaken for a
A)
tree fern
done
clear
B)
monocot plant
done
clear
C)
dicot plant
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 190) A plant which is first terrestrial and then? becomes epiphyte (space parasite)
A)
Vanda
done
clear
B)
Cuscuta
done
clear
C)
Rafflesia
done
clear
D)
Santalum
done
clear
View Answer play_arrow
question_answer 191) Endogenous and exogenous spores both can be traced in
A)
Mucor
done
clear
B)
Agaricus
done
clear
C)
Pemcillium
done
clear
D)
Rhizopus
done
clear
View Answer play_arrow
question_answer 192) Yeast is a good source of
A)
protein
done
clear
B)
riboflavin (vitamin\[-{{B}_{2}}\])
done
clear
C)
sugar
done
clear
D)
ascorbic acid (vitamin-C)
done
clear
View Answer play_arrow
question_answer 193) Witches broom of legume is caused by
A)
virus
done
clear
B)
bacteria
done
clear
C)
fungus
done
clear
D)
mycoplasma
done
clear
View Answer play_arrow
question_answer 194) In angiosperm, the archegonium is replaced by
A)
egg cell
done
clear
B)
female gametophyte
done
clear
C)
pollars and egg apparatus
done
clear
D)
synergids
done
clear
View Answer play_arrow
question_answer 195) Which of the following statements is correct?
A)
Producers produce energy
done
clear
B)
Consumers consume energy
done
clear
C)
Decomposers decompose energy
done
clear
D)
Producers transform energy
done
clear
View Answer play_arrow
question_answer 196) Browning of cauliflower is a
A)
fungal disease
done
clear
B)
bacterial disease
done
clear
C)
physiological disease
done
clear
D)
viral disease
done
clear
View Answer play_arrow
question_answer 197) Best biofertilizer for paddy fields is
A)
Rhizobium
done
clear
B)
Azolla
done
clear
C)
Anthoceros
done
clear
D)
Bacillus polymyxa
done
clear
View Answer play_arrow
question_answer 198) First man made cereal is
A)
Secale cereale
done
clear
B)
Triticale
done
clear
C)
Triticum aestivum
done
clear
D)
maximum dwarf, high yield wheat
done
clear
View Answer play_arrow
question_answer 199) In which of the following fruits, the edible part is aril?
A)
Apple
done
clear
B)
Pomegranate
done
clear
C)
Orange
done
clear
D)
Litchi
done
clear
View Answer play_arrow
question_answer 200) Breeding of crops with high levels of minerals, vitamins and proteins is called
A)
somatic hybridization
done
clear
B)
bio fortification
done
clear
C)
bio magnifications
done
clear
D)
micro propagation
done
clear
View Answer play_arrow