A) - 0.35 V
B) - 1.17 V
C) + 0.35 V
D) + 1.17 V
Correct Answer: C
Solution :
In this reaction,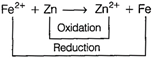 \[EMF={{E}_{cathode}}-{{E}_{anode}}\] \[=-\,0.41-(-0.76)=+\,0.35\,V\]
\[EMF={{E}_{cathode}}-{{E}_{anode}}\] \[=-\,0.41-(-0.76)=+\,0.35\,V\]
You need to login to perform this action.
You will be redirected in
3 sec
