question_answer 1) An electric generator is based on
A)
Faradays law of electromagnetic induction
done
clear
B)
motion of charged particles in electromagnetic field
done
clear
C)
Newtons laws of motion
done
clear
D)
fission of uranium by slow neutrons
done
clear
View Answer play_arrow
question_answer 2) Which of the following can be zero, when a panicle is in motion for some time
A)
Distance
done
clear
B)
Displacement
done
clear
C)
Speed
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 3) An object of mass 10 kg moves at a constant speed of 10 m/s. A constant force, that acts for 4 s on the object, gives it a speed 2 m/s in opposite direction. The force acting on the object is
A)
-3 N
done
clear
B)
-30 N
done
clear
C)
3 N
done
clear
D)
30 N
done
clear
View Answer play_arrow
question_answer 4) A car of mass 1000 kg moves on a circular track of radius 20 m. If the coefficient of friction is 0.64, then the maximum velocity with which the car can move is
A)
22.4 m/s
done
clear
B)
5.6 m/s
done
clear
C)
11.2 m/s
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 5) A particle is executing SHM. Then, the graph of velocity as a function of displacement is a/an
A)
straight line
done
clear
B)
circle
done
clear
C)
ellipse
done
clear
D)
hyperbola
done
clear
View Answer play_arrow
question_answer 6) A long spring, when stretched by a distance x, has potential energy U. On increasing the stretching to rue, the potential energy of the spring will be
A)
\[\frac{U}{n}\]
done
clear
B)
\[nU\]
done
clear
C)
\[{{n}^{2}}U\]
done
clear
D)
\[\frac{U}{{{n}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 7) A body cools from \[50{}^\circ C\] to \[49{}^\circ C\] in 5 s. How long will it take to cool from \[40{}^\circ C\] to\[39{}^\circ C\]? Assume temperature of surroundings to be \[30{}^\circ C\] and Newtons law of cooling is valid
A)
2.5 s
done
clear
B)
10 s
done
clear
C)
20 s
done
clear
D)
5 s
done
clear
View Answer play_arrow
question_answer 8) A sound has an intensity of\[2\times {{10}^{-8}}W\text{/}{{m}^{2}}\]. Its intensity level in decibels is\[\left( {{\log }_{10}}2=0.3 \right)\]
A)
23
done
clear
B)
3
done
clear
C)
43
done
clear
D)
4.3
done
clear
View Answer play_arrow
question_answer 9) A ray reflected successively from two plane mirrors inclined at a certain angle undergoes a deviation of \[240{}^\circ \]. Then, the number of images observable is
A)
3
done
clear
B)
5
done
clear
C)
7
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 10) A concave mirror of focal length f in vacuum is placed in a medium of refractive index 2. Its focal length in the medium is
A)
\[\frac{f}{2}\]
done
clear
B)
\[f\]
done
clear
C)
\[2f\]
done
clear
D)
\[4f\]
done
clear
View Answer play_arrow
question_answer 11) In the lowest energy level of hydrogen atom, the electron has the angular momentum
A)
\[\frac{\pi }{h}\]
done
clear
B)
\[\frac{h}{\pi }\]
done
clear
C)
\[\frac{h}{2\pi }\]
done
clear
D)
\[\frac{2\pi }{h}\]
done
clear
View Answer play_arrow
question_answer 12) Three identical charges are placed at the comers of an equilateral triangle. If the force between any two charges is F, then the net force on each will be
A)
\[\sqrt{2}\]F
done
clear
B)
2 F
done
clear
C)
\[\sqrt{2}\]F
done
clear
D)
3 F
done
clear
View Answer play_arrow
question_answer 13) The resistance of the fuse wire is
A)
Low
done
clear
B)
moderate
done
clear
C)
zero
done
clear
D)
very high
done
clear
View Answer play_arrow
question_answer 14) If 1A of current is passed through \[CuS{{O}_{4}}\]solution for 10 s, the number of copper atoms deposite at the cathode will be about
A)
\[1.6\times {{10}^{20}}\]
done
clear
B)
\[8\times {{10}^{19}}\]
done
clear
C)
\[3.1\times {{10}^{19}}\]
done
clear
D)
\[6.2\times {{10}^{19}}\]
done
clear
View Answer play_arrow
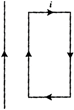
question_answer 15)
A rectangular loop carrying a current i is situated near a long straight wire such that the wire is parallel to one of the sides of the loop and is in the plane of the loop. If a steady current I is established in the wire as shown in the figure, the loop will
A)
rotate about an axis parallel to the wire
done
clear
B)
move away from the wire
done
clear
C)
move towards the wire
done
clear
D)
remain stationary
done
clear
View Answer play_arrow
question_answer 16) Two points A and B are situated at distance \[x\] and 2\[x\] respectively from the nearer pole of a magnet 2 cm long. The ratio of magnetic field at A and B is:
A)
4 : 1 exactly
done
clear
B)
4 : 1 approximately
done
clear
C)
8 : 1 exactly
done
clear
D)
8 : 1 approximately
done
clear
View Answer play_arrow
question_answer 17) The inductance of a coil is proportional to
A)
its length
done
clear
B)
the number of turns
done
clear
C)
the resistance of the coil
done
clear
D)
the square of the number of turns
done
clear
View Answer play_arrow
question_answer 18) On increasing the reverse bias to a large value in a p-n junction diode current
A)
increases slowly
done
clear
B)
remains fixed
done
clear
C)
suddenly increases
done
clear
D)
decreases slowly
done
clear
View Answer play_arrow
question_answer 19) The half-value period of a radioactive nucleide is 3 h. In 9 h its activity will be reduced by a factor of
A)
\[\frac{1}{9}\]
done
clear
B)
\[\frac{1}{27}\]
done
clear
C)
\[\frac{1}{6}\]
done
clear
D)
\[\frac{1}{8}\]
done
clear
View Answer play_arrow
question_answer 20) An n-type semiconductor is formed by adding impurity materials
A)
aluminium, boron or selenium
done
clear
B)
aluminium, boron or indium
done
clear
C)
phosphorus, antimony or arsenic
done
clear
D)
cobalt, aluminium or selenium
done
clear
View Answer play_arrow
question_answer 21) According to Bohrs theory (assuming infinite mass of the nucleus), the frequency of the second line of the Balmer series is:
A)
\[6.16\times {{10}^{14}}Hz\]
done
clear
B)
\[6.16\times {{10}^{13}}Hz\]
done
clear
C)
\[6.16\times {{10}^{10}}Hz\]
done
clear
D)
\[6.16\times {{10}^{16}}Hz\]
done
clear
View Answer play_arrow
question_answer 22) A dip needle in a plane perpendicular to magnetic meridian will remain
A)
vertical
done
clear
B)
horizontal
done
clear
C)
in any direction
done
clear
D)
at an angle of dip to the horizontal
done
clear
View Answer play_arrow
question_answer 23) If the end A of a wire is irradiated with a-q and the other end B is irradiated with p-n then
A)
a current will flow from A to B
done
clear
B)
there will be no current in the wire
done
clear
C)
a current will flow from B task q
done
clear
D)
a current will flow from each end to the midpoint of the wire
done
clear
View Answer play_arrow
question_answer 24) A least distance of distinct vision is 25 cm. The focal length of a convex lens is 5 cm. It can act as a simple microscope of magnifying power
A)
4
done
clear
B)
5
done
clear
C)
6
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 25) A person standing in front of a mirror finds his image larger than himself. This implies that the mirror is
A)
plane
done
clear
B)
convex
done
clear
C)
concave
done
clear
D)
cylindrical with bulging side outwards
done
clear
View Answer play_arrow
question_answer 26) A Carnot engine working between 300 and 600 K has a work output of 800 joule per cycle. The amount of heat energy supplied from the source to engine in each cycle is
A)
800 J
done
clear
B)
1600 J
done
clear
C)
3200 J
done
clear
D)
6400 J
done
clear
View Answer play_arrow
question_answer 27) A body floats with one third of its vote outside water and 3/4 of its volume outside another liquid. The density of other liquid is
A)
\[\frac{9}{4}g/cc\]
done
clear
B)
\[\frac{4}{9}g/cc\]
done
clear
C)
\[\frac{8}{3}g/cc\]
done
clear
D)
\[\frac{3}{8}g/cc\]
done
clear
View Answer play_arrow
question_answer 28) If the maximum velocity and acceleration particle executing SHM are equal in magnitude the time period will be
A)
1.57 s
done
clear
B)
3.14 s
done
clear
C)
6.28 s
done
clear
D)
12.56 s
done
clear
View Answer play_arrow
question_answer 29) The angle of projection at which the horizontal range and the maximum height a projectile are equal is
A)
\[30{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
\[76{}^\circ \]
done
clear
View Answer play_arrow
question_answer 30) The largest and the shortest distances of the earth from the sun are \[{{r}_{1}}\] and \[{{r}_{2}}\]. Its distance from the sun when it is at the perpendicular the major axis of the orbit drawn from the sun, is
A)
\[\frac{{{r}_{1}}+{{r}_{2}}}{4}\]
done
clear
B)
\[\frac{{{r}_{1}}{{r}_{2}}}{{{r}_{1}}+{{r}_{2}}}\]
done
clear
C)
\[\frac{2{{r}_{1}}{{r}_{2}}}{{{r}_{1}}+{{r}_{2}}}\]
done
clear
D)
\[\frac{{{r}_{1}}+{{r}_{2}}}{3}\]
done
clear
View Answer play_arrow
question_answer 31) If the heat of solution for one mole of KCl in 20 moles of water and 200 moles of water was + 3.80 kcal and +4.40 kcal respectively, then the heat of dilution is
A)
+ 8.20 kcal
done
clear
B)
- 8.2 kcal
done
clear
C)
+ 0.60 kcal
done
clear
D)
- 0.60 kcal
done
clear
View Answer play_arrow
question_answer 32) The size of an octahedral void formed in a closest packed lattice as compared to tetrahedral void is
A)
equal
done
clear
B)
smaller
done
clear
C)
larger
done
clear
D)
not definite
done
clear
View Answer play_arrow
question_answer 33) A substance will be deliquescent if its vapour pressure is
A)
equal to the atmospheric pressure
done
clear
B)
equal to that of water vapour in the air
done
clear
C)
greater than that of water vapour in the air
done
clear
D)
less than that of water vapour in the air
done
clear
View Answer play_arrow
question_answer 34) The weight of iron which will be converted into its oxide \[(F{{e}_{3}}{{O}_{4}})\]by the action of 18 g of steam on it will be (at. wt. of Fe = 56)
A)
168 g
done
clear
B)
84 g
done
clear
C)
42 g
done
clear
D)
21 g
done
clear
View Answer play_arrow
question_answer 35) Cupellation process is used in the metallurgy of
A)
Cu
done
clear
B)
Ag
done
clear
C)
Al
done
clear
D)
Fe
done
clear
View Answer play_arrow
question_answer 36) Which of the following two are is structural?
A)
\[Xe{{F}_{2}},\,IF_{2}^{-}\]
done
clear
B)
\[N{{H}_{3}},\,B{{F}_{3}}\]
done
clear
C)
\[CO_{3}^{2-},\,\,SO_{3}^{2-}\]
done
clear
D)
\[PC{{l}_{5}},\,IC{{l}_{5}}\]
done
clear
View Answer play_arrow
question_answer 37) If one litre of air is passed repeatedly over heated copper and magnesium till no further reduction in volume takes place. The volume finally obtained would be approximately
A)
800 mL
done
clear
B)
200 mL
done
clear
C)
10 mL
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 38) Which one of the following transition metal ions is diamagnetic?
A)
\[C{{o}^{2+}}\]
done
clear
B)
\[N{{i}^{2+}}\]
done
clear
C)
\[C{{u}^{2+}}\]
done
clear
D)
\[Z{{n}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 39) The right order of the solubility of sulphates of alkaline earth metals in water is
A)
Be > Ca > Mg > Ba > Sr
done
clear
B)
Mg > Be > Ba > Ca > Sr
done
clear
C)
Be > Mg > Ca > Sr > Ba
done
clear
D)
Mg > Ca > Ba > Be > Sr
done
clear
View Answer play_arrow
question_answer 40) On adding \[AlC{{l}_{3}}\] to water
A)
the ionization of water increases
done
clear
B)
the ionization of water decreases
done
clear
C)
the ionization of water remains constant
done
clear
D)
the ionic product of water increases
done
clear
View Answer play_arrow
question_answer 41) In nitroprusside ion, the iron and NO exist as\[F{{e}^{2+}}\] and \[N{{O}^{+}}\] rather than \[F{{e}^{3+}}\]and\[NO\]. These forms can be differentiated by
A)
estimating the concentration of iron
done
clear
B)
measuring the concentration of \[C{{N}^{-}}\]
done
clear
C)
measuring the solid state magnetic moment
done
clear
D)
thermally decomposing the compound
done
clear
View Answer play_arrow
question_answer 42) Which of the following is not an organometallic compound?
A)
Ethyl magnesium bromide
done
clear
B)
Tetraethyl lead
done
clear
C)
Sodium ethoxide
done
clear
D)
Trimethyl aluminium
done
clear
View Answer play_arrow
question_answer 43) 8.2 L of an ideal gas weigh 9.0 at 300 K and 1 atm pressure. The molecular mass of gas is
A)
9
done
clear
B)
27
done
clear
C)
54
done
clear
D)
81
done
clear
View Answer play_arrow
question_answer 44) Which of the following liberates methane on treatment with water?
A)
Silicon carbide
done
clear
B)
Calcium carbide
done
clear
C)
Beryllium carbide
done
clear
D)
Magnesium carbide
done
clear
View Answer play_arrow
question_answer 45) Inert pair effect plays an important role in case of
A)
phosphorus
done
clear
B)
bismuth
done
clear
C)
antimony
done
clear
D)
arsenic
done
clear
View Answer play_arrow
question_answer 46)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 47) Pick out the correct statement An alloy of Na + K is
A)
liquid at room temperature
done
clear
B)
used in specially designed thermometer
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 48) Schiffs base and Schiffs reagent are
A)
structural isomers
done
clear
B)
anomers
done
clear
C)
diastereomers
done
clear
D)
entirely different compounds
done
clear
View Answer play_arrow
question_answer 49) Propene, \[C{{H}_{3}}CH=C{{H}_{2}}\]can be converted into 1-propanol by oxidation. Indicate which set of reagents amongst the following is ideal to effect the above conversion?
A)
\[KMn{{O}_{4}}\](alkaline)
done
clear
B)
Osmium tetroxide \[(Os{{O}_{4}}\text{/}C{{H}_{2}}C{{l}_{2}})\]
done
clear
C)
\[{{B}_{2}}{{H}_{6}}\] and alk. \[{{H}_{2}}{{O}_{2}}\]
done
clear
D)
\[{{O}_{3}}\text{/}Zn\]
done
clear
View Answer play_arrow
question_answer 50) When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives
A)
2-chloropropane
done
clear
B)
allyl chloride
done
clear
C)
no reaction
done
clear
D)
n-propyl chloride
done
clear
View Answer play_arrow
question_answer 51) An ester is boiled with KOH. The product is cooled and acidified with concentrated HCl. A white crystalline acid separates. The ester is
A)
methyl acetate
done
clear
B)
ethyl acetate
done
clear
C)
ethyl formate
done
clear
D)
ethyl benzoate
done
clear
View Answer play_arrow
question_answer 52) A non-copper alloy is
A)
solder
done
clear
B)
brass
done
clear
C)
bronze
done
clear
D)
belt metal
done
clear
View Answer play_arrow
question_answer 53) Which of the following is a condensation homopolymer?
A)
Nylon-6
done
clear
B)
Nylon-66
done
clear
C)
Nylon-610
done
clear
D)
Dad-on
done
clear
View Answer play_arrow
question_answer 54) A simple method to remove peroxides from ethers is to treat them with an aqueous solution of
A)
\[KI\]
done
clear
B)
\[KCNS\]
done
clear
C)
\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\]
done
clear
D)
\[B{{r}_{2}}\]
done
clear
View Answer play_arrow
question_answer 55) Isobutyl bromide may be obtained from isobutylene and HBr in the presence of
A)
peroxide
done
clear
B)
hydroquinone
done
clear
C)
diphenyl amine
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 56) Which of the following has lowest octane number?
A)
Iso-octane
done
clear
B)
n-heptane
done
clear
C)
n-hexane
done
clear
D)
n-hexadecane
done
clear
View Answer play_arrow
question_answer 57) Which of the following is the most reactive towards ring nitration?
A)
Benzene
done
clear
B)
Mesitylene
done
clear
C)
Toluene
done
clear
D)
m-xylene
done
clear
View Answer play_arrow
question_answer 58) A solution of 500 mL of 0.2 M KOH and 500 mL of 0.2 M HCl is mixed and stirred; the rise in temperature is \[{{T}_{1}}\]. The experiment is repeated using 250 mL each of solution, the temperature rise is \[{{T}_{2}}\]. Which of the following is true?
A)
\[{{T}_{1}}={{T}_{2}}\]
done
clear
B)
\[{{T}_{1}}=2{{T}_{2}}\]
done
clear
C)
\[{{T}_{1}}=4{{T}_{2}}\]
done
clear
D)
\[{{T}_{2}}=9{{T}_{1}}\]
done
clear
View Answer play_arrow
question_answer 59) Bones glow in dark because
A)
they contain shining material
done
clear
B)
they contain red phosphorus
done
clear
C)
they contain white phosphorus which undergoes slow combustion in contact with air
done
clear
D)
white phosphorus changes to red phosphorus
done
clear
View Answer play_arrow
question_answer 60) Superconductors are derived from compounds of
A)
p-block elements
done
clear
B)
lanthanides
done
clear
C)
acrinides
done
clear
D)
transition elements
done
clear
View Answer play_arrow
question_answer 61) The first live healthy cloned mammal of the world was
A)
Molly sheep
done
clear
B)
Dolly sheep
done
clear
C)
Polly sheep
done
clear
D)
A monkey
done
clear
View Answer play_arrow
question_answer 62) The maintenance of internal favourable conditions, by a self-regulated mechanism, inspite of the fact that there are changes in the environment is called
A)
entropy
done
clear
B)
homeostates
done
clear
C)
enthalpy
done
clear
D)
steady state
done
clear
View Answer play_arrow
question_answer 63) Two morphologically similar closely related sympatric populations which are reproductively isolated and cant introduced to produce fertile hybrid are called
A)
sibling species
done
clear
B)
allopatric species
done
clear
C)
morphospecies
done
clear
D)
sympatric species
done
clear
View Answer play_arrow
question_answer 64) Free living non-symbiotic aerobic non-photosynthetic nitrogen fixing bacteria in soils is
A)
Azotobacter
done
clear
B)
Rhizobium
done
clear
C)
Closridium
done
clear
D)
Anabaena
done
clear
View Answer play_arrow
question_answer 65) When the mycelium of Rhizopus oryzae grows submerged in a nutritive medium such as sugar solution the young coenocytic hyphae develop septa and divide into short mulrinucleate segment known as
A)
oidia
done
clear
B)
endospores
done
clear
C)
exospores
done
clear
D)
conidia
done
clear
View Answer play_arrow
question_answer 66) Viruses cannot multiply of their own because they
A)
are dead
done
clear
B)
do not have sex organ and gametes
done
clear
C)
lack genetic material
done
clear
D)
lack cellular machinery to use their own genetic material
done
clear
View Answer play_arrow
question_answer 67) A structure absent in angiosperm is
A)
archegonium
done
clear
B)
pistil
done
clear
C)
megagametophyte
done
clear
D)
micro- gametophyte
done
clear
View Answer play_arrow
question_answer 68) What is mitoplast?
A)
Another name of mitochondria
done
clear
B)
Membraneless mitochondria
done
clear
C)
Mitochondria without outer membrane
done
clear
D)
Mitochondria without inner membrane
done
clear
View Answer play_arrow
question_answer 69) Synaptonemal complex is a ribonucleo protein structure reported by Moses 1956. It is visible from
A)
leptotene through diplotene
done
clear
B)
pachytene through diplotene
done
clear
C)
zygotene through pachytene
done
clear
D)
diplotene through metaphase
done
clear
View Answer play_arrow
question_answer 70) A tree growing in Indian Botanical Garden, Sibpur (Howrah, Calcutta) with age over 200 yr, circumference 404 m, prop roots 1,600 and whose main stem has decayed is
A)
Ficus benghalensis
done
clear
B)
Ficus religiosa
done
clear
C)
Eucalyptus regnans
done
clear
D)
No such tree exist
done
clear
View Answer play_arrow
question_answer 71) The number of pollen grains produced by head inflorescence of Asteraceae (Compositae) having 10 flowers. If each anther produces 20 pollen grains are
A)
300
done
clear
B)
500
done
clear
C)
800
done
clear
D)
1,000
done
clear
View Answer play_arrow
question_answer 72) Ovary is obliquely placed in/distortion of ovary occurs in
A)
Ranunculaceae
done
clear
B)
Brassicaceae
done
clear
C)
Compositae
done
clear
D)
Solanaceae
done
clear
View Answer play_arrow
question_answer 73) Seeds which are able to withstand reduction in moisture and temperature are called:
A)
dormant seeds
done
clear
B)
orthodox seeds
done
clear
C)
recalcitrant seeds
done
clear
D)
non-viable seeds
done
clear
View Answer play_arrow
question_answer 74) Trophocytes, mycetocytes, oenocytes and urate cells, are found in the fat body of cockroach. Which statement is true?
A)
Trophocytes contain reserve food
done
clear
B)
Mycetocytes contains symbiotic bacteria
done
clear
C)
Oenocytes secrete wax and urace cells contains uric acid
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 75) Cockroach has a stomadaeal valve between
A)
crop and gizzard
done
clear
B)
gizzard and mesenteron
done
clear
C)
mesenteron and ileum
done
clear
D)
ileum and colon
done
clear
View Answer play_arrow
question_answer 76) Spermathecae of earthworm take pan in
A)
collection of sperms of other animals
done
clear
B)
collection of sperms of the same animals
done
clear
C)
sperm maturation
done
clear
D)
fertilization
done
clear
View Answer play_arrow
question_answer 77) Which of the following has the highest water potential?
A)
1 M salt solution
done
clear
B)
1M sugar solution
done
clear
C)
Distilled water
done
clear
D)
1M sugar solution with 2-3 bars pressure applied to it
done
clear
View Answer play_arrow
question_answer 78) \[{{C}_{4}}\] plants can absorb \[C{{O}_{2}}\]from
A)
its much low concentration
done
clear
B)
its much high concentration
done
clear
C)
carbonates
done
clear
D)
bicarbonates
done
clear
View Answer play_arrow
question_answer 79) Which of the following physiological effects is caused in plants by gibberellic acid?
A)
Shortening of genetically tall plants
done
clear
B)
Rooting in stem cuttings
done
clear
C)
Elongation of genetically dwarf plants
done
clear
D)
Yellowing of young leaves
done
clear
View Answer play_arrow
question_answer 80) Red drop is
A)
drop in flowering of SDP when red light is given in the dark period
done
clear
B)
drop in flowering in LDP when dark period is interrupted by red light
done
clear
C)
fall in the rate of photosynthesis when monochromatic light of red region is given to the plant
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 81) LSD is derived from ergot, an extract got from fruiting body of fungus Claviceps purpurea, however, bhang, ganja and charas are obtained from the dried .......... of Cannatris indica
A)
leaves and flower
done
clear
B)
leaves
done
clear
C)
flowers
done
clear
D)
stems
done
clear
View Answer play_arrow
question_answer 82) Which is correct match?
A)
Wilt of pigeon pea \[\to \]Phytophthora infestans
done
clear
B)
Late blight of potato\[\to \] Fusarium udum
done
clear
C)
Citrus canker \[\to \]Xanrhomonos axonopodis
done
clear
D)
Soft rot of sugarcare \[\to \]Anguina tritici
done
clear
View Answer play_arrow
question_answer 83) Multiple shoots are produced in meristem culture because the culture medium contains
A)
cytokinin for overcoming apical dominance
done
clear
B)
auxin for proliferation of meristem
done
clear
C)
auxin for overcoming apical dominance
done
clear
D)
\[G{{A}_{3}}\]for proliferation of meristem
done
clear
View Answer play_arrow
question_answer 84) Pancoats syndrome is associated with
A)
neophobia
done
clear
B)
neophrenia
done
clear
C)
neoplasm
done
clear
D)
neophelium
done
clear
View Answer play_arrow
question_answer 85) Bile can be prevented to pass into duodenum by
A)
pyloric valve
done
clear
B)
sphincter of Boyden
done
clear
C)
sphincter of Oddi
done
clear
D)
cardiac sphincter
done
clear
View Answer play_arrow
question_answer 86) In lungs, air is separated from venous blood by
A)
squamous epithelium + tunica externa of blood vessel
done
clear
B)
squamous epithelium + endothelium of blood vessel
done
clear
C)
transitional epithelium + tunica medica of blood vessel
done
clear
D)
columnar epithelium + 3 layered wall of blood vessel
done
clear
View Answer play_arrow
question_answer 87) Bicarbonates formed inside erythrocytes passes out into plasma while chloride of plasma pass into erythrocytes. The phenomenon is called
A)
bicarbonate shift
done
clear
B)
Hamburger phenomenon
done
clear
C)
carbochlorosis
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 88) In mammals the opening of post caval in the right auricle is guarded by
A)
mitral valve
done
clear
B)
the besius valve
done
clear
C)
eustachius valve
done
clear
D)
bicupid valve
done
clear
View Answer play_arrow
question_answer 89) Abnormal rise in white blood cells is
A)
leukaemia
done
clear
B)
polycythemia
done
clear
C)
anaemia
done
clear
D)
pneumonia
done
clear
View Answer play_arrow
question_answer 90) The kidneys not only remove the waste product from the blood but also play a very important role in maintaining
A)
equilibrium of the body
done
clear
B)
temperature of the body
done
clear
C)
constant composition of the blood irrespective of the nature of the food or fluid intake
done
clear
D)
blood pressure constant
done
clear
View Answer play_arrow