question_answer 1)
What is the current through an ideal p-n junction diode shown in the figure below?
A)
Zero
done
clear
B)
10 mA
done
clear
C)
20 mA
done
clear
D)
50 mA
done
clear
View Answer play_arrow
question_answer 2) Absorption of X-rays is maximum in which the following material sheets of sar thickness?
A)
Cu
done
clear
B)
Au
done
clear
C)
Be
done
clear
D)
Pb
done
clear
View Answer play_arrow
question_answer 3) A car of mass m is moving with momentum P. If\[\mu \]be the coefficient of friction between the tyres and the road, what will be stoppin distance due to friction alone?
A)
\[\frac{{{P}^{2}}}{2\mu g}\]
done
clear
B)
\[\frac{{{P}^{2}}}{2\,m\mu g}\]
done
clear
C)
\[\frac{{{P}^{2}}}{2\,{{m}^{2}}\mu g}\]
done
clear
D)
\[\frac{{{P}^{2}}}{2\,mg}\]
done
clear
View Answer play_arrow
question_answer 4) The electron emitted in beta radiation originates from
A)
inner orbits of atom
done
clear
B)
free electron existing in nuclei
done
clear
C)
decay of neutron in the nucleus
done
clear
D)
photon escaping from the nucleus
done
clear
View Answer play_arrow
question_answer 5) Two equal and opposite charge ( +q and -q) axe situated at x distance from each other, the of potential at very far point will depend upon
A)
only on \[q\]
done
clear
B)
only on \[x\]
done
clear
C)
on\[qx\]
done
clear
D)
on\[\frac{q}{x}\]
done
clear
View Answer play_arrow
question_answer 6) A common hydrometer reads specific gravity of liquids, compared to the 1.6 mark of the stem the mark 1.5 will be
A)
upwards
done
clear
B)
downwards
done
clear
C)
in the same place
done
clear
D)
may be upwards or downward, depending upon the hydrometer
done
clear
View Answer play_arrow
question_answer 7) Each of the two points charges are doubled and their distance is halver force of interaction becomes n times , where n is
A)
4
done
clear
B)
1
done
clear
C)
1/16
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 8) Two bodies of different masses of 2 kg and 4 kg moving with velocity 2 m/s and 10 m/s towards each other due to mutual gravitational attraction.What is the velocity of their centre of mass?
A)
5 m/s
done
clear
B)
6 m/s
done
clear
C)
8 m/s
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 9) A voltmetre of range 2 V and resistance 300\[\Omega \] cannot be converted into ammeter of range
A)
1 A
done
clear
B)
1 mA
done
clear
C)
100 mA
done
clear
D)
10 mA
done
clear
View Answer play_arrow
question_answer 10) The ratio of intensity at the centre of a bright fringe to the intensity at a point distant one fourth of the distance between two successive bright fringes will be
A)
4
done
clear
B)
3
done
clear
C)
2
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 11) What should be the velocity of an electron so that its momentum becomes equal to that of a photon of wavelength 5200\[\overset{\text{o}}{\mathop{\text{A}}}\,\]?
A)
700 m/s
done
clear
B)
1000 m/s
done
clear
C)
1400 m/s
done
clear
D)
2000 m/s
done
clear
View Answer play_arrow
question_answer 12) The unit of thermal conductance is
A)
\[W{{K}^{-1}}\]
done
clear
B)
\[J{{K}^{-2}}\]
done
clear
C)
\[WK\]
done
clear
D)
\[JK\]
done
clear
View Answer play_arrow
question_answer 13)
The current flowing in the given circuit is 0.1A. The potential difference between the points x and y is
A)
4.0 V
done
clear
B)
3.0 V
done
clear
C)
2.5 V
done
clear
D)
2.0 V
done
clear
View Answer play_arrow
question_answer 14) An electron is moving in a circle of radius r in a uniform magnetic field B. Suddenly, the field is reduced to B/2. The radius of the circle now becames
A)
\[\frac{r}{2}\]
done
clear
B)
\[\frac{r}{4}\]
done
clear
C)
\[2r\]
done
clear
D)
\[4r\]
done
clear
View Answer play_arrow
question_answer 15) Which one of the following phenomena is used in optical fibres?
A)
Scattering
done
clear
B)
Successive reflections
done
clear
C)
Refraction
done
clear
D)
Total internal reflection
done
clear
View Answer play_arrow
question_answer 16) The essential distinction between X-rays and \[\gamma \]-rays is that
A)
\[\gamma \]-rays have smaller wavelength than X-rays
done
clear
B)
\[\gamma \]-rays emanate from nucleus which X-rays
done
clear
C)
\[\gamma \]-rays have greater ionising power than X-rays
done
clear
D)
\[\gamma \]-rays are more penetrating than X-rays
done
clear
View Answer play_arrow
question_answer 17) In the Boolean algebra\[\overline{A}\cdot \overline{B}\]is same as
A)
\[\overline{A+B}\]
done
clear
B)
\[A\cdot B\]
done
clear
C)
\[\overline{A\cdot B}\]
done
clear
D)
\[A+B\]
done
clear
View Answer play_arrow
question_answer 18) Radius of orbit of satellite of the earth is R. Its kinetic energy is proportional to
A)
\[\frac{1}{R}\]
done
clear
B)
\[\frac{1}{\sqrt{R}}\]
done
clear
C)
\[R\]
done
clear
D)
\[\frac{1}{{{R}^{3/2}}}\]
done
clear
View Answer play_arrow
question_answer 19) A conducting sphere of radius JR = 20cm is given a charge\[Q=16\mu C.\] What is E at centre?
A)
\[3.6\times {{10}^{6}}N\text{/}C\]
done
clear
B)
\[1.8\times {{10}^{6}}N\text{/}C\]
done
clear
C)
Zero
done
clear
D)
\[0.9\times {{10}^{6}}N\text{/}C\]
done
clear
View Answer play_arrow
question_answer 20) The maximum range of a gun on horizontal terrain is 16 km, if \[g=10\,m\text{/}{{s}^{2}}.\] What must be the muzzle velocity of the shell?
A)
200 m/s
done
clear
B)
100 m/s
done
clear
C)
400 m/s
done
clear
D)
300 m/s
done
clear
View Answer play_arrow
question_answer 21) A solid Sphere of mass 2 kg rolls up a \[30{}^\circ \] incline with an initial speed 10 m/s. The maximum height reached by the sphere is \[(g=10\,m\text{/}{{s}^{2}})\]
A)
3.5 m
done
clear
B)
7.0 m
done
clear
C)
10.5 m
done
clear
D)
14.0 m
done
clear
View Answer play_arrow
question_answer 22) A toroidal solenoid with an air case has an average radius of 15\[cm\], area of cross-section 12\[c{{m}^{2}}\] and 1200 turns. Ignoring the field variation across the cross-section of the toroid, the self-inductance of the toroid is
A)
4.6 mH
done
clear
B)
6.9 mH
done
clear
C)
2.3 mH
done
clear
D)
9.2 mH
done
clear
View Answer play_arrow
question_answer 23) The transfer ratio \[\beta \] of a transistor is 50. The input resistance of the transistor when used in the common emitter made is\[1\,k\Omega \]. The peal value of the collecter alternating current for an input peak voltage of 0.01 V is
A)
100\[\mu A\]
done
clear
B)
500\[\mu A\]
done
clear
C)
0.01\[\mu A\]
done
clear
D)
0.25\[\mu A\]
done
clear
View Answer play_arrow
question_answer 24) A vessel has 6 kg of hydrogen at pressure p and temperature 500 K . A small hole is made in it so, that hydrogen leaks out. How much hydrogen leaks out, if the final pressure is p/2 and temperature falls to 300 K?
A)
2g
done
clear
B)
3g
done
clear
C)
4g
done
clear
D)
1g
done
clear
View Answer play_arrow
question_answer 25) A coil has an inductance of 0.7 H and is joined in series with a resistance of 220 . When an alternating emf of 220 V at 50\[{{C}_{ps}}\]is applied to it, then the wattless component of the current in the circuits is
A)
5 A
done
clear
B)
0.5 A
done
clear
C)
0.7 A
done
clear
D)
7 A
done
clear
View Answer play_arrow
question_answer 26) In interference pattern , the energy is
A)
created at the maximum
done
clear
B)
destroyed at the minimum
done
clear
C)
conserved but redistributed
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 27) An eye specialist prescribes spectacles havin? a combination of a convex lens of focal length 40 cm contact with a concave lens of length 25cm. The power of this lens combination will be
A)
+1.5 D
done
clear
B)
-1.5 D
done
clear
C)
6.67 D
done
clear
D)
-6.67 D
done
clear
View Answer play_arrow
question_answer 28) The magnitude of magnetic induction for a current carrying toroid of uniform cross-section is
A)
uniform over the whole cross-section
done
clear
B)
maximum on the outer edge
done
clear
C)
maximum on the inner edge
done
clear
D)
maximum of the centre of cross-section
done
clear
View Answer play_arrow
question_answer 29) Two similar . heater coils separately take 10 min to boil a certain amount of water. If both coils are connected in series, time taken to boil the same amount of the water will be
A)
15 min
done
clear
B)
20 min
done
clear
C)
7.5 min
done
clear
D)
25 min
done
clear
View Answer play_arrow
question_answer 30) Isogonic lines are those for which
A)
declination is the same at all places on the line
done
clear
B)
angle.of dip is the same at the place on the line
done
clear
C)
the value of horizontal components of the earths magnetic field is the same
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 31) What will happen to the weight of the body at the south pole, if the earth stops rotating about its polar axis?
A)
No change
done
clear
B)
Increases
done
clear
C)
decreases but does not become zero
done
clear
D)
reduces tozero
done
clear
View Answer play_arrow
question_answer 32) Pure silicon at 300K has equal electron\[({{n}_{e}})\]and hole \[({{n}_{h}})\]concentration of \[1.5\times {{10}^{16}}{{m}^{-3}}.\] Doping by indium increase \[({{n}_{h}})\] to \[4.5\times {{10}^{22}}{{m}^{-3}}.\] The\[({{n}_{e}})\]in the doped silicon is
A)
\[9\times {{10}^{5}}\]
done
clear
B)
\[5\times {{10}^{9}}\]
done
clear
C)
\[2.25\times {{10}^{11}}\]
done
clear
D)
\[3\times {{10}^{19}}\]
done
clear
View Answer play_arrow
question_answer 33) The value of P so that the vector \[2\hat{i}-\hat{j}+\hat{k},\]\[\hat{i}+2\hat{j}-3\hat{k},\] and \[3\hat{i}+P\hat{j}-5\hat{k}\] are coplanar should be
A)
16
done
clear
B)
-4
done
clear
C)
4
done
clear
D)
-8
done
clear
View Answer play_arrow
question_answer 34) An engine develops 20 Hp. When rotating at a speed of 1800 rev/min. The torque that it delivers is
A)
400 N-m
done
clear
B)
60 N-m
done
clear
C)
40 N-m
done
clear
D)
80 N-m
done
clear
View Answer play_arrow
question_answer 35) A body of mass 10 kg falls from a height of 5m (g = 10\[m\text{/}{{s}^{2}}\]) and is stopped with in one-tenth of a second on the ground. The force of interaction is
A)
100 N
done
clear
B)
Zero
done
clear
C)
1000 N
done
clear
D)
1100 N
done
clear
View Answer play_arrow
question_answer 36) A convex mirror is used to form an image of a real object. Then , the incorrect statement is
A)
the image lies between the focus
done
clear
B)
the image is diminished in size
done
clear
C)
the image is erect
done
clear
D)
the image is real
done
clear
View Answer play_arrow
question_answer 37) The surface charge density of the earth is
A)
\[{{10}^{-9}}c{{m}^{-2}}\]
done
clear
B)
\[{{10}^{-6}}c{{m}^{-2}}\]
done
clear
C)
\[-{{10}^{-9}}c{{m}^{-2}}\]
done
clear
D)
\[-100\,c{{m}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 38) A body of specific heat\[0.2\,kcal\text{/}kg{}^\circ C\]is heated through\[100{}^\circ C\]. The percentage increase in its mass is
A)
\[9%\]
done
clear
B)
\[9.3\times {{10}^{-11}}%\]
done
clear
C)
\[10%\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 39) Three circular concentric wires of radii a, 2a and 3a are carrying current \[3I,\,\,2I,\]and\[I\]in same manner. The magnetic field at the common centre is
A)
\[\frac{13{{\mu }_{0}}I}{6a}\]
done
clear
B)
\[\frac{{{\mu }_{0}}I}{6a}\]
done
clear
C)
\[\frac{{{\mu }_{0}}I}{a}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 40)
A)
\[\frac{20}{7}V\]
done
clear
B)
\[\frac{40}{7}V\]
done
clear
C)
\[\frac{10}{7}V\]
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 41) A raft of wood of mass 120 kg floats in water. The weight that can be put on the raft to make it just sink, should be draft \[{{d}_{raft}}=600\,kg\text{/}{{m}^{3}}\]
A)
80 kg
done
clear
B)
50 kg
done
clear
C)
60 kg
done
clear
D)
30 kg
done
clear
View Answer play_arrow
question_answer 42) A monatomic gas supplied the heat Q very slowly keeping the pressure constant. The work done by the gas will be
A)
\[\frac{2}{3}Q\]
done
clear
B)
\[\frac{3}{5}Q\]
done
clear
C)
\[\frac{2}{5}Q\]
done
clear
D)
\[\frac{1}{5}Q\]
done
clear
View Answer play_arrow
question_answer 43) An L, C circuit is in the state of resonance \[C=0.1\mu F\] and\[L=0.25\,H\]. Neglecting ohmic resistance of circuit. What is the frequency of oscillations?
A)
1007 Hz
done
clear
B)
100 Hz
done
clear
C)
109 Hz
done
clear
D)
500 Hz
done
clear
View Answer play_arrow
question_answer 44) When temperature of an ideal gas is increased from\[27{}^\circ C\]and\[227{}^\circ C,\]its rms speed is changed from 400 m/s to\[{{V}_{s}}\]. The \[{{V}_{s}}\] is
A)
516 m/s
done
clear
B)
450 m/s
done
clear
C)
310 m/s
done
clear
D)
746 m/s
done
clear
View Answer play_arrow
question_answer 45) The coefficient of volumetric expansion of mercury is \[18\times {{10}^{-5}}\text{/}{}^\circ C\]. A thermometre bulb has value of \[{{10}^{-6}}{{m}^{3}}\] and cross-section of stem is \[0.002\,c{{m}^{2}}\] assuming the bulb is tilled mercury at\[{}^\circ C\]. The length of mercury at \[100{}^\circ C\] is
A)
18 cm
done
clear
B)
4.5 cm
done
clear
C)
2.25 cm
done
clear
D)
9 cm
done
clear
View Answer play_arrow
question_answer 46) Kinetic energy of an electron accelerated in a potential difference of 100 V, will be
A)
\[1.6\times {{10}^{-21}}J\]
done
clear
B)
\[1.6\times {{10}^{-23}}J\]
done
clear
C)
\[1.6\times {{10}^{-10}}J\]
done
clear
D)
\[1.6\times {{10}^{-16}}J\]
done
clear
View Answer play_arrow
question_answer 47)
In an equilateral triangle ABC, \[{{F}_{1}},\,\,{{F}_{2}}\] and \[{{F}_{3}}\] are three forces acting along the sides AB, BC and AC as shown in the given figure. What should be the magnitude of \[{{F}_{3}},\] so that the total torque about is zero?
A)
2 N
done
clear
B)
4 N
done
clear
C)
6 N
done
clear
D)
8 N
done
clear
View Answer play_arrow
question_answer 48) If the ratio of specific heats of a gas at constant pressure to that at constant volume is \[\gamma ,\] the change in internal energy of a gas. When, the volume changes from V to 2V at constant pressure is
A)
\[pV\]
done
clear
B)
\[\frac{R}{\gamma -1}\]
done
clear
C)
\[\frac{pV}{\gamma -1}\]
done
clear
D)
\[\frac{\gamma \,pV}{\gamma -1}\]
done
clear
View Answer play_arrow
question_answer 49) A \[2{}^\circ C\] rise in temperature is observed in a conductor by parsing a current. When the current is triped, the rise in temperature will be
A)
\[9{}^\circ C\]
done
clear
B)
\[18{}^\circ C\]
done
clear
C)
\[27{}^\circ C\]
done
clear
D)
\[36{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 50)
A conducting rod AC of length \[4\,l\] is rotated about a point O in a uniform magnetic field B directed into the paper \[AO=l\] and \[OC=3\,l,\] then
A)
\[{{V}_{A}}-{{V}_{O}}=\frac{B\omega \,{{l}^{2}}}{2}\]
done
clear
B)
\[{{V}_{O}}-{{V}_{C}}=\frac{9}{2}B\omega \,{{l}^{2}}\]
done
clear
C)
\[{{V}_{A}}-{{V}_{C}}=4B\omega \,{{l}^{2}}\]
done
clear
D)
\[{{V}_{C}}-{{V}_{O}}=\frac{9}{2}B\omega \,{{l}^{2}}\]
done
clear
View Answer play_arrow
question_answer 51) The decay constant of a radio isotopes is\[\lambda \]. If \[{{A}_{1}}\] and \[{{A}_{2}}\] are its activities at times \[{{t}_{1}}\] and \[{{t}_{2}}\] respectively . The number of nuclei, which have decayed during the time \[({{t}_{1}}-{{t}_{2}})\]
A)
\[{{A}_{1}}{{t}_{1}}-{{A}_{2}}{{t}_{2}}\]
done
clear
B)
\[{{A}_{1}}-{{A}_{2}}\]
done
clear
C)
\[\left( \frac{{{A}_{1}}-{{A}_{2}}}{\lambda } \right)\]
done
clear
D)
\[\lambda \,({{A}_{1}}-{{A}_{2}})\]
done
clear
View Answer play_arrow
question_answer 52) Energy gap between valence band and conduction band of a semiconductor is
A)
zero
done
clear
B)
infinite
done
clear
C)
1 eV
done
clear
D)
10 eV
done
clear
View Answer play_arrow
question_answer 53) An alpha nucleus of energy \[\frac{1}{2}m{{v}^{2}}\] bombards a heavy nuclear target of charge Ze. Then, the distance of closer approach for the alpha nucleus will be proportional to
A)
\[\frac{1}{Ze}\]
done
clear
B)
\[{{V}^{2}}\]
done
clear
C)
\[\frac{1}{m}\]
done
clear
D)
\[\frac{1}{{{V}^{4}}}\]
done
clear
View Answer play_arrow
question_answer 54) A gramophone record is revolving with an angular velocity \[\omega \,.\] A coin is placed at a distance \[r\] from the centre of the record .The static coefficient of friction is \[\mu \]. The coin will revolve with the record, if
A)
\[r=\mu g{{\omega }^{2}}\]
done
clear
B)
\[r<\frac{{{\omega }^{2}}}{\mu g}\]
done
clear
C)
\[r\le \frac{\mu g}{{{\omega }^{2}}}\]
done
clear
D)
\[r\ge \frac{\mu g}{{{\omega }^{2}}}\]
done
clear
View Answer play_arrow
question_answer 55) The excess pressure inside a spherical soap bubble of radius 1cm is balanced by a column of oil (specific gravity = 0.8), 2 mm high , the surface tension of the bubble is
A)
3.92 N/m
done
clear
B)
0.0392 N/m
done
clear
C)
0.392 N/m
done
clear
D)
0.00392 N/m
done
clear
View Answer play_arrow
question_answer 56) A body of mass \[{{m}_{1}}=4\,kg\] moves at \[5\,\hat{i}\,m\text{/}s\] and another body of mass \[{{m}_{2}}=2\,kg\] moves at\[10\,\hat{i}\,m\text{/}s\]. The kinetic energy of centre of mass is
A)
\[\frac{200}{3}J\]
done
clear
B)
\[\frac{500}{3}J\]
done
clear
C)
\[\frac{400}{3}J\]
done
clear
D)
\[\frac{800}{3}J\]
done
clear
View Answer play_arrow
question_answer 57) Charge passing through a conductor of crosssection area \[A=0.3\,{{m}^{2}}\] is given by \[q=3{{t}^{2}}+5t+2\] coulomb, where\[t\]is in second. What is the value of drift velocity at \[t=2\,s\] (Given \[n=2\times {{10}^{25}}/{{m}^{3}}\])
A)
\[0.77\times {{10}^{-5}}m\text{/}s\]
done
clear
B)
\[1.77\times {{10}^{-5}}m\text{/}s\]
done
clear
C)
\[2.08\times {{10}^{-5}}m\text{/}s\]
done
clear
D)
\[0.57\times {{10}^{-5}}m\text{/}s\]
done
clear
View Answer play_arrow
question_answer 58) The thermo emf of a thermocouple is given by \[E=aT+b{{T}^{2}},\] where \[\frac{a}{b}=-\,200{}^\circ C.\] If the cold function is kept at \[30{}^\circ C,\] then the inversion temperature is (E in volt, T in centigrade)
A)
103 K
done
clear
B)
143 K
done
clear
C)
333 K
done
clear
D)
443 K
done
clear
View Answer play_arrow
question_answer 59) Two coherent sources whose intensity ratio is 64 : 1 produce interference fringes. The ratio of intensities of maxima and minima is
A)
9: 7
done
clear
B)
8 : 1
done
clear
C)
81 : 49
done
clear
D)
81 : 7
done
clear
View Answer play_arrow
question_answer 60) The frequency of vibration in a vibration magnetometer of the combination of two bar magnets of magnetic moments \[{{M}_{1}}\] and \[{{M}_{2}}\]is 6 Hz when like poles are tied and it is 2 Hz when the unlike poles are tied together , then the ratio \[{{M}_{1}}:{{M}_{2}}\]is
A)
4 : 5
done
clear
B)
5 : 4
done
clear
C)
1 : 3
done
clear
D)
3 : 1
done
clear
View Answer play_arrow
question_answer 61) Vapour pressure of \[CC{{l}_{4}}\] at \[25{}^\circ C\] is 143 mm of Hg and 0.5 g of a non-volatile solute (mol. wt. = 65) is dissolved in 100 mL \[CC{{l}_{4}}\,.\] Find the vapour pressure of the solution. (Density of\[CC{{l}_{4}}\,=1.58\,g\text{/}c{{m}^{3}}\]).
A)
94.39 mm
done
clear
B)
141.93 mm
done
clear
C)
134.44 mm
done
clear
D)
199.34 mm
done
clear
View Answer play_arrow
question_answer 62) \[MnO_{4}^{-}+8{{H}^{+}}+5{{e}^{-}}\xrightarrow[{}]{{}}M{{n}^{2+}}+4{{H}_{2}}O;\]\[E{}^\circ =1.51\,\,V\] \[Mn{{O}_{2}}+4{{H}^{+}}+2{{e}^{-}}\xrightarrow[{}]{{}}M{{n}^{2+}}+2{{H}_{2}}O;\]\[E{}^\circ =1.23\,\,V\]
A)
1.70 V
done
clear
B)
0.91 V
done
clear
C)
1.37 V
done
clear
D)
0.548 V
done
clear
View Answer play_arrow
question_answer 63) The direct conversion of A to B is difficult, hence it is carried out by the following shown path \[\underset{A}{\mathop{\underset{\uparrow }{\mathop{C}}\,}}\,\xrightarrow{{}}\underset{B}{\mathop{\underset{\downarrow }{\mathop{D}}\,}}\,\] Given, \[\Delta {{S}_{(A\to C)}}=50\,\,eu\] \[\Delta {{S}_{(C\to D)}}=30\,\,eu\] \[\Delta {{S}_{(D\to B)}}=20\,\,eu\]where eu is entropy unit, then\[\Delta {{S}_{(A\to B)}}\]is
A)
+ 100 eu
done
clear
B)
+ 60 eu
done
clear
C)
- 100 eu
done
clear
D)
- 60 eu
done
clear
View Answer play_arrow
question_answer 64) The solubility product of\[A{{g}_{2}}Cr{{O}_{4}}\]is\[32\times {{10}^{-12}}.\] What is the concentration of\[CrO_{4}^{-}\]ions in that solution?
A)
\[2\times {{10}^{-4}}M\]
done
clear
B)
\[16\times {{10}^{-4}}M\]
done
clear
C)
\[8\times {{10}^{-4}}M\]
done
clear
D)
\[8\times {{10}^{-8}}M\]
done
clear
View Answer play_arrow
question_answer 65) The oxidation number of\[N\]and\[Cl\]in \[NOCl{{O}_{4}}\] respectively are
A)
+2 and +7
done
clear
B)
+3 and +7
done
clear
C)
-3 and +7
done
clear
D)
+2 and -7
done
clear
View Answer play_arrow
question_answer 66) Which of the following statement is not true about amorphous solids?
A)
On heating they may become crystalline at certain temperature
done
clear
B)
They may become crystalline on keeping for long time
done
clear
C)
Amorphous solids can be moulded by heating
done
clear
D)
They are an isotropic in nature
done
clear
View Answer play_arrow
question_answer 67) An electron, a proton and an alpha particle have KE of 16E, 4E and E respectively. What is the qualitative order of their de-Broglie wavelengths?
A)
\[{{\lambda }_{e}}>{{\lambda }_{p}}>{{\lambda }_{\alpha }}\]
done
clear
B)
\[{{\lambda }_{p}}={{\lambda }_{\alpha }}>{{\lambda }_{e}}\]
done
clear
C)
\[{{\lambda }_{p}}<{{\lambda }_{e}}>{{\lambda }_{\alpha }}\]
done
clear
D)
\[{{\lambda }_{\alpha }}<{{\lambda }_{e}}\approx {{\lambda }_{p}}\]
done
clear
View Answer play_arrow
question_answer 68)
Peroxide ion I. has five completely filled anti-bonding molecular orbitals II. is diamagnetic III. has bond order one IV. is isoelectronic with neon
Which of these are correct?
A)
II and III
done
clear
B)
I, II and IV
done
clear
C)
I, III and IV
done
clear
D)
I and IV
done
clear
View Answer play_arrow
question_answer 69)
Which of the following statements are correct? I. Order of a reaction can be known from experimental result and not from the stoichiometry of reaction. II. Overall molecularity of a reaction may be determined in a manner similar to overall order of reaction. III. Overall order of reaction,\[{{A}^{m}}+{{B}^{n}}\xrightarrow{{}}A{{B}_{x}}\,\text{is}\,(m+n)\] IV. Molecularity of a reaction refers to (i) molecularity of each of the elementary steps (slow steps) in a multistep reaction. (ii) molecularity of that particular step in a single step reaction.
Select the correct answer by using the codes given below.
A)
I, III and IV
done
clear
B)
I, II and III
done
clear
C)
II, III and IV
done
clear
D)
I, II and IV
done
clear
View Answer play_arrow
question_answer 70) The stability of hydrides of carbon family is in the order
A)
\[C{{H}_{4}}>Si{{H}_{4}}>Ge{{H}_{4}}>Sn{{H}_{4}}>Pb{{H}_{4}}\]
done
clear
B)
\[C{{H}_{4}}<Si{{H}_{4}}<Ge{{H}_{4}}<Sn{{H}_{4}}<Pb{{H}_{4}}\]
done
clear
C)
\[C{{H}_{4}}>Sn{{H}_{4}}>Ge{{H}_{4}}>Si{{H}_{4}}>Pb{{H}_{4}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 71)
The IUPAC name of compound
A)
hexane-1,2,5-tricarbonitrile
done
clear
B)
hexane-1,3,6-tricarbonitrile
done
clear
C)
butane-1,2,4-tricarbonitrile
done
clear
D)
butane-1,3,4-tricarbonitrile
done
clear
View Answer play_arrow
question_answer 72) Which of the following compounds cannot be prepared singly by the Wurtz reaction?
A)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CHC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
D)
All of the above can be prepared
done
clear
View Answer play_arrow
question_answer 73)
Match the following Column I and Column II. Column I Column II A. \[RN{{H}_{2}}\xrightarrow{KMn{{O}_{4}}}\] 1. \[{{R}_{3}}N\to O\] B. \[{{R}_{2}}NH\xrightarrow{{{H}_{2}}S{{O}_{5}}}\] 2. \[{{R}_{3}}CN{{O}_{2}}\] C. \[{{R}_{3}}N\xrightarrow{{{H}_{2}}S{{O}_{5}}}\] 3. \[{{R}_{3}}CHO\] D. \[{{R}_{3}}CN{{H}_{2}}\xrightarrow{{{H}_{2}}S{{O}_{5}}}\] 4. \[{{R}_{2}}NOH\]
Codes
A)
A-3 B-1 C-4 D-2
done
clear
B)
A-2 B-3 C-4 D-1
done
clear
C)
A-3 B-2 C-1 D-4
done
clear
D)
A-1 B-2 C-3 D-4
done
clear
View Answer play_arrow
question_answer 74) The polymer used in making synthetic hair wigs is made up of
A)
\[C{{H}_{2}}=CHCl\]
done
clear
B)
\[C{{H}_{2}}=CHCOOC{{H}_{3}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{2}}=CH-CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 75) Which one is not a constituent of nucleic acid?
A)
Uracil
done
clear
B)
Guanidine
done
clear
C)
Phosphoric acid
done
clear
D)
Ribose sugar
done
clear
View Answer play_arrow
question_answer 76) 1.520 g of the hydroxide of a metal on ignition gave 0.995 g of oxide. The equivalent weight of metal is
A)
1.520
done
clear
B)
0.995
done
clear
C)
19.00
done
clear
D)
9.00
done
clear
View Answer play_arrow
question_answer 77) Deionised water is obtained by passing hard water through
A)
anion exchanger
done
clear
B)
zeolite
done
clear
C)
cation exchanger
done
clear
D)
Both anion and cation exchanger
done
clear
View Answer play_arrow
question_answer 78) A dark brown solid \[X\]reacts with\[N{{H}_{3}}\]to form a mild explosive which decomposes to give a violet coloured gas. \[X\]also reacts with \[{{H}_{2}}\] to give an acid\[Y\cdot Y\]can also be prepared by heating its salt with\[{{H}_{3}}P{{O}_{4}}\,.\]\[X\]and\[Y\]are
A)
\[C{{l}_{2}},HCl\]
done
clear
B)
\[S{{O}_{2}},{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
\[B{{r}_{2}},HBr\]
done
clear
D)
\[{{I}_{2}},HI\]
done
clear
View Answer play_arrow
question_answer 79) Amongst the following, the lowest degree of para-magnetism per mole of the compound at 298 K will be shown by
A)
\[MnS{{O}_{4}}\cdot 4{{H}_{2}}O\]
done
clear
B)
\[NiS{{O}_{4}}\cdot 6{{H}_{2}}O\]
done
clear
C)
\[FeS{{O}_{4}}\cdot 6{{H}_{2}}O\]
done
clear
D)
\[CuS{{O}_{4}}\cdot 5{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 80)
The final product IV in the sequence of reactions,
A)
\[C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{C}}\,}}\,HOC{{H}_{2}}C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{C}}\,}}\,HC{{H}_{2}}C{{H}_{2}}Br\]
done
clear
C)
\[C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
D)
\[C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{C}}\,}}\,HOC{{H}_{2}}C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 81) When sodium is heated with moist air, then the product obtained is
A)
\[N{{a}_{2}}{{O}_{2}}\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
C)
\[NaOH\]
done
clear
D)
\[N{{a}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 82) Calculate the total pressure in a 10.0 L cylinder which contains 0.4 g helium, 1.6 g oxygen and 1.4 g nitrogen at\[27{}^\circ C\].
A)
0.492 atm
done
clear
B)
49.2 atm
done
clear
C)
4.92 atm
done
clear
D)
0.0492 atm
done
clear
View Answer play_arrow
question_answer 83) The species\[{{C}_{2}}\]
A)
has one o bond and one\[\pi \]bond
done
clear
B)
has both n bonds
done
clear
C)
has both o bonds
done
clear
D)
does not exist
done
clear
View Answer play_arrow
question_answer 84) Which among the following is correct for \[_{5}B\] in normal state?
A)
\[\overset{2s}{\mathop{}}\,\,\,\,\overset{2p}{\mathop{}}\,:\]Against Hunds rule
done
clear
B)
\[\,\,\,:\]Against Aufbau principle as well as Hunds rule
done
clear
C)
\[\,\,\,:\]Violation of Paulis exclusion principle and not Hunds rule
done
clear
D)
\[\,\,\,:\]Against Aufbau principle
done
clear
View Answer play_arrow
question_answer 85) The number of coulombs required to reduce 12.3 g of nitrobenzene to aniline, is
A)
96500 C
done
clear
B)
5790 C
done
clear
C)
95700 C
done
clear
D)
57900 C
done
clear
View Answer play_arrow
question_answer 86)
The curve showing the variation of pressure with temperature for a given amount of adsorption is called
A)
adsorption isobar
done
clear
B)
adsorption isotherm
done
clear
C)
adsorption isostere
done
clear
D)
adsorption isochore
done
clear
View Answer play_arrow
question_answer 87) Ore pitch blende is the main source of
A)
Ra
done
clear
B)
Th
done
clear
C)
Mg
done
clear
D)
Ce
done
clear
View Answer play_arrow
question_answer 88) In which one of the following pairs, the radius of the second species is greater than that of the first?
A)
\[Na,\,Mg\]
done
clear
B)
\[{{O}^{2-}},\,{{N}^{3-}}\]
done
clear
C)
\[L{{i}^{+}},\,B{{e}^{2+}}\]
done
clear
D)
\[B{{a}^{2+}},\,S{{r}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 89) The free energy change for the following reactions are given below. \[{{C}_{2}}{{H}_{2}}(g)+\frac{5}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}2C{{O}_{2}}(g)+{{H}_{2}}O(l);\]\[\Delta G{}^\circ =-1234\,kJ\] \[C(s)+{{O}_{2}}(g)\xrightarrow{{}}C{{O}_{2}}(g);\] \[\Delta G{}^\circ =-\,394\,kJ\]\[{{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}{{H}_{2}}O(l);\] \[\Delta G{}^\circ =-\,237\,kJ\] What is the standard free energy change for the reaction? \[{{H}_{2}}(g)+2C(s)\xrightarrow{{}}{{C}_{2}}{{H}_{2}}(g)\]
A)
\[-\,209\text{ }kJ\]
done
clear
B)
\[-\,2259\text{ }kJ\]
done
clear
C)
\[+\,2259\text{ }kJ\]
done
clear
D)
\[209\text{ }kJ\]
done
clear
View Answer play_arrow
question_answer 90)
Match following Column I and Column II. Column I Column II A. Rain cloud 1. Sol B. Milk of magnesia 2. Foam C. Whipped cream 3. Micelles D. Soap in water 4. Aerosol
Codes
A)
A-1 B-2 C-3 D-4
done
clear
B)
A-4 B-1 C-2 D-3
done
clear
C)
A-4 B-2 C-3 D-1
done
clear
D)
A-3 B-1 C-2 D-4
done
clear
View Answer play_arrow
question_answer 91) The total number of possible isomers for the complex compound \[[Cu{{(N{{H}_{3}})}_{4}}]\,[PtC{{l}_{4}}]\]
A)
6
done
clear
B)
5
done
clear
C)
4
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 92)
In which of the compounds given below there is more than one kind of hybridisation (\[sp,\]\[s{{p}^{2}},\]\[s{{p}^{3}}\]) for carbon? I. \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\] II. \[C{{H}_{3}}CH=CH-C{{H}_{3}}\] III. \[C{{H}_{2}}=CH-CH=C{{H}_{2}}\] IV. \[H-C-C\equiv C-H\]
A)
II and IV
done
clear
B)
I and IV
done
clear
C)
II and III
done
clear
D)
Only II
done
clear
View Answer play_arrow
question_answer 93) If a compound on analysis was found to contain C = 18.5%, H = 1.55%, Cl = 55.04% and O = 24.81%, then its empirical formula is
A)
\[C{{H}_{2}}OCl\]
done
clear
B)
\[C{{H}_{2}}Cl{{O}_{2}}\]
done
clear
C)
\[ClC{{H}_{2}}O\]
done
clear
D)
\[CHClO\]
done
clear
View Answer play_arrow
question_answer 94) Which of the following is not a biopolymer?
A)
Proteins
done
clear
B)
Rubber
done
clear
C)
Cellulose
done
clear
D)
RNA
done
clear
View Answer play_arrow
question_answer 95) Initiation of polypeptide chain is through
A)
lysine
done
clear
B)
glycine
done
clear
C)
leucine
done
clear
D)
methionine
done
clear
View Answer play_arrow
question_answer 96) Which of the following statement is not true about the drug barbital?
A)
It causes addiction
done
clear
B)
It is a non-hypnotic drug
done
clear
C)
It is a tranquillizer
done
clear
D)
It is used in sleeping-pills
done
clear
View Answer play_arrow
question_answer 97) A compound liberates \[C{{O}_{2}}\]with \[NaHC{{O}_{3}}\]and also gives colour with neutral\[FeC{{l}_{3}}\]solution. The compound can be
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 98) A solution of D-glucose in water rotates the plane of polarised light
A)
to the left
done
clear
B)
to the right
done
clear
C)
to either side
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 99) Nitroethane can exhibit one of the following kind of isomerism
A)
metamerism
done
clear
B)
optical activity
done
clear
C)
tautomerism
done
clear
D)
position isomerism
done
clear
View Answer play_arrow
question_answer 100) The addition of HBr to an alkene in the presence of peroxide is the example of
A)
electrophilic addition reaction
done
clear
B)
nucleophilic addition reaction
done
clear
C)
free radical addition reaction
done
clear
D)
the formation of carbocation as an intermediate
done
clear
View Answer play_arrow
question_answer 101) The end product in the below reaction is\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\xrightarrow{HN{{O}_{2}}}A\xrightarrow{PC{{l}_{5}}}B\xrightarrow{N{{H}_{3}}}C\]
A)
ethyl cyanide
done
clear
B)
ethyl amine
done
clear
C)
methyl amine
done
clear
D)
acetamide
done
clear
View Answer play_arrow
question_answer 102) When excess of\[{{C}_{6}}{{H}_{6}}\]reacts with\[C{{H}_{2}}C{{l}_{2}}\]in the presence of anhydrous\[AlC{{l}_{3}},\]the following compound is obtained
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 103) Which reaction is not feasible?
A)
\[2KI+B{{r}_{2}}\xrightarrow{{}}2KBr+{{I}_{2}}\]
done
clear
B)
\[2KBr+{{I}_{2}}\xrightarrow{{}}2KI+B{{r}_{2}}\]
done
clear
C)
\[2KBr+C{{l}_{2}}\xrightarrow{{}}2KCl+B{{r}_{2}}\]
done
clear
D)
\[2{{H}_{2}}O+2{{F}_{2}}\xrightarrow{{}}4HF+{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 104) Given the limiting molar conductivity as \[\Lambda _{m}^{\infty }(HCl)=425.9\,\,{{\Omega }^{-1}}\,c{{m}^{2}}\,mo{{l}^{-1}}\] \[\Lambda _{m}^{\infty }(NaCl)=126.4\,\,{{\Omega }^{-1}}\,c{{m}^{2}}\,mo{{l}^{-1}}\] \[\Lambda _{m}^{\infty }(C{{H}_{3}}COONa)=91\,\,{{\Omega }^{-1}}\,c{{m}^{2}}\,mo{{l}^{-1}}\] The molar conductivity, at infinite dilution, of acetic acid (in \[{{\Omega }^{-1}}c{{m}^{2}}\,mo{{l}^{-1}}\]) will be
A)
481.5
done
clear
B)
390.5
done
clear
C)
299.5
done
clear
D)
516.9
done
clear
View Answer play_arrow
question_answer 105) If 5.85 g of \[NaCl\](molecular weight 58.5) is dissolved in water and the solution is made up to 0.5 L, the molarity of the solution will be
A)
0.1
done
clear
B)
0.2
done
clear
C)
0.3
done
clear
D)
0.4
done
clear
View Answer play_arrow
question_answer 106) What is the equilibrium expression for the reaction,\[{{P}_{4}}(s)+5{{O}_{2}}(g){{P}_{4}}{{O}_{10}}(s)?\]
A)
\[{{K}_{C}}=\frac{1}{{{[{{O}_{2}}]}^{5}}}\]
done
clear
B)
\[{{K}_{C}}={{[{{O}_{2}}]}^{5}}\]
done
clear
C)
\[{{K}_{C}}=\frac{[{{P}_{4}}{{O}_{10}}]}{5\,[{{P}_{4}}]\,[{{O}_{2}}]}\]
done
clear
D)
\[{{K}_{C}}=\frac{[{{P}_{4}}{{O}_{10}}]}{[{{P}_{4}}]\,{{[{{O}_{2}}]}^{5}}}\]
done
clear
View Answer play_arrow
question_answer 107)
Which of the following reactions do not involve oxidation reduction? I. \[2Rb+2{{H}_{2}}O\xrightarrow{{}}2RbOH+{{H}_{2}}\] II. \[2Cu{{I}_{2}}\xrightarrow{{}}2CuI+{{I}_{2}}\] III. \[N{{H}_{4}}Br+KOH\xrightarrow[{}]{{}}KBr+N{{H}_{3}}+{{H}_{2}}O\] IV. \[4KCN+Fe{{(CN)}_{2}}\xrightarrow{{}}{{K}_{4}}[Fe{{(CN)}_{6}}]\]
A)
I and II
done
clear
B)
I and III
done
clear
C)
I, III and IV
done
clear
D)
III and IV
done
clear
View Answer play_arrow
question_answer 108) There are two isotopes of an element with atomic mass z. Heavier one has atomic mass z + 2 and lighter one has z - 1, then abundance of lighter one is
A)
66.6%
done
clear
B)
96.7%
done
clear
C)
6.67%
done
clear
D)
33.3%
done
clear
View Answer play_arrow
question_answer 109) How many gram of sucrose (mol. wt. = 342) should be dissolved in 100 g water in order to produce a solution with a \[105.0{}^\circ C\] difference between the freezing point and boiling temperature? \[[{{k}_{f}}=1.860{}^\circ C\text{/}m,\,{{k}_{b}}=0.151{}^\circ C\text{/}m]\]
A)
34.2 g
done
clear
B)
72 g
done
clear
C)
342 g
done
clear
D)
460 g
done
clear
View Answer play_arrow
question_answer 110) The statement that is true for the long form of the periodic table is
A)
it reflects the sequence of filling the electrons in the order of sub-energy levels s, p, d and f
done
clear
B)
it helps to predict the stable valency states of the elements
done
clear
C)
it reflects trends in physical and chemical properties of the elements
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 111) When carbon monoxide is passed over solid caustic soda heated to \[200{}^\circ C,\]it forms
A)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
B)
\[C{{H}_{3}}COONa\]
done
clear
C)
\[NaHC{{O}_{3}}\]
done
clear
D)
\[HCOONa\]
done
clear
View Answer play_arrow
question_answer 112) The half-life of two samples are 0.1 and 0.8 s. Their respective concentration are 400 and 50 respectively. The order of the reaction is
A)
0
done
clear
B)
2
done
clear
C)
1
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 113) What are the species A and B in the following\[Cr{{O}_{3}}+{{H}_{2}}O\xrightarrow{{}}A\xrightarrow{O{{H}^{-}}}B\]
A)
\[{{H}_{2}}Cr{{O}_{4}},\,{{H}_{2}}C{{r}_{2}}{{O}_{7}}\]
done
clear
B)
\[{{H}_{2}}C{{r}_{2}}{{O}_{7}},\,C{{r}_{2}}{{O}_{3}}\]
done
clear
C)
\[CrO_{4}^{2-},\,CrO_{7}^{2-}\]
done
clear
D)
\[{{H}_{2}}Cr{{O}_{4}},\,CrO_{4}^{2-}\]
done
clear
View Answer play_arrow
question_answer 114) Which of the following reactions will not yield p-tert butylphenol?
A)
\[\text{Phenol}+C{{H}_{3}}-\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C}}\,}}\,=C{{H}_{2}}\xrightarrow{{{H}^{+}}}\]
done
clear
B)
\[\text{Phenol}+{{(C{{H}_{3}})}_{3}}COH\xrightarrow{{{H}^{+}}}\]
done
clear
C)
\[\text{Phenol}+{{(C{{H}_{3}})}_{3}}C\cdot Cl\xrightarrow{AlC{{l}_{3}}}\]
done
clear
D)
\[\text{Phenol}+CHC{{l}_{3}}\xrightarrow{NaOH}\]
done
clear
View Answer play_arrow
question_answer 115) Formic acid and acetic acid are distinguished by
A)
\[NaHC{{O}_{3}}\]
done
clear
B)
\[FeC{{l}_{3}}\]
done
clear
C)
Victor Meyer test
done
clear
D)
Tollens reagent
done
clear
View Answer play_arrow
question_answer 116) As it passes into food chain, the concentration of DDT
A)
remains same
done
clear
B)
decreases
done
clear
C)
increases
done
clear
D)
unpredictable
done
clear
View Answer play_arrow
question_answer 117) The final product of the following sequence of reaction is\[\underset{C{{H}_{2}}}{\overset{C{{H}_{2}}}{\mathop{||}}}\,\xrightarrow[CC{{l}_{4}}]{B{{r}_{2}}}A\xrightarrow[{}]{KCN}B\xrightarrow[{}]{{{H}^{+}}/{{H}_{2}}O}C\]
A)
\[\underset{C{{H}_{2}}-COOH}{\overset{C{{H}_{2}}-COOH}{\mathop{|}}}\,\]
done
clear
B)
\[\underset{C{{H}_{2}}-Br}{\overset{C{{H}_{2}}-Br}{\mathop{|}}}\,\]
done
clear
C)
\[\underset{C{{H}_{2}}-CN}{\overset{C{{H}_{2}}-COOH}{\mathop{|}}}\,\]
done
clear
D)
\[\underset{C{{H}_{2}}-CN}{\overset{C{{H}_{2}}-CN}{\mathop{|}}}\,\]
done
clear
View Answer play_arrow
question_answer 118) Identify the set of reagent / reaction conditions X and Y in the following set of transformations. \[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}Br\xrightarrow{X}\text{Product}\xrightarrow{Y}\]\[C{{H}_{3}}-\underset{Br}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{3}}\]
A)
X = dilute aqueous \[NaOH,\]\[20{}^\circ C;\]Y = HBr/acetic acid, \[20{}^\circ C\]
done
clear
B)
X = concentrated alcoholic\[NaOH,\]\[80{}^\circ C;\]Y = HBr/acetic acid, \[20{}^\circ C\]
done
clear
C)
X = dilute aqueous \[NaOH,\] 20°C; Y =\[B{{r}_{2}}\text{/}CHC{{l}_{3}},\,0{}^\circ C\]
done
clear
D)
X = concentrated alcoholic\[NaOH,\]\[80{}^\circ C;\]Y = \[B{{r}_{2}}\text{/}CHC{{l}_{3}},\,0{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 119) The slag obtained during the extraction of copper from copper pyrites is composed of
A)
\[C{{u}_{2}}S\]
done
clear
B)
\[Si{{O}_{2}}\]
done
clear
C)
\[CuSi{{O}_{3}}\]
done
clear
D)
\[FeSi{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 120) An\[{{S}_{N}}2\]reaction at an asymmetric carbon of a compound always gives
A)
a mixture of diastereomers
done
clear
B)
a single stereoisomer
done
clear
C)
an enantiomer of the substrate
done
clear
D)
a product with opposite optical rotation
done
clear
View Answer play_arrow
question_answer 121) During the life cycle of Fasciola hepatica (liver fluke) infects its intermediate host and primary host at the following larval stage respectively
A)
redia and miraeidium
done
clear
B)
cercaria and redia
done
clear
C)
metacercaria and cercaria
done
clear
D)
miraeidium and metacercaria
done
clear
View Answer play_arrow
question_answer 122) Which type of ribosomes are found in Nostoc cells?
A)
50 S
done
clear
B)
60 S
done
clear
C)
70 S
done
clear
D)
80 S
done
clear
View Answer play_arrow
question_answer 123) Bone marrow is absent in the bones of
A)
Pisces
done
clear
B)
Amphibia
done
clear
C)
Reptilia
done
clear
D)
Aves
done
clear
View Answer play_arrow
question_answer 124) Formation .of sex cells was first seen in
A)
pteridophyte
done
clear
B)
bryophytes
done
clear
C)
gymnosperms
done
clear
D)
angiosperms
done
clear
View Answer play_arrow
question_answer 125) Most plant cells are surrounded by cell wall. There are some exceptions, e.g.,
A)
root hairs
done
clear
B)
bacteria
done
clear
C)
stem hairs
done
clear
D)
gametes
done
clear
View Answer play_arrow
question_answer 126) Which is not correct for cyclic photo-phosphorylation?
A)
No \[{{O}_{2}}\]given off
done
clear
B)
No\[NaDP{{H}_{2}}\]synthesized
done
clear
C)
No water consumed
done
clear
D)
PS-I and PS-II are involved
done
clear
View Answer play_arrow
question_answer 127) Dental formula of rat is
A)
\[\frac{0033}{3133}\]
done
clear
B)
\[\frac{1003}{1003}\]
done
clear
C)
\[\frac{2123}{2123}\]
done
clear
D)
\[\frac{3131}{3121}\]
done
clear
View Answer play_arrow
question_answer 128) In which segment of earthworm is clitellum present?
A)
5th to 9th segment
done
clear
B)
14th to 16th segment
done
clear
C)
1st segment
done
clear
D)
15th to 26th segment
done
clear
View Answer play_arrow
question_answer 129) Which of the following protein in blood is responsible for anti-coagulation?
A)
Heparin
done
clear
B)
Histamine
done
clear
C)
Hirudin
done
clear
D)
EDTA
done
clear
View Answer play_arrow
question_answer 130) Polar bodies are produced during the formation of
A)
sperm
done
clear
B)
spermatocytes
done
clear
C)
oogonlum
done
clear
D)
secondary oocyte
done
clear
View Answer play_arrow
question_answer 131) Mestrual bleeding occurs due to
A)
decrease in level of progesterone
done
clear
B)
decrease in level of GH
done
clear
C)
decrease in level of FSH
done
clear
D)
decrease in level of LH
done
clear
View Answer play_arrow
question_answer 132) Organic evolution is
A)
continuous process
done
clear
B)
discontinuous process
done
clear
C)
initially discontinuous but now continuous
done
clear
D)
initially continuous but now discontinuous
done
clear
View Answer play_arrow
question_answer 133) In ECG, P-R interval corresponds to
A)
time delay in AV-node
done
clear
B)
SA-nodal conduction time
done
clear
C)
increased ventricular contraction
done
clear
D)
time interval between onset of ventricular contraction
done
clear
View Answer play_arrow
question_answer 134) Which of following plants material is widely used in the preparation of culture medium?
A)
Pinus longifolia
done
clear
B)
Cocos nucifera
done
clear
C)
Borassus flabellifer
done
clear
D)
Cycas revolute
done
clear
View Answer play_arrow
question_answer 135) Formation of diploid embryo sac from diploid vegetative structure is called
A)
diplospory
done
clear
B)
apospory
done
clear
C)
adventive embryony
done
clear
D)
apomixes
done
clear
View Answer play_arrow
question_answer 136) A pure tall pea plant was reared in soil poor in the nutrition and reached the size of pure dwarf nea plant, if this plant is selfed, then the genotype in the\[{{F}_{1}}\text{-}\]generation is most likely to be
A)
all tall
done
clear
B)
50% tall: 50% .dwarf
done
clear
C)
all dwarf
done
clear
D)
Not possible: to predict
done
clear
View Answer play_arrow
question_answer 137) Instead of chromosome which of the following has only DNA
A)
Anabaena and \[E.coli\]
done
clear
B)
Volvox
done
clear
C)
Chlamydomonas
done
clear
D)
Plasmodiophora and Cystopus
done
clear
View Answer play_arrow
question_answer 138) Who is credited to show that Viruses are the cause of cancer?
A)
GJ Mendel
done
clear
B)
HG Khorana
done
clear
C)
MS Swaminathan
done
clear
D)
Dulbecco
done
clear
View Answer play_arrow
question_answer 139) DNA nucleotides are attached by
A)
hydrogen bond
done
clear
B)
covalent bond
done
clear
C)
van der Waals forces
done
clear
D)
electrovalent bond
done
clear
View Answer play_arrow
question_answer 140) The condition in which the potassium level is increased, is known as
A)
hyper cholesterolemia
done
clear
B)
hyper kalaemia
done
clear
C)
osteomalacia
done
clear
D)
hyper excitability
done
clear
View Answer play_arrow
question_answer 141) Apnea is
A)
absence of breathing
done
clear
B)
decreased ventilation
done
clear
C)
increased ventilation
done
clear
D)
laboured breathing
done
clear
View Answer play_arrow
question_answer 142) Embryoids are
A)
highly active embryos
done
clear
B)
embryos produced from reproductive cells
done
clear
C)
embryos produced from somatic cell through tissue culture
done
clear
D)
dead embryos
done
clear
View Answer play_arrow
question_answer 143) The dried fruit used for making musical instruments is
A)
snake gourd
done
clear
B)
bitter gourd
done
clear
C)
bottle gourd
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 144) A ribozyme is
A)
an enzyme associated with ribosome
done
clear
B)
a catalytic RNA
done
clear
C)
an enzyme that helps in ribose synthesis
done
clear
D)
An enzyme that joins ribose with adenine
done
clear
View Answer play_arrow
question_answer 145) What is true about cleavage in the fertilized egg in humans?
A)
It is meroblastic
done
clear
B)
To starts when the egg reaches uterus
done
clear
C)
It is identical to the normal mitosis
done
clear
D)
It starts while the egg is in fallopian tube
done
clear
View Answer play_arrow
question_answer 146) The bond associated with the secondary structure of protein molecules are
A)
peptide bond
done
clear
B)
phosphodiester bond
done
clear
C)
hydrogen bond
done
clear
D)
energy rich bond
done
clear
View Answer play_arrow
question_answer 147) Which enzyme is useful as colour brightening agent in detergent industry?
A)
Amylase
done
clear
B)
Lipase
done
clear
C)
Protease
done
clear
D)
Cellulase
done
clear
View Answer play_arrow
question_answer 148) The first organisms appears on earth were
A)
Photoautotrophs
done
clear
B)
Chemoautotrophs
done
clear
C)
Chemoheterotrophs
done
clear
D)
Coacervaters
done
clear
View Answer play_arrow
question_answer 149) Law of independent assortment can be explained with the help of
A)
dihybrid cross
done
clear
B)
monohybrid cross
done
clear
C)
test-cross
done
clear
D)
back-cross
done
clear
View Answer play_arrow
question_answer 150) Hamburgers phenomenon explains
A)
formation of \[HCO_{3}^{-}\]
done
clear
B)
chloride shift
done
clear
C)
oxygen saturation of haemoglobin
done
clear
D)
breathing mechanism
done
clear
View Answer play_arrow
question_answer 151) Deuterostome condition and indeterminate radial cleavage are the characteristics of
A)
chordates and arthropods
done
clear
B)
chordates and echinoderms
done
clear
C)
arthropods and echinoderms
done
clear
D)
chordates, arthropods and annelids
done
clear
View Answer play_arrow
question_answer 152) The prokaryotic genome differ from eukaryotic genome because
A)
the DNA is complexed with histone in prokaryotes
done
clear
B)
the DNA is circular and single stranded in prokaryotes
done
clear
C)
repetitive sequences are present in eukaryotes
done
clear
D)
genes in the former case are organised into operons
done
clear
View Answer play_arrow
question_answer 153) Analogous organs have a
A)
common embryonic origin but perform different functions
done
clear
B)
common embryonic origin and perform similar
done
clear
C)
different embryonic origin, and perform different function
done
clear
D)
different embryonic origin but perform similar function
done
clear
View Answer play_arrow
question_answer 154) Transcription unit
A)
starts with TATA box
done
clear
B)
starts with palindromes and end with rho factor
done
clear
C)
starts with promoter and end with terminator region.
done
clear
D)
starts with CAAT region
done
clear
View Answer play_arrow
question_answer 155) The name explant is given to
A)
plants produced by tissue culture
done
clear
B)
plants produced by rDNA technology
done
clear
C)
a cell of cells used in tissue culture technique
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 156) Parallelism is
A)
adaptive convergence
done
clear
B)
adaptive divergence
done
clear
C)
adaptive convergence of for off species
done
clear
D)
adaptive convergence of closely related species
done
clear
View Answer play_arrow
question_answer 157) Homeostasis is
A)
tendency of biological system to change with change in environment
done
clear
B)
tendency of biological system to resist changes
done
clear
C)
disturbances of self-regulatory system and natural control
done
clear
D)
biotic material used in homeopathy
done
clear
View Answer play_arrow
question_answer 158) The presence of gill slits in the embryos of all vertebrates supports the theory of
A)
metamorphosis
done
clear
B)
biogenesis
done
clear
C)
organic evolution
done
clear
D)
recapitulation
done
clear
View Answer play_arrow
question_answer 159) The presence of more diversity at the junction of territories of two different habitats in known as
A)
bottle neck effect
done
clear
B)
edge effect
done
clear
C)
junction effect
done
clear
D)
Pasteur effect
done
clear
View Answer play_arrow
question_answer 160) During the dark reaction of photosynthesis, which of the following takes place?
A)
Molecular \[{{O}_{2}}\]is released
done
clear
B)
PGAL synthesis and reduction of\[NADP{{H}_{2}}\]
done
clear
C)
ATP is formed
done
clear
D)
\[{{H}_{2}}\] is released
done
clear
View Answer play_arrow
question_answer 161) At a particular locus the frequency of A allele is 0.6 and that of a is 0.4. What would be the frequency of heterozygotes in a random mating population at equilibrium?
A)
0.36
done
clear
B)
0.16
done
clear
C)
0.24
done
clear
D)
0.48
done
clear
View Answer play_arrow
question_answer 162) Shola forests are found in
A)
Eastern coast of Orissa
done
clear
B)
North-East Himalaya
done
clear
C)
Western Ghats (Kerala)
done
clear
D)
Deccan plateau
done
clear
View Answer play_arrow
question_answer 163) On the basis of cranial capacity which one is near to modern man?
A)
Cro-magnon man
done
clear
B)
Java man
done
clear
C)
Peking man
done
clear
D)
Neanderthal man
done
clear
View Answer play_arrow
question_answer 164)
Identify the correctly matched pair/pairs of the germ layers and their derivatives I. Ectoderm - Epidermis II. Endoderm - Dermis III. Mesoderm - Muscles IV. Mesoderm - Notochord V. Endoderm - Enamel of teeth
A)
I and IV
done
clear
B)
I and II
done
clear
C)
I, III and IV
done
clear
D)
I, II, III, and IV
done
clear
View Answer play_arrow
question_answer 165) Co-acervates were formed by
A)
radiation
done
clear
B)
polymerization
done
clear
C)
Polymerisation and aggregation
done
clear
D)
DNA coiling
done
clear
View Answer play_arrow
question_answer 166)
Match List I with List II. List-I (Discoveries) List-II (Scientist) A PCR technologies 1. G Kohler and C Milstein B. DNA fingerprinting 2. Mc Clintock C. Hybridoma technology 3. K Mullis D. Jumping genes 4. Allec Jeffreys
Which one code the following is correctly matched?
A)
A-2 B-1 C-4 D-3
done
clear
B)
A-2 B-4 C-1 D-3
done
clear
C)
A-3 B-1 C-4 D-2
done
clear
D)
A-3 B-4 C-1 D-2
done
clear
View Answer play_arrow
question_answer 167) Metabolic .water is the one
A)
used during trans amination
done
clear
B)
used during photosynthesis
done
clear
C)
produced during anaerobic utilisation of glucose
done
clear
D)
produced during condensation or polymerization
done
clear
View Answer play_arrow
question_answer 168) One gene one enzyme hypothesis was proposed by
A)
Jacob and Monad
done
clear
B)
Beadle and Tatum
done
clear
C)
Watson and Crick
done
clear
D)
Garrod and Jensen
done
clear
View Answer play_arrow
question_answer 169) In which of the following plants does the stem performs the function of storage and perennation?
A)
Wheat
done
clear
B)
Ground nut
done
clear
C)
Raddish
done
clear
D)
Ginger
done
clear
View Answer play_arrow
question_answer 170) Ketone bodies are the by products in metabolism of
A)
carbohydrate
done
clear
B)
protein
done
clear
C)
fat
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 171) Bromelain is an enzyme extracted from
A)
Ficus
done
clear
B)
papaya
done
clear
C)
pineapple
done
clear
D)
yeast
done
clear
View Answer play_arrow
question_answer 172) Resolving power of a light microscope is
A)
0.2 micrometer \[(\mu m)\]
done
clear
B)
0.4 micrometer \[(\mu m)\]
done
clear
C)
1 angstrom \[(\overset{\text{o}}{\mathop{A}}\,)\]
done
clear
D)
10 angstrom \[(\overset{\text{o}}{\mathop{A}}\,)\]
done
clear
View Answer play_arrow
question_answer 173) An alkaloid, which arrest cell division is obtained from
A)
Chrysanthamum
done
clear
B)
Colchicum
done
clear
C)
Dalbergia
done
clear
D)
Crocus
done
clear
View Answer play_arrow
question_answer 174) Mark the wrong match
A)
Bowmans capsule-Glomerular filteration
done
clear
B)
DCT-Absorption of glucose
done
clear
C)
Loop of Henle-Concentration of urine
done
clear
D)
PCT-Absorption of \[N{{a}^{+}}\]and \[{{K}^{+}}\]ion
done
clear
View Answer play_arrow
question_answer 175)
Match the Column I and Column II. Column-I (Biological molecule) Column-II (Functions) A. Glycogen 1. Hormone B. Globulin 2. Biocatalyst C. Steroid 3. Antibody D. Thrombin 4. Storage product
Which the following in correctly matched.
A)
A-3 B-2 C-4 D-1
done
clear
B)
A-4 B-2 C-1 D-3
done
clear
C)
A-2 B-4 C-3 D-1
done
clear
D)
A-4 B-3 C-1 D-2
done
clear
View Answer play_arrow
question_answer 176) An increase in the \[{{P}_{50}}\]of an oxyhaemoglobin curve would result from a decrease in
A)
oxygen
done
clear
B)
pH
done
clear
C)
metabolism
done
clear
D)
temperature
done
clear
View Answer play_arrow
question_answer 177) In germinating seeds, the fatty acids are degraded exclusively in the
A)
peroxisome
done
clear
B)
mitochondria
done
clear
C)
proplastids
done
clear
D)
glyoxysome
done
clear
View Answer play_arrow
question_answer 178) Which of the following decreases during aerobic exercise?
A)
Mean blood pressure
done
clear
B)
Circulating blood volume
done
clear
C)
Cerebral blood flow
done
clear
D)
Skin temperature
done
clear
View Answer play_arrow
question_answer 179) Which enzyme is used to remove the introns?
A)
Ligase
done
clear
B)
Ribozyme
done
clear
C)
RNA protease
done
clear
D)
Carbinozyme
done
clear
View Answer play_arrow
question_answer 180) Cyclosporine is used as
A)
allergic
done
clear
B)
immuno suppressant
done
clear
C)
prophylactic for viruses
done
clear
D)
prophylactic for marasmus.
done
clear
View Answer play_arrow
question_answer 181) Which one of the following is not a quantitative credit control technique?
A)
Bank Rate
done
clear
B)
Cash Reserve Ratio (CRR)
done
clear
C)
Increase of interest rate on saving deposit
done
clear
D)
Statutory Liquidity Ratio (SLR)
done
clear
View Answer play_arrow
question_answer 182) Interest on public debt is a part of
A)
transfer payments by the enterprises
done
clear
B)
transfer payments by the government
done
clear
C)
national income
done
clear
D)
interest payment by house holds
done
clear
View Answer play_arrow
question_answer 183) A Trade Policy consists of
A)
Export- Import Policy
done
clear
B)
Licensing Policy
done
clear
C)
Foreign Exchange Policy
done
clear
D)
Balance of Payment Policy
done
clear
View Answer play_arrow
question_answer 184) Which one of the following Bills must be passed by each House of the Indian Parliament separately by special majority?
A)
Ordinary Bill
done
clear
B)
Money Bill
done
clear
C)
Finance Bill
done
clear
D)
Constitution Amendment Bill
done
clear
View Answer play_arrow
question_answer 185) Who sang Sare Jahan Se Achchha Hindostan Hamara of Iqbal and Jan-Gana-Mana of Rabindra Nath Tagore in the Central Assembly at midnight of 14/15 August, 1947?
A)
Rameshwarj Nehru
done
clear
B)
Meera Ben
done
clear
C)
Sucheta Kriplani
done
clear
D)
MS Subbalaxmi
done
clear
View Answer play_arrow
question_answer 186) In what ways does the Indian Parliament exercise control over the administration?
A)
Through Parliamentary Committees
done
clear
B)
Through Consultative Committees of various Ministers
done
clear
C)
By mailing the administrations send periodic reports
done
clear
D)
By compelling the executive to issue writs
done
clear
View Answer play_arrow
question_answer 187) What could be the maximum time limit of Zero Hour?
A)
30 minutes
done
clear
B)
1 hour
done
clear
C)
2 hours
done
clear
D)
Indefinite period
done
clear
View Answer play_arrow
question_answer 188) Under whose leadership was the Congress Socialist Party founded in 1934?
A)
Jawahar Lal Nehru and Mahatma Gandhi
done
clear
B)
Acharya Narendra Dev and Jai Prakash Narayan
done
clear
C)
Subhash Chandra Bose and P C Joshi
done
clear
D)
Saifuddin Kitchlew and Rajendra Prasad
done
clear
View Answer play_arrow
question_answer 189) The immortal National Song Bande Mataram has been written by
A)
Rabindranath Tagore
done
clear
B)
Sharat Chandra Chattopadhyaya
done
clear
C)
Bankim Chandra Chattopadhyaya
done
clear
D)
Surendranath Bandopadhyaya
done
clear
View Answer play_arrow
question_answer 190) Who, among the following, repealed the Vernacular Press Act?
A)
Lord Dufferin
done
clear
B)
Lord Ripon
done
clear
C)
Lord Curzon
done
clear
D)
Lord Hardinge
done
clear
View Answer play_arrow
question_answer 191) Who established Shanti Niketan?
A)
Jawahar Lal Nehru
done
clear
B)
Bal Gangadhar filak
done
clear
C)
Rabindra Nath Tagore
done
clear
D)
Mohandas Karamchand Gandhi
done
clear
View Answer play_arrow
question_answer 192) Seamounts may be
A)
mountain abruptly rising from the ocean floor
done
clear
B)
mountains resulting out of diastropic activity from the ocean floor
done
clear
C)
mountains resulting from the changes inside the ocean floor.
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 193) The Caspian Sea , the largest inland sea or lake in the world, is located
A)
wholly in the continent of Europe
done
clear
B)
wholly in the continent of Asia
done
clear
C)
partly in Europe and partly in Asia
done
clear
D)
partly in Africa and partly in Asia
done
clear
View Answer play_arrow
question_answer 194) Coral reefs are formed by
A)
volcanic rocks
done
clear
B)
marine sediments
done
clear
C)
chlorine Material precipitated from sea water
done
clear
D)
tiny colonial marine animals which construct limestone selection material
done
clear
View Answer play_arrow
question_answer 195) Which one is responsible for red rot of sugarcane?
A)
Colletotrichum falcatum
done
clear
B)
Albugo Candida
done
clear
C)
Fusarium oxysporufn
done
clear
D)
Claviceps purpurea
done
clear
View Answer play_arrow
question_answer 196) Plant are made disease resistance by
A)
thermal action
done
clear
B)
harmonal action
done
clear
C)
by cross breeding with wild varity
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 197) Respiration occur in fishes through
A)
lung
done
clear
B)
gill
done
clear
C)
nostril
done
clear
D)
scles
done
clear
View Answer play_arrow
question_answer 198) Metallic solids are always opaque because
A)
they reflect all the incident light
done
clear
B)
they scatter all the incident light
done
clear
C)
the incident light is readily absorbed by free electrons in a metal
done
clear
D)
the energy band traps the incident light
done
clear
View Answer play_arrow
question_answer 199) Which of the following is not a semiconductor?
A)
Germinium
done
clear
B)
Silicon
done
clear
C)
Arsenic
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 200) In photoelectric effect, the number of photo electrons emitted is proportional to
A)
intensity of incident beam
done
clear
B)
frequency of incident beam
done
clear
C)
velocity of incident beam
done
clear
D)
work function of photo cathode
done
clear
View Answer play_arrow
question_answer 201) Which of the following is a popular programming language for developing multimedia web pages?
A)
COBOL
done
clear
B)
Java
done
clear
C)
BASIC
done
clear
D)
Assembler
done
clear
View Answer play_arrow
question_answer 202) An example of an autocatalytic reaction is
A)
hydrogenation of oil
done
clear
B)
decomposition of nitroglycerine
done
clear
C)
oxidation of\[N{{a}_{2}}As{{O}_{3}}\] in presence of \[N{{a}_{2}}S{{O}_{3}}\]
done
clear
D)
thermal decomposition of\[KCl{{O}_{3}}\]in presence of \[Mn{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 203) Domestic cooking gas consists of mostly
A)
methane and ethane
done
clear
B)
liquified butane and iso butante
done
clear
C)
ethylene and carbon monoxide
done
clear
D)
hydrogen and acetylene
done
clear
View Answer play_arrow
question_answer 204) Which of the following animals is protected in Kaziranga Sanctuary of Asom?
A)
Indian Bison
done
clear
B)
Indian Lion
done
clear
C)
Indian Rhinoceros
done
clear
D)
Indian Elephant
done
clear
View Answer play_arrow
question_answer 205) Most waste generated in the house can be recycled but there are certain items that cannot be recycled. In the list given below, which item is not recyclable?
A)
Newspaper
done
clear
B)
Plastic bags
done
clear
C)
Old Medicines
done
clear
D)
Glass bottles
done
clear
View Answer play_arrow
question_answer 206) Durand Cup is associated with the game of
A)
Football
done
clear
B)
Polo
done
clear
C)
Cricket
done
clear
D)
Hockey
done
clear
View Answer play_arrow
question_answer 207) Name the President of India who was elected unopposed?
A)
Dr Shankar Dayal Sharma
done
clear
B)
Dr Neelam Sanjiva Reddy
done
clear
C)
Dr Fakhruddina Ali Ahmed
done
clear
D)
Dr Zakir Hussain
done
clear
View Answer play_arrow
question_answer 208) The First Asian Secretary General of UNO was
A)
Vijay Laxmi Pandit
done
clear
B)
U Thant
done
clear
C)
Trygve Lie
done
clear
D)
Kurt Wal Dheum
done
clear
View Answer play_arrow
question_answer 209) Which is not an official language of UNO?
A)
English
done
clear
B)
Chinese
done
clear
C)
French.
done
clear
D)
Hindi
done
clear
View Answer play_arrow
question_answer 210) Who among the following is not a recipient of the Bharatiya Gyanpith Award?
A)
M G K Menon
done
clear
B)
Sunderlal Bahuguna
done
clear
C)
Aruna Asaf Ali
done
clear
D)
MF Hussain
done
clear
View Answer play_arrow
question_answer 211) When is National Girl Child Day of India?
A)
January 25
done
clear
B)
January 24
done
clear
C)
October 24
done
clear
D)
November 14
done
clear
View Answer play_arrow
question_answer 212) The First Asian Games were held in
A)
Manila
done
clear
B)
Tokyo
done
clear
C)
Jakarta
done
clear
D)
New Delhi
done
clear
View Answer play_arrow
question_answer 213) MODVAT stands for
A)
ad valorem tax on output
done
clear
B)
deduction of cost of inputs from the value of output
done
clear
C)
reduction in import duties
done
clear
D)
imposition of tax on professions
done
clear
View Answer play_arrow
question_answer 214) What is the percentage of the total workforce employed in agriculture sector in India?
A)
About 50%
done
clear
B)
About 56%
done
clear
C)
About 60%
done
clear
D)
About 64%
done
clear
View Answer play_arrow
question_answer 215) The Indian Constitution is
A)
federal
done
clear
B)
unitary
done
clear
C)
a happy mixture of the federal and unitary
done
clear
D)
federal in normal times and unitary in times of emergency
done
clear
View Answer play_arrow
question_answer 216) The overall objective of Fundamental Rights are to ensure
A)
Democratic Government
done
clear
B)
Individual Liberty
done
clear
C)
Judicial in Dependence
done
clear
D)
Secularism
done
clear
View Answer play_arrow
question_answer 217) Harihar and Bukka laid the foundation of the Vijayanagar empire under the influence of
A)
Jnaneshvera
done
clear
B)
Tukaram
done
clear
C)
Ramanuj
done
clear
D)
Vidyaranya
done
clear
View Answer play_arrow
question_answer 218) Which of the following Vedas provides information about the civilization of the Early Vedic Age?
A)
Rigveda
done
clear
B)
Yajurveda
done
clear
C)
Atharvaveda
done
clear
D)
Samaveda
done
clear
View Answer play_arrow
question_answer 219) Which of the following is a block mountain?
A)
Rocky
done
clear
B)
Vosgas
done
clear
C)
Alps
done
clear
D)
Andes
done
clear
View Answer play_arrow
question_answer 220) The fertility of the soil can be increased by growing
A)
cereals
done
clear
B)
fibre crops
done
clear
C)
legumes
done
clear
D)
root crops
done
clear
View Answer play_arrow
question_answer 221) The term global warming means
A)
quick melting of polar ice sheets
done
clear
B)
depletion of ozone layer
done
clear
C)
high temperature in torrid zone
done
clear
D)
general rise in temperature due to carbon dioxide
done
clear
View Answer play_arrow
question_answer 222) The substance most commonly used as a food preservatives
A)
sodium carbonate
done
clear
B)
tartaric acid
done
clear
C)
acetic acid
done
clear
D)
benzoic acid
done
clear
View Answer play_arrow
question_answer 223) Digital Computers use a ..... system to encode date and programs.
A)
semi conductor
done
clear
B)
decimal
done
clear
C)
binary
done
clear
D)
RAM
done
clear
View Answer play_arrow
question_answer 224) The Earth is almost spherical in shape was stated by
A)
Ptolemy
done
clear
B)
Aryabhatta
done
clear
C)
Pythagoras
done
clear
D)
Copernicus
done
clear
View Answer play_arrow
question_answer 225) Which one of the following does a TV remote control unit use to operate a TV set?
A)
Light waves
done
clear
B)
Sound waves
done
clear
C)
Micro waves
done
clear
D)
Radio waves
done
clear
View Answer play_arrow
question_answer 226) Who directed the film Bombay?
A)
Shyam Senegal
done
clear
B)
Meera Nair
done
clear
C)
Shekhar Kapoor
done
clear
D)
Mani Ratnam
done
clear
View Answer play_arrow
question_answer 227) The first writer to use Urdu as the medium of poetic expression was
A)
Amir Khusrau
done
clear
B)
Mirza Ghalib
done
clear
C)
Bahadur Shah Zafar
done
clear
D)
Faiz
done
clear
View Answer play_arrow
question_answer 228) Cricket was an Olympic event at which of the following Olympics?
A)
Landon, 1908
done
clear
B)
Amsterdam, 1928
done
clear
C)
Paris, 1900
done
clear
D)
Melbourne, 1956
done
clear
View Answer play_arrow
question_answer 229) The decade 1991-2000 was celebrated as
A)
SAARC Decade of the Girl Child
done
clear
B)
SAARC Decade of the Handicapped
done
clear
C)
UN Decade of the Youth
done
clear
D)
UN Decade of the Handicapped
done
clear
View Answer play_arrow
question_answer 230) Alice in Wonderland is written by
A)
Lewis Carrol
done
clear
B)
Chester Bowles
done
clear
C)
Charles Dickens
done
clear
D)
Jonathan Swift
done
clear
View Answer play_arrow
question_answer 231) Who wrote the novel Mrignayani?
A)
Vrandavan Lal Verma
done
clear
B)
Acharya Chatursen
done
clear
C)
Amrat Lal Nugar
done
clear
D)
Bhagwati Charan Verma
done
clear
View Answer play_arrow
question_answer 232) The term National Income represents
A)
Gross National Product at market price- depreciation
done
clear
B)
Gross National Product at market price - depreciation + net factor income from abroad
done
clear
C)
Gross National Product at market price - depreciation and indirect taxes + subsidies
done
clear
D)
Gross National Product at market prices - net factor income from abroad
done
clear
View Answer play_arrow
question_answer 233) The Final Authority in India to adopt the Five Year Plan for the country vest in
A)
the Planning Commission
done
clear
B)
the National Development Council
done
clear
C)
the Union Cabinet
done
clear
D)
the Parliament
done
clear
View Answer play_arrow
question_answer 234) What is the correct order of towns on the basis of population-size (Census 2001)?
A)
Greater Mumbai, Delhi, Kolkata, Chennai, Bengaluru
done
clear
B)
Greater Mumbai, Delhi, Kolkata, Bengaluru, Chennai
done
clear
C)
Kolkata, Greater Mumbai, Delhi, Chennai, Hyderabad
done
clear
D)
Delhi, Greater Mumbai, Kolkata, Chennai, Hyderabad
done
clear
View Answer play_arrow
question_answer 235) Which one of the following is a basic feature of the Presidential Government?
A)
Rigid Constitution
done
clear
B)
Single Executive
done
clear
C)
Supremacy of the Legislature
done
clear
D)
Residual Powers of the States
done
clear
View Answer play_arrow
question_answer 236) The office of Lokpal and Lokayukta in India is based on which one of the following?
A)
Parliamentary Commissioner of UK
done
clear
B)
Ombudsman in Scandinavia
done
clear
C)
Procurator General of Russia
done
clear
D)
Council of State in France
done
clear
View Answer play_arrow
question_answer 237) The Indus Valley people traded with the
A)
Chinese
done
clear
B)
Mesopotamians
done
clear
C)
Parthians
done
clear
D)
Romans
done
clear
View Answer play_arrow
question_answer 238) The word Aryan means
A)
of good family
done
clear
B)
cultivator
done
clear
C)
pastoral society
done
clear
D)
brahmachari
done
clear
View Answer play_arrow
question_answer 239) Gandhara School of Art came into existence in
A)
Hinayana Sect
done
clear
B)
Mahayana Sect
done
clear
C)
Vaishnava Sect
done
clear
D)
Shaiva Sect
done
clear
View Answer play_arrow
question_answer 240) The difference in the duration of day and night increase as one moves from
A)
West to East
done
clear
B)
East and West of the prime Meridian
done
clear
C)
Poles to Equator
done
clear
D)
Equator to Poles
done
clear
View Answer play_arrow
question_answer 241) One Astronomical unit is the Average distance between
A)
the Earth and the Sun
done
clear
B)
the Earth and the Moon
done
clear
C)
the Jupiter and the Sun
done
clear
D)
the Pluto and the Sun
done
clear
View Answer play_arrow
question_answer 242) Emu is an example of
A)
Active Volcano
done
clear
B)
Dormant Volcano
done
clear
C)
Extinct Volcano
done
clear
D)
Plateau in a Volcano region
done
clear
View Answer play_arrow
question_answer 243) Branch of agriculture concerned with the production of crops
A)
Agrotology
done
clear
B)
Agronomy
done
clear
C)
Agrostology
done
clear
D)
Anthropology
done
clear
View Answer play_arrow
question_answer 244) Bacteria which convert atmospheric nitrogen into nitrogenous compound are called
A)
denitrifying bacteria
done
clear
B)
nitrogen fixing bacteria
done
clear
C)
nitrifying bacteria
done
clear
D)
decomposing bacteria
done
clear
View Answer play_arrow
question_answer 245) National Flower of India is
A)
Lotus
done
clear
B)
Rafflesia
done
clear
C)
Lorenthus
done
clear
D)
Rose
done
clear
View Answer play_arrow
question_answer 246) A man inside an artificial satellite feels weightlessness because, the force of attraction due to Earth, becomes
A)
zero in comparison of Earths surface
done
clear
B)
balanced by the force of attraction due to Moon
done
clear
C)
equal to centripeated force
done
clear
D)
non-effective due to particular design of the Satellite
done
clear
View Answer play_arrow
question_answer 247) All of the logic and Mathematical calculations done by the computer happen in/on the
A)
system board
done
clear
B)
central control unit
done
clear
C)
central processing unit
done
clear
D)
mother board
done
clear
View Answer play_arrow
question_answer 248) The specific aim of ecology is to study
A)
how to best preserve diversity
done
clear
B)
pollution
done
clear
C)
the distribution patterns of plants and animals on Earth
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 249) Which one of the following is a pair of endangered species?
A)
Garden lizard and maxican poppy
done
clear
B)
Rhesus monkey and sal tree
done
clear
C)
Indian peacock and carrot grass
done
clear
D)
Hornbill and Indian aconite
done
clear
View Answer play_arrow
question_answer 250) Indra Nooyi is the Chief Executive Officer of which company?
A)
Pepsi
done
clear
B)
Coca Cola
done
clear
C)
LG
done
clear
D)
Samsung
done
clear
View Answer play_arrow
question_answer 251) Who among the following is the creator of the Rock Garden in Chandigarh?
A)
Le Corbousier
done
clear
B)
Pupul Jayakar
done
clear
C)
Nem Chand
done
clear
D)
Kamala Devi Chattopodhyay
done
clear
View Answer play_arrow
question_answer 252) NABARD is associated with the development of
A)
agricultural sector and rural area
done
clear
B)
heavy industries
done
clear
C)
banking sector
done
clear
D)
real estates
done
clear
View Answer play_arrow
question_answer 253) Famous play Macbeth is written by
A)
Leo Tolstoy
done
clear
B)
William Shakespeare
done
clear
C)
John Milton
done
clear
D)
Charles Dickens
done
clear
View Answer play_arrow
question_answer 254) Dr. Rabindranath Tagore received the Nobel Prize for his work
A)
Evening Songs
done
clear
B)
Morning Songs
done
clear
C)
Gitanjali
done
clear
D)
Gora
done
clear
View Answer play_arrow
question_answer 255) Which day is celebrated as United Nations Day every year?
A)
7th April
done
clear
B)
25th August
done
clear
C)
24th October
done
clear
D)
27th December
done
clear
View Answer play_arrow
question_answer 256) Wood is related to charcoal. In the same way, as coal is related to
A)
fire
done
clear
B)
smoke
done
clear
C)
coke
done
clear
D)
ash
done
clear
View Answer play_arrow
question_answer 257) Arrange the following words according to English dictionary. 1. Brook 2. Bandit 3. Boisterous 4. Baffle
A)
2, 4, 3, 1
done
clear
B)
1, 2, 3, 4
done
clear
C)
4, 1, 3, 2
done
clear
D)
4, 2, 3, 1
done
clear
View Answer play_arrow
question_answer 258) Which one of the following letter series when sequentially placed at the gaps in the given letter series shall complete it? _ _aba_ _ba_ab
A)
abbba
done
clear
B)
abbab
done
clear
C)
baabb
done
clear
D)
bbaba
done
clear
View Answer play_arrow
question_answer 259) Find the wrong number in the given series. 46080, 3840, 384, 48, 24, 2, 1
A)
384
done
clear
B)
48
done
clear
C)
24
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 260) Pointing out to a lady, Rajan said, She is the daughter of the woman who is the mother of the husband of my mother. Who is the lady to Rajan?
A)
Aunt
done
clear
B)
Grand daughter
done
clear
C)
Daughter
done
clear
D)
Sister
done
clear
View Answer play_arrow
question_answer 261) Nitin ranks 18th in a class of 49 students. What is his rank from the last?
A)
18
done
clear
B)
19
done
clear
C)
31
done
clear
D)
32
done
clear
View Answer play_arrow
question_answer 262) Select the word which cannot be formed from the original word. CONCENTRATE
A)
TREAT
done
clear
B)
REASON
done
clear
C)
CENTRE
done
clear
D)
CONCERN
done
clear
View Answer play_arrow
question_answer 263) If ROBUST is written as QNATRS, which word would be written as ZXCMP in the following?
A)
BZEOR
done
clear
B)
AYDNQ
done
clear
C)
AWDLQ
done
clear
D)
YYBNO
done
clear
View Answer play_arrow
question_answer 264) The total number of digits used in numbering, the pages of a book having 366 pages is
A)
732
done
clear
B)
990
done
clear
C)
1098
done
clear
D)
1305
done
clear
View Answer play_arrow
question_answer 265) Directions: Find out the missing numbers. \[\begin{matrix} 8 & 17 & 33 \\ 12 & 5 & 29 \\ 10 & 13 & ? \\ \end{matrix}\]
A)
9
done
clear
B)
23
done
clear
C)
33
done
clear
D)
43
done
clear
View Answer play_arrow
question_answer 266) Directions: Find out the missing numbers. \[\begin{matrix} 963 & 2 & 844 \\ 464 & ? & 903 \\ \end{matrix}\]
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 267)
The four positions of a dice are given below
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 268)
Which answer figure will complete the pattern of question figure? Question Figure
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 269)
Select the figure in which questions figure is hidden embedded? Question Figure
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 270)
A piece of paper is folded and cut as shown below in the question figure. From the given answer figures, indicate how it will appear when opened? Question Figures
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 271) If the sum of a number of two digits and a numbers formed by reversing the digits is 99, then what is the sum of the digits of the original number?
A)
9
done
clear
B)
81
done
clear
C)
Cannot be known
done
clear
D)
18
done
clear
View Answer play_arrow
question_answer 272) What is the unit number in the value of\[{{(729)}^{59}}\]?
A)
9
done
clear
B)
8
done
clear
C)
7
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 273) \[{{(92)}^{2}}-{{(85)}^{2}}\]is divisible by
A)
17
done
clear
B)
11
done
clear
C)
10
done
clear
D)
59
done
clear
View Answer play_arrow
question_answer 274) The HCF of two numbers is 8. Which one of the following can never be their LCM?
A)
48
done
clear
B)
56
done
clear
C)
60
done
clear
D)
24
done
clear
View Answer play_arrow
question_answer 275) If the diagonal and the area of a rectangle are 25 m and 168\[{{m}^{2}}\], what is the length of the rectangle?
A)
17 m
done
clear
B)
31 m
done
clear
C)
12 m
done
clear
D)
24 m
done
clear
View Answer play_arrow
question_answer 276) What will be the area of a circle whose perimeter is 88 cm?
A)
576 \[c{{m}^{2}}\]
done
clear
B)
616 \[c{{m}^{2}}\]
done
clear
C)
636 \[c{{m}^{2}}\]
done
clear
D)
Cannot be determined
done
clear
View Answer play_arrow
question_answer 277) One angle of a right angle triangle is\[35{}^\circ \]. Which of the following is one of its angles?
A)
45
done
clear
B)
60
done
clear
C)
55
done
clear
D)
Cannot be determined
done
clear
View Answer play_arrow
question_answer 278) If 45 men can complete a work in 30 days working 12 h a day, in how many days will 60 men complete the work, working 10 h a day?
A)
31
done
clear
B)
29
done
clear
C)
25
done
clear
D)
27
done
clear
View Answer play_arrow
question_answer 279) A merchant marks his goods up by 60% and then offers a discount on the market price. If the final selling price after the discount results in the merchant making no profit or loss, what was the percentage discount offered by the merchant?
A)
60
done
clear
B)
40
done
clear
C)
37.5
done
clear
D)
Depends on the cost price
done
clear
View Answer play_arrow
question_answer 280) The ratio between boys and girls in a school is 4 : 6 respectively. If the number of boys is increased by 200 the ratio becomes 5 : 6 respectively. How many girls are there in the school?
A)
1200
done
clear
B)
800
done
clear
C)
1000
done
clear
D)
Cannot be determined
done
clear
View Answer play_arrow
question_answer 281) Average cost of 5 apples and 4 mangoes is Rs.36. The average cost of 7 apples and 8 mangoes is Rs.48. Find the total cost of 24 apples and 24 mangoes.
A)
1044
done
clear
B)
2088
done
clear
C)
720
done
clear
D)
324
done
clear
View Answer play_arrow
question_answer 282) If \[x+\frac{1}{x}=3,\] the value of \[{{x}^{5}}+\frac{1}{{{x}^{5}}}\] is
A)
122
done
clear
B)
118
done
clear
C)
123
done
clear
D)
120
done
clear
View Answer play_arrow
question_answer 283) A mixture of 40 L of milk and water contain 10% water. How much water must be added to make water 20% in the new mixture?
A)
10 L
done
clear
B)
7 L
done
clear
C)
5 L
done
clear
D)
3 L
done
clear
View Answer play_arrow
question_answer 284) The speed of a motor boat itself is 20 km/h and the rate of flow of the river is 4 km/h. Moving with the stream the boat went 120 km. What distance will the boat cover during the same time going against the stream?
A)
80
done
clear
B)
180
done
clear
C)
60
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 285) If an amount of Rs. 85602 is distributed equally amongst 33 persons. How much amount would each person get?
A)
Rs. 2594
done
clear
B)
Rs. 2954
done
clear
C)
Rs. 2549
done
clear
D)
Rs. 2495
done
clear
View Answer play_arrow
question_answer 286)
Directions: In each of the following questions six words are given which are denoted by A, B, C, D, E and F. By using all the six words, each only once, you have to frame a meaningful and grammatically correct sentence. The correct order of words is the answer. Choose from the four alternatives the one having the correct order of words. A. eat B. does C. raw D. not E. meat F. man
A)
FBDAEC
done
clear
B)
BDAFCE
done
clear
C)
FBADCE
done
clear
D)
FBDACE
done
clear
View Answer play_arrow
question_answer 287)
Directions: In each of the following questions six words are given which are denoted by A, B, C, D, E and F. By using all the six words, each only once, you have to frame a meaningful and grammatically correct sentence. The correct order of words is the answer. Choose from the four alternatives the one having the correct order of words. A. different B. there C. of D. vehicles E. kinds F. are
A)
BFEACD
done
clear
B)
AECDBF
done
clear
C)
DCAEBF
done
clear
D)
BFAECD
done
clear
View Answer play_arrow
question_answer 288)
Directions: In each of the following questions six words are given which are denoted by A, B, C, D, E and F. By using all the six words, each only once, you have to frame a meaningful and grammatically correct sentence. The correct order of words is the answer. Choose from the four alternatives the one having the correct order of words. A. attitude B. an C. swadeshi D. mind E. is F. of
A)
BAFDEC
done
clear
B)
CEBAFD
done
clear
C)
CEAFBD
done
clear
D)
BAFDCE
done
clear
View Answer play_arrow
question_answer 289) Directions: Read each sentence to find out whether there is any error in it. The error, if any, will be in one part of the sentence. The number of that part (a, b, c) is the answer. If there is no error, the answer is , i.e., No error. (Ignore errors of punctuation, if any).
A)
Most of the popular tele-serials are not only illogical
done
clear
B)
/ in their story line
done
clear
C)
/but also crude in their presentation,
done
clear
D)
/No error
done
clear
View Answer play_arrow
question_answer 290) Directions: Read each sentence to find out whether there is any error in it. The error, if any, will be in one part of the sentence. The number of that part (a, b, c) is the answer. If there is no error, the answer is , i.e., No error. (Ignore errors of punctuation, if any).
A)
I am trying to convince him
done
clear
B)
/for the last two days to come
done
clear
C)
/and live with me till his fathers anger cools down.
done
clear
D)
/ No error
done
clear
View Answer play_arrow
question_answer 291) Directions: Read each sentence to find out whether there is any error in it. The error, if any, will be in one part of the sentence. The number of that part (a, b, c) is the answer. If there is no error, the answer is , i.e., No error. (Ignore errors of punctuation, if any).
A)
It is a pity that a son born from very good parents
done
clear
B)
/should live a life of
done
clear
C)
/misery and deprivation of the worst order.
done
clear
D)
/No error
done
clear
View Answer play_arrow
question_answer 292) Directions: Pick out the most effective word from the given words to -fill in the blanks to make the sentence meaningfully complete. Countless Indians today use neem twigs, called datun, as ............. toothbrushes.
A)
durable
done
clear
B)
extended
done
clear
C)
saturated
done
clear
D)
disposable
done
clear
View Answer play_arrow
question_answer 293) Directions: Pick out the most effective word from the given words to -fill in the blanks to make the sentence meaningfully complete. I have always admired his ............. knowledge and scholarship.
A)
highest
done
clear
B)
flexible
done
clear
C)
provocative
done
clear
D)
profound
done
clear
View Answer play_arrow
question_answer 294) Directions: Pick out the most effective word from the given words to -fill in the blanks to make the sentence meaningfully complete. The fight for liberation brings .............. the best and the noblest qualities in mankind.
A)
with
done
clear
B)
in
done
clear
C)
for
done
clear
D)
out
done
clear
View Answer play_arrow
question_answer 295) Directions: In each sentence given below one word has been printed in bold. Below the sentence four words are suggested one of which can replace the word printed in bold without changing the meaning of the sentence. Find out the appropriate word in each case. In a hole below a mango tree, a snake was staying.
A)
halting
done
clear
B)
stopping
done
clear
C)
living
done
clear
D)
sitting
done
clear
View Answer play_arrow
question_answer 296) Directions: In each sentence given below one word has been printed in bold. Below the sentence four words are suggested one of which can replace the word printed in bold without changing the meaning of the sentence. Find out the appropriate word in each case. It was a useless attempt on her part to appear in this examination.
A)
fruitful
done
clear
B)
unjustified
done
clear
C)
future
done
clear
D)
futile
done
clear
View Answer play_arrow
question_answer 297) Directions: In each sentence given below one word has been printed in bold. Below the sentence four words are suggested one of which can replace the word printed in bold without changing the meaning of the sentence. Find out the appropriate word in each case. He was confident that his plan would work well.
A)
doubtful
done
clear
B)
careful
done
clear
C)
sure
done
clear
D)
upset
done
clear
View Answer play_arrow
question_answer 298) Directions: In each question given below, three words which are numbered, and have been printed of which one may be wrongly spelt. The number of that word is the answer. If all the three words are correctly spelt mark i.e., All correct as the answer.
A)
Barricade
done
clear
B)
Checkmate
done
clear
C)
Accesible
done
clear
D)
All correct
done
clear
View Answer play_arrow
question_answer 299) Directions: In each question given below, three words which are numbered, and have been printed of which one may be wrongly spelt. The number of that word is the answer. If all the three words are correctly spelt mark i.e., All correct as the answer.
A)
Providential
done
clear
B)
Sattelite
done
clear
C)
Precedence
done
clear
D)
All correct
done
clear
View Answer play_arrow
question_answer 300) Directions: In each question given below, three words which are numbered, and have been printed of which one may be wrongly spelt. The number of that word is the answer. If all the three words are correctly spelt mark i.e., All correct as the answer.
A)
Cacophony
done
clear
B)
Exacerbate
done
clear
C)
Particuler
done
clear
D)
All correct
done
clear
View Answer play_arrow
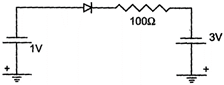
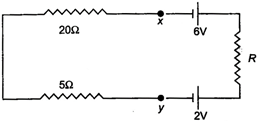
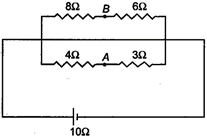
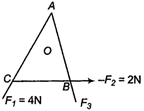
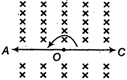
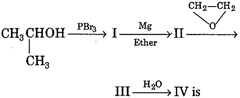
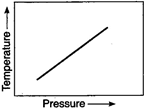
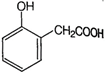
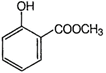
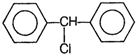
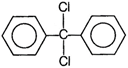
 Which number is on the face opposite to 6?
Which number is on the face opposite to 6?
 Answer Figures
Answer Figures
 Answer Figures
Answer Figures

 Answer Figures
Answer Figures