question_answer 1) A raindrop with radius 1.5 mm falls from a cloud at a height 1200 m from ground. The density of water is 1000 \[kg{{m}^{-3}}\] and density of air is 1.2\[kg{{m}^{-3}}\]. Assume the drop was spherical throughout the fall and there is no air drag. The impact speed of the drop will be
A)
27 \[km{{s}^{-1}}\]
done
clear
B)
550 \[km{{s}^{-1}}\]
done
clear
C)
zero
done
clear
D)
129 \[km{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 2) A man is standing on an international space station, which is orbiting earth at an altitude 520 km with a constant speed 7.6 \[km{{s}^{-1}}\]. If the mans weight is 50 kg, his acceleration is
A)
7.6 \[km{{s}^{-2}}\]
done
clear
B)
7.6 \[km{{s}^{-2}}\]
done
clear
C)
8.4 \[km{{s}^{-2}}\]
done
clear
D)
10 \[km{{s}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 3) A rope of mass 0.1 kg is connected at the same height of two opposite walls. It is allowed to hang under its own weight. At [he contact point between the rope and the wall, the rope makes an angle \[\theta ={{10}^{o}}\] with respect to horizontal. The tension in the rope at its midpoint between the walls is
A)
2.78 N
done
clear
B)
2.56 N
done
clear
C)
2.82 N
done
clear
D)
2.71 N
done
clear
View Answer play_arrow
question_answer 4) A boat crosses a river from port A to port B, which are just on the opposite sides. The speed of the water is \[{{v}_{W}}\] and that of boat is Vg relative to water. Assume \[{{v}_{B}}=2{{v}_{W.}}\] What is the time taken by the boat, if it has to cross the river directly on the AB line?
A)
\[\frac{2D}{{{v}_{B}}\sqrt{3}}\]
done
clear
B)
\[\frac{\sqrt{3}D}{2{{v}_{B}}}\]
done
clear
C)
\[\frac{D}{{{v}_{B}}\sqrt{2}}\]
done
clear
D)
\[\frac{D\sqrt{2}}{{{v}_{B}}}\]
done
clear
View Answer play_arrow
question_answer 5) Raindrops are falling from a certain height. Assume all raindrops are spherical and have same drag coefficient. The impact speed of large raindrops compared to that of small raindrops is
A)
greater
done
clear
B)
smaller
done
clear
C)
same
done
clear
D)
depends on height
done
clear
View Answer play_arrow
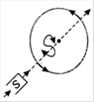
question_answer 6) Top of the stratosphere has an electric field E (in units of \[V{{m}^{-1}}\]) nearly equal to
A)
0
done
clear
B)
10
done
clear
C)
100
done
clear
D)
1000
done
clear
View Answer play_arrow
question_answer 7) The surface charge density (in \[C{{m}^{-2}}\]) of the earth is about
A)
\[{{10}^{-9}}\]
done
clear
B)
\[-{{10}^{-9}}\]
done
clear
C)
\[{{10}^{-9}}\]
done
clear
D)
\[-{{10}^{-9}}\]
done
clear
View Answer play_arrow
question_answer 8) Gausss law is valid for
A)
any closed surface
done
clear
B)
only regular closed surfaces
done
clear
C)
any open surface
done
clear
D)
only irregular open surfaces
done
clear
View Answer play_arrow
question_answer 9) One of the following is not a property of field lines
A)
field lines are continuous curves without any breaks
done
clear
B)
two field lines cannot cross each other
done
clear
C)
field lines start at positive charges and end at negative charges
done
clear
D)
they form closed loops
done
clear
View Answer play_arrow
question_answer 10) Nichrome or Manganin is widely used in wire bound standard resistors because of their
A)
temperature independent resistivity
done
clear
B)
very weakly temperature dependent resistivity
done
clear
C)
strong dependence of resistivity with temperature
done
clear
D)
mechanical strength
done
clear
View Answer play_arrow
question_answer 11) A galvanometer coil has a resistance of \[10\,\,\Omega \]and the meter shows full scale deflection for a current of 1 mA. The shunt resistance required to convert the galvanometer into an ammeter of range 0-100 mA is about
A)
\[10\,\,\Omega \]
done
clear
B)
\[1\,\,\Omega \]
done
clear
C)
\[0.1\,\,\Omega \]
done
clear
D)
\[0.01\,\,\Omega \]
done
clear
View Answer play_arrow
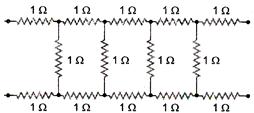
question_answer 12)
The current (in ampere) drawn from a 12 V supply by the infinite network shown in the following figure is
A)
\[2.7\]
done
clear
B)
\[3.3\]
done
clear
C)
\[4.4\]
done
clear
D)
\[5.2\]
done
clear
View Answer play_arrow
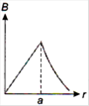
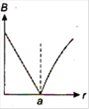
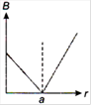
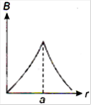
question_answer 13) A long straight wire of a circular cross-section (radius\[a\]) carries a steady current\[I\]and the current\[I\]is uniformly distributed across this cross-section. Which of the following plots represents the variation of magnitude of magnetic field B with distance r from the centre of the wire?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 14) The gyromagnetic ratio of an electron of charge e and mass m is equal to
A)
\[\frac{{{e}^{2}}}{2m}\]
done
clear
B)
\[\frac{{{e}^{2}}}{2{{m}^{2}}}\]
done
clear
C)
\[\frac{e}{4m}\]
done
clear
D)
\[\frac{e}{2m}\]
done
clear
View Answer play_arrow
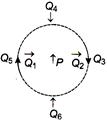
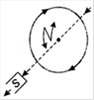
question_answer 15)
The figure below shows the various positions (labelled by subscripts) of small magnetized needles P and Q. The arrows show the direction of their magnetic moment. Which configuration corresponds to the lowest potential energy of all the configurations show?
A)
\[P{{Q}_{3}}\]
done
clear
B)
\[P{{Q}_{4}}\]
done
clear
C)
\[P{{Q}_{5}}\]
done
clear
D)
\[P{{Q}_{6}}\]
done
clear
View Answer play_arrow
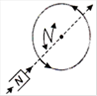
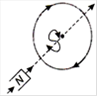
question_answer 16) Which of the following figures correctly depicts the Lenzs law? The arrows show the movement of the labelled pole of a bar magnet into a closed circular loop and the arrows on the circle show the direction of the induced current
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 17) An AC voltage is applied to a pure inductor L, drives a current in the inductor. The current in the inductor would be
A)
ahead of the voltage by \[\pi /2\]
done
clear
B)
lagging the voltage by \[\pi /2\]
done
clear
C)
ahead of the voltage by \[\pi /4\]
done
clear
D)
lagging the voltage by \[3\pi /4\]
done
clear
View Answer play_arrow
question_answer 18) The radiation pressure (in\[N{{m}^{-2}}\]) of the visible light is of the order of
A)
\[{{10}^{-2}}\]
done
clear
B)
\[{{10}^{-4}}\]
done
clear
C)
\[{{10}^{-6}}\]
done
clear
D)
\[{{10}^{-8}}\]
done
clear
View Answer play_arrow
question_answer 19) The critical angle for total internal reflection in diamond is \[{{24.5}^{o}}\]. The refractive index of the diamond is
A)
2.45
done
clear
B)
1.41
done
clear
C)
2.59
done
clear
D)
1.59
done
clear
View Answer play_arrow
question_answer 20) When a glass lens with n = 1.47 is immersed in a trough of liquid, it looks to be disappeared. The liquid in the trough could be
A)
water
done
clear
B)
kerosene
done
clear
C)
glycerine
done
clear
D)
alcohol
done
clear
View Answer play_arrow
question_answer 21) In Youngs double slit experiment, two slits are made 5 mm apart and the screen is placed 2 m away. What is the fringe separation when light of wavelength 500 nm is used?
A)
0.002 mm
done
clear
B)
0.02 mm
done
clear
C)
0.2mm
done
clear
D)
2mm
done
clear
View Answer play_arrow
question_answer 22) For what distance is ray optics a good approximation when the aperture is 4 mm wide and the wavelength is 500 nm?
A)
32m
done
clear
B)
64m
done
clear
C)
16m
done
clear
D)
8m
done
clear
View Answer play_arrow
question_answer 23) Which of the following metal thermionically emit an electron at a relatively lowest temperature among them?
A)
Platinum
done
clear
B)
Copper
done
clear
C)
Aluminium
done
clear
D)
Molybdenum
done
clear
View Answer play_arrow
question_answer 24) Among the following four spectral regions, the photon has the highest energy in
A)
infrared
done
clear
B)
violet
done
clear
C)
red
done
clear
D)
blue
done
clear
View Answer play_arrow
question_answer 25) Which of these particles (having the same kinetic energy) has the largest de-Broglie wavelength?
A)
Electron
done
clear
B)
Alpha particle
done
clear
C)
Proton
done
clear
D)
Neutron
done
clear
View Answer play_arrow
question_answer 26) The radius of an electron orbit in a hydrogen atom is of the order of
A)
\[{{10}^{-8}}\,\,m\]
done
clear
B)
\[{{10}^{-9}}\,\,m\]
done
clear
C)
\[{{10}^{-11}}\,\,m\]
done
clear
D)
\[{{10}^{-10}}\,\,m\]
done
clear
View Answer play_arrow
question_answer 27) The size of the nucleus of an atom of mass number A is proportional to
A)
\[{{A}^{3/4}}\]
done
clear
B)
\[{{A}^{2/3}}\]
done
clear
C)
\[{{A}^{1/3}}\]
done
clear
D)
\[{{A}^{5/3}}\]
done
clear
View Answer play_arrow
question_answer 28) A radioactive isotope has a half-life of 2 yr. How long will it take the activity to reduce to 3% of its original value?
A)
4.8 yr
done
clear
B)
7 yr
done
clear
C)
10 yr
done
clear
D)
9.6 yr
done
clear
View Answer play_arrow
question_answer 29) A p-n photodiode is fabricated from a semiconductor with band gap of\[2.8eV\]. Which of the following wavelength it can detect?
A)
950 nm
done
clear
B)
820 nm
done
clear
C)
580 nm
done
clear
D)
440 nm
done
clear
View Answer play_arrow
question_answer 30) A n-p-n transistor having AC, current gain of 50 is to be used to make an amplifier of power gain of 300. What will be the voltage gain of the amplifier?
A)
8.5
done
clear
B)
6
done
clear
C)
4
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 31) A water molecule has an electric dipole moment \[6.4\times {{10}^{-30}}Cm\] when it is in vapour state. The distance in metre between the centre of positive and negative charge of the molecule is
A)
\[4\times {{10}^{-10}}\]
done
clear
B)
\[4\times {{10}^{-11}}\]
done
clear
C)
\[4\times {{10}^{-12}}\]
done
clear
D)
\[4\times {{10}^{-13}}\]
done
clear
View Answer play_arrow
question_answer 32) The radius of the rear wheel of bicycle is twice that of the front wheel. When the bicycle is moving, the angular speed of the rear wheel compared to that of the front is
A)
greater
done
clear
B)
smaller
done
clear
C)
same
done
clear
D)
exact double
done
clear
View Answer play_arrow
question_answer 33) A uniform rod of length \[L\] and mass 18 kg is made to rest on two measuring scale at its two ends. A uniform block of mass 2.7 kg is placed on the rod at a distance \[L/4\] from the left end. The force experienced by the measuring scale on the right end is
A)
18 N
done
clear
B)
96 N
done
clear
C)
29 N
done
clear
D)
45 N
done
clear
View Answer play_arrow
question_answer 34) You drive a car at a speed of \[70\,\,km{{h}^{-1}}\] in a straight road for 8.4 km, and then the car runs out of petrol. You walk for 30 min to reach a petrol pump at a distance of 2 km. The average velocity from the beginning of your drive till you reach the petrol pump is
A)
16.8 \[km{{h}^{-1}}\]
done
clear
B)
35 \[km{{h}^{-1}}\]
done
clear
C)
64 \[km{{h}^{-1}}\]
done
clear
D)
18.6 \[km{{h}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 35) Two iron blocks of equal mass but with double surface area slide down an inclined plane with friction coefficient \[\mu .\] If the first block with surface area A experiences a frictional force\[f\] then the second block with surface area 2 A will experience a frictional force
A)
\[\frac{f}{2}\]
done
clear
B)
\[f\]
done
clear
C)
\[2f\]
done
clear
D)
\[4f\]
done
clear
View Answer play_arrow
question_answer 36) A point mass m is placed inside a spherical shell of radius R and mass M at a distance \[\frac{R}{2}\]from the centre of the shell. The gravitational force exerted by the shell on the point mass is
A)
\[\frac{GMm}{{{R}^{2}}}\]
done
clear
B)
\[\frac{GMm}{{{R}^{2}}}\]
done
clear
C)
zero
done
clear
D)
\[\frac{4GMm}{{{R}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 37) The motion of a particle executing SHM in one dimension is described by \[x=-0.5\,\,\,\sin \,(2t+\pi /4),\] where \[x\] is in metre and t in second. The frequency of oscillation in Hz is
A)
\[2\]
done
clear
B)
\[\pi \]
done
clear
C)
\[\pi /2\]
done
clear
D)
\[1/\pi \]
done
clear
View Answer play_arrow
question_answer 38) Two stars of mass \[{{m}_{1}}\] and \[{{m}_{2}}\] are parts of a binary star system. The radii of their orbits are \[{{r}_{1}}\] and \[{{r}_{2}}\] respectively, measured from the centre of mass of the system. The magnitude of gravitational force \[{{m}_{1}}\] exerts on m, is
A)
\[\frac{{{m}_{1}}{{m}_{2}}G}{{{({{r}_{1}}+{{r}_{2}})}^{2}}}\]
done
clear
B)
\[\frac{{{m}_{1}}G}{{{({{r}_{1}}+{{r}_{2}})}^{2}}}\]
done
clear
C)
\[\frac{{{m}_{2}}G}{{{({{r}_{1}}+{{r}_{2}})}^{2}}}\]
done
clear
D)
\[\frac{({{m}_{1}}+{{m}_{2}})}{{{({{r}_{1}}+{{r}_{2}})}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 39) On the centre of a frictionless table a small hole is made, through which a weightless string of length \[2l\] is inserted. On the two ends of the string two balls of the same mass m are attached. Arrangement is made in such a way that half of the string is on the table top and half is hanging below. The ball on the table top is made to move in a circular path with a constant speed v. What is the centripetal acceleration of the moving ball?
A)
\[mvl\]
done
clear
B)
\[g\]
done
clear
C)
zero
done
clear
D)
\[2\,mvl\]
done
clear
View Answer play_arrow
question_answer 40) Tom and Dick are running forward with the same speed. They are throwing a rubber ball to each other at a constant speed v as seen by the thrower. According to Sam who is standing on the ground the speed of ball is
A)
same as v
done
clear
B)
greater than v
done
clear
C)
less than v
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 41) A ball moves in a frictionless inclined table without slipping. The work done by the table surface on the ball is
A)
positive
done
clear
B)
negative
done
clear
C)
zero
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 42) A synchronous satellite goes around the earth once in every 24 h. What is the radius of orbit of the synchronous satellite in terms of the earths radius? (Given mass of the earth, \[{{m}_{e}}=5.98\times {{10}^{24}}\,kg,\] radius of the earth, \[{{r}_{e}}=6.37\times {{10}^{6}}\,m,\] Universal constant of gravitation, \[G=6.67\times {{10}^{-11}}N-{{m}^{2}}k{{g}^{-2}})\]
A)
\[2.4\,{{r}_{e}}\]
done
clear
B)
\[3.6\,{{r}_{e}}\]
done
clear
C)
\[4.8\,{{r}_{e}}\]
done
clear
D)
\[6.6\,{{r}_{e}}\]
done
clear
View Answer play_arrow
question_answer 43) Two cylinders of equal size are filled with equal amount of ideal diatomic gas at room temperature. Both the cylinders are fitted with pistons. In cylinder A the piston is free to move, while in cylinder B the piston is fixed. When same amount of heat is supplied to both the cylinders, the temperature of the gas in cylinder A raises by 20 K. What will be the rise is temperature of the gas in cylinder B?
A)
28 K
done
clear
B)
20 K
done
clear
C)
15 K
done
clear
D)
10 K
done
clear
View Answer play_arrow
question_answer 44) An ideal gas is made to go through a cyclic thermodynamical process in four steps. The amount of heat involved are \[{{Q}_{1}}=600\,J,\] \[{{Q}_{2}}=-400\,J,\,\,\,\,\,{{Q}_{3}}=-300\,J\] and \[{{Q}_{4}}=-300\,J\] respectively. The corresponding works involved are \[{{W}_{1}}=300\,J,\,\,\,{{W}_{2}}=-200\,J,\,\,\,{{W}_{3}}=-150\,J\] and , \[{{W}_{4}}\]What is the value of \[{{W}_{4}}\]?
A)
\[-20\,J\]
done
clear
B)
\[100\,J\]
done
clear
C)
\[150\,J\]
done
clear
D)
\[50\,J\]
done
clear
View Answer play_arrow
question_answer 45) The angle subtended by a coin of radius 1 cm held at a distance of 80 cm from your eyes is
A)
\[{{1.43}^{o}}\]
done
clear
B)
\[{{0.72}^{o}}\]
done
clear
C)
\[{{0.0125}^{o}}\]
done
clear
D)
\[{{0.025}^{o}}\]
done
clear
View Answer play_arrow
question_answer 46)
The three initial and final position of a man on the x-axis are given as (i) (8 m, 7 m) (ii) (7 m, 3 m) and (iii) (7 m, 3 m)
Which pair gives the negative displacement?
A)
(i)
done
clear
B)
(ii)
done
clear
C)
(iii)
done
clear
D)
(i) and (iii)
done
clear
View Answer play_arrow
question_answer 47) A bird flies from \[(-3\,m,\,4m,\,-3\,m)\] to \[(7\,m,\,-2\,m,\,-3\,m)\] in xyz coordinates. The birds S displacement in unit vectors is given by
A)
\[(4\mathbf{\hat{i}}+2\mathbf{\hat{j}}-6\mathbf{\hat{k}})\]
done
clear
B)
\[(10\mathbf{\hat{i}}-6\mathbf{\hat{j}})\]
done
clear
C)
\[(4\mathbf{\hat{i}}-2\mathbf{\hat{j}})\]
done
clear
D)
\[(10\mathbf{\hat{i}}+6\mathbf{\hat{j}}-6\mathbf{\hat{k}})\]
done
clear
View Answer play_arrow
question_answer 48) A coastguard ship locates a pirate ship at a distance 560 m. It fires a cannon ball with an initial speed 82 \[m{{s}^{-1}}\]. At what angle from horizontal the ball must be fired so that it hits the pirate ship?
A)
\[{{54}^{o}}\]
done
clear
B)
\[{{125}^{o}}\]
done
clear
C)
\[{{27}^{o}}\]
done
clear
D)
\[{{18}^{o}}\]
done
clear
View Answer play_arrow
question_answer 49) An object moves at a constant speed along a circular path in horizontal XY plane, with the centre at the origin. When the object is a\[x=-2m,\] its velocity is \[(-4m{{s}^{-1}})\mathbf{\hat{j}}.\] What is the objects acceleration when it is \[y=2\,m\]?
A)
\[-(8\,m{{s}^{-2}})\,\hat{j}\]
done
clear
B)
\[(8\,m{{s}^{-2}})\,\hat{i}\]
done
clear
C)
\[(-4\,m{{s}^{-2}})\,\hat{j}\]
done
clear
D)
\[(4\,m{{s}^{-2}})\,\hat{i}\]
done
clear
View Answer play_arrow
question_answer 50) A block is lying static on the floor. The maximum value of static frictional force on the block is 10 N. If a horizontal force of 8 N is applied to the block, what will be the frictional force on the block?
A)
2N
done
clear
B)
18 N
done
clear
C)
8N
done
clear
D)
10 N
done
clear
View Answer play_arrow
question_answer 51) Chlorobenzene is ...... reactive than benzene towards electrophilic substitution and directs the incoming electrophile to the ...... position.
A)
more, ortho/para
done
clear
B)
less, ortho/para
done
clear
C)
more, meta
done
clear
D)
less, meta
done
clear
View Answer play_arrow
question_answer 52) When acetyl chloride reacts with sodium propionate/the product formed is
A)
acetic anhydride
done
clear
B)
acetic propionic anhydride
done
clear
C)
n-propyl acetate
done
clear
D)
pent-2,4-dione
done
clear
View Answer play_arrow
question_answer 53) In the reaction below, X is \[{{C}_{6}}{{H}_{5}}MgBr+C{{H}_{3}}OH\xrightarrow{{}}\,\,\,X\]
A)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{6}}OH\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}OC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 54) Which of the following compounds will show geometrical isomerism?
A)
Cyclohexene
done
clear
B)
2 hexene
done
clear
C)
3 -hexyne
done
clear
D)
1,1-diphenylethylene
done
clear
View Answer play_arrow
question_answer 55) Which of the following reactions involves carbon-carbon bond formation?
A)
Reimer-Tiemann reaction
done
clear
B)
Hydroboration-oxidation
done
clear
C)
Cannizaro reaction
done
clear
D)
Reaction of primary alcohols with PCC
done
clear
View Answer play_arrow
question_answer 56) Aldol condensation does not occur between
A)
two different aldehydes
done
clear
B)
two different ketones
done
clear
C)
an aldehyde and a ketone
done
clear
D)
an aldehyde and an ester
done
clear
View Answer play_arrow
question_answer 57) Which of the following statements is not true?
A)
Pheromones are secreted outside the body by the insects
done
clear
B)
Aspirin is analgesic and anti-pyretic
done
clear
C)
Sucrose is a dipeptide commonly known as aspartame
done
clear
D)
The DNA assists in the synthesis of RNA molecules
done
clear
View Answer play_arrow
question_answer 58) In which of the following reactions, the product obtained is chiral?
A)
\[C{{H}_{3}}COC{{H}_{3}}\xrightarrow{NaB{{H}_{4}}}\]
done
clear
B)
\[C{{H}_{3}}COCl\xrightarrow{Resonmund\text{ }reduction}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}COC{{H}_{2}}C{{H}_{3}}\xrightarrow{Sn,\,HCl}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}COC{{H}_{3}}\xrightarrow{LiAl{{H}_{4}}}\]
done
clear
View Answer play_arrow
question_answer 59) Which of the following compounds gives blood red colouration when its Lassaignes extract is treated with acid and ferric chloride?
A)
Thiourea
done
clear
B)
Diphenyl sulphide
done
clear
C)
Phenyl hydrazine
done
clear
D)
Benzamide
done
clear
View Answer play_arrow
question_answer 60) Which of the following statements is not correct?
A)
Allergic conditions are cured by anti-histamines
done
clear
B)
Hormones are continuously produced but not stored in the body
done
clear
C)
The function of the white blood cells is to protect the body against infections
done
clear
D)
Catabolism involves degradation of molecules
done
clear
View Answer play_arrow
question_answer 61) m-bromoaniline can be prepared by
A)
\[{{C}_{6}}{{H}_{6}}\xrightarrow[{{H}_{2}}S{{O}_{4}}]{HN{{O}_{3}}}\,\,\,\xrightarrow[2.\,\,NaOH,\,\,{{H}_{2}}O]{1.\,\,Sn-HCl}\xrightarrow[{{H}_{2}}O]{B{{r}_{2}}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{6}}\xrightarrow[FeB{{r}_{3}}]{B{{r}_{2}}}\,\xrightarrow[{{H}_{2}}S{{O}_{4}}]{HN{{O}_{3}}}\xrightarrow[Pt]{{{H}_{2}}}\]
done
clear
C)
\[m-Br{{C}_{6}}{{H}_{4}}COOH\xrightarrow{SOC{{l}_{2}}}\xrightarrow{N{{H}_{3}}}\]\[\xrightarrow[{{H}^{+}}]{B{{r}_{2}},\,\,NaN{{H}_{2}}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\xrightarrow[C{{u}_{2}}B{{r}_{2}}]{NaN{{O}_{2}},HCl}\xrightarrow{NaN{{H}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 62) In the reaction below, X is Neo-pentyl alcohol \[\xrightarrow{{{H}_{2}}S{{O}_{4}}}\,\,X\]
A)
2-methylpentane
done
clear
B)
2-methylpent-2-ene
done
clear
C)
2-methylbut-2-ene
done
clear
D)
neo-pentane
done
clear
View Answer play_arrow
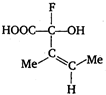
question_answer 63)
The configuration of the chiral centre and the geometry of the double bond in the following molecule can be described by
A)
R and E
done
clear
B)
S and E
done
clear
C)
R and Z
done
clear
D)
S and Z
done
clear
View Answer play_arrow
question_answer 64) Which polymers occur naturally?
A)
Starch and nylon
done
clear
B)
Starch and cellulose
done
clear
C)
Proteins and nylon
done
clear
D)
Proteins and PVC
done
clear
View Answer play_arrow
question_answer 65) Calculate the work done when 1 mol of an ideal gas is compressed reversibly from 1.0 bar to 4.00 bar at a constant temperature of 300 K.
A)
4.01 kJ
done
clear
B)
-8.02 kJ
done
clear
C)
18.02 kJ
done
clear
D)
-14.01 kJ
done
clear
View Answer play_arrow
question_answer 66) The enthalpy of neutralisation of oxalic acid by a strong base is -25.4 kcal \[mo{{l}^{-1}}\]. The enthalpy of neutralisation of strong acid and strong base is -13.7 kcal \[equi{{v}^{-1}}\]. The enthalpy of dissociation of \[{{H}_{2}}{{C}_{2}}{{O}_{2}}\rightleftharpoons \,2{{H}^{+}}+{{C}_{2}}O_{4}^{2-}\] is
A)
1.0 kcal \[mo{{l}^{-1}}\]
done
clear
B)
2.0 kcal \[mo{{l}^{-1}}\]
done
clear
C)
18.55 kcal \[mo{{l}^{-1}}\]
done
clear
D)
11.7 kcal \[mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 67) At the equilibrium of the reaction\[2X(g)+Y(g)\xrightarrow{{}}{{X}_{2}}Y(g)\], the number of moles of \[{{X}_{2}}Y\]at equilibrium is affected by the
A)
temperature and pressure
done
clear
B)
temperature only
done
clear
C)
pressure only
done
clear
D)
temperature, pressure and catalyst used
done
clear
View Answer play_arrow
question_answer 68) For a first order reaction, the time required for 99.9% of the reaction to take place is nearly
A)
10 times that required for half of the reaction
done
clear
B)
100 times that required for two-third of the reaction
done
clear
C)
10 times that required for one-fourth of the reaction
done
clear
D)
20 times that required for half of the reaction
done
clear
View Answer play_arrow
question_answer 69) An endothermic reaction has a positive internal energy change AE. In such a case, what is the minimum value that the activation energy can have?
A)
\[\Delta E\]
done
clear
B)
\[\Delta E=\Delta H+\Delta {{n}_{g}}RT\]
done
clear
C)
\[\Delta E=\Delta H-\Delta {{n}_{g}}RT\]
done
clear
D)
\[\Delta E={{E}_{a}}+RT\]
done
clear
View Answer play_arrow
question_answer 70) A compound contains two types of atoms X and Y. It crystallizes in a cubic lattice with atoms X at the comers of the unit cell and atoms Y at the body centres. The simplest possible formula of this compound is
A)
\[{{X}_{3}}Y\]
done
clear
B)
\[{{X}_{2}}Y\]
done
clear
C)
\[XY\]
done
clear
D)
\[X{{Y}_{8}}\]
done
clear
View Answer play_arrow
question_answer 71) Which of the following halogens does not exhibit a positive oxidation number in its compounds?
A)
I
done
clear
B)
Br
done
clear
C)
\[Cl\]
done
clear
D)
F
done
clear
View Answer play_arrow
question_answer 72) Among the following, the strongest conjugate base is
A)
\[NO_{3}^{-}\]
done
clear
B)
\[C{{l}^{-}}\]
done
clear
C)
\[SO_{4}^{2-}\]
done
clear
D)
\[C{{H}_{3}}CO{{O}^{-}}\]
done
clear
View Answer play_arrow
question_answer 73) Determine the pH of the solution that results from the addition of 20.00 mL of 0.01 M\[Ca{{(OH)}_{2}}\] to \[30.00\,\,mL\] of 0.001 M HCl
A)
11.30
done
clear
B)
10.53
done
clear
C)
2.70
done
clear
D)
8.35
done
clear
View Answer play_arrow
question_answer 74) Adsorption is an exothermic process. The amount of substance adsorbed should
A)
increase with decrease in temperature
done
clear
B)
increase with increase in temperature
done
clear
C)
decrease with decrease in temperature
done
clear
D)
decrease with increase in temperature
done
clear
View Answer play_arrow
question_answer 75) Fog is a colloidal solution of
A)
liquid particles dispersed in gas
done
clear
B)
gaseous particles dispersed in a liquid
done
clear
C)
solid particles dispersed in a liquid
done
clear
D)
solid particles dispersed in gas
done
clear
View Answer play_arrow
question_answer 76) The correct set of quantum numbers for the unpaired electron of a chlorine atom is
A)
2, 0, 0, + 1/2
done
clear
B)
2, 1, -1, +1/2
done
clear
C)
3, 1, -1, \[\pm \] 1/2
done
clear
D)
3, 0, 0, \[\pm \] ½
done
clear
View Answer play_arrow
question_answer 77) The temperature at which real gases obey the ideal gas laws over a wide range of pressures is called
A)
critical temperature
done
clear
B)
inversion temperature
done
clear
C)
Boyle temperature
done
clear
D)
reduced temperature
done
clear
View Answer play_arrow
question_answer 78) Common salt obtained from sea-water contains 95% NaCI by mass. The approximate number of molecules present in 10.0 g of the salt is
A)
1021
done
clear
B)
1022
done
clear
C)
1023
done
clear
D)
1024
done
clear
View Answer play_arrow
question_answer 79) In the redox reaction, \[xKMn{{O}_{4}}+yN{{H}_{3}}\xrightarrow{{}}KN{{O}_{3}}+Mn{{O}_{2}}\]\[+KOH+{{H}_{2}}O\]x and y are
A)
\[x=4,\,y=6\]
done
clear
B)
\[x=3,\,y=8\]
done
clear
C)
\[x=8,\,y=6\]
done
clear
D)
\[x=8,\,y=3\]
done
clear
View Answer play_arrow
question_answer 80) Which of the following aqueous solutions has the highest boiling point?
A)
\[0.1\,M\,KN{{O}_{3}}\]
done
clear
B)
\[0.1\,M\,N{{a}_{3}}P{{O}_{4}}\]
done
clear
C)
\[0.1\,M\,BaC{{l}_{2}}\]
done
clear
D)
\[0.1\,M\,{{K}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 81) The values of electronegativity of atoms A and are 1.2 and 4.0 respectively. The per cent ionic character of the A - B bond is
A)
50%
done
clear
B)
72.24%
done
clear
C)
55.3%
done
clear
D)
43%
done
clear
View Answer play_arrow
question_answer 82) 100 mL of \[P{{H}_{3}}\] on heating forms P and \[{{H}_{2}}\], the volume change in the reaction is
A)
an increase of 50 mL
done
clear
B)
an increase of 100 mL
done
clear
C)
an increase of 150 mL
done
clear
D)
a decrease of 50 mL
done
clear
View Answer play_arrow
question_answer 83) The common features among the species\[C{{N}^{-}}\], CO and \[N{{O}^{+}}\] are
A)
bond order three and iso-electronic
done
clear
B)
bond order three and weak-field ligands
done
clear
C)
bond order two and \[\pi \]-acceptor
done
clear
D)
iso-electronic and weak-field ligands
done
clear
View Answer play_arrow
question_answer 84) The magnitude of crystal field stabilization energy (CFSE or A^) in tetrahedral complexes is considerably less than in the octahedral field, because
A)
there are only four ligands instead of six, so the ligand field is only 2/3 the size, hence the \[{{\Delta }_{t}}\] is only 2/3 the size
done
clear
B)
the direction of the orbitals does not coincide with the direction of the ligands. This reduces the crystal field stabilisation energy \[({{\Delta }_{t}})\] by further 2/3
done
clear
C)
both points a and b are correct
done
clear
D)
both points a and b are wrong
done
clear
View Answer play_arrow
question_answer 85) The role of phosphate in detergent powder is to
A)
control pH level of the detergent water mixture
done
clear
B)
remove \[C{{a}^{2+}}\] and \[M{{g}^{2+}}\] ions from the water that causes the hardness of water
done
clear
C)
provide whiteness to the fabrics
done
clear
D)
form solid detergent as phosphate-less detergents are liquid in nature
done
clear
View Answer play_arrow
question_answer 86) If \[{{I}_{2}}\] is dissolved in aqueous KI, the intense yellow species, \[I_{3}^{-}\], is formed. The structure of \[I_{3}^{-}\] ion is
A)
square pyramidal
done
clear
B)
trigonal bipyramidal
done
clear
C)
octahedral
done
clear
D)
pentagonal bipyramidal
done
clear
View Answer play_arrow
question_answer 87) In the change of ]^0+ to NO, the electron is added to the
A)
\[\sigma \] orbital
done
clear
B)
\[\pi \] orbital
done
clear
C)
\[{{\sigma }^{*}}\] orbital
done
clear
D)
\[{{\pi }^{*}}\] orbital
done
clear
View Answer play_arrow
question_answer 88) Iron has an oxidation number of +3, in which of the following compounds?
A)
\[Fe{{(N{{O}_{3}})}_{2}}\]
done
clear
B)
\[Fe{{C}_{2}}{{O}_{4}}\]
done
clear
C)
\[[Fe{{({{H}_{2}}O)}_{6}}]\,C{{l}_{3}}\]
done
clear
D)
\[{{(N{{H}_{4}})}_{2}}S{{O}_{4}}\,\,FeS{{O}_{4}}\,\,6{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 89) The expected spin-only magnetic moments for \[{{[Fe{{(CN)}_{6}}]}^{4-}}\] and \[{{[Fe{{F}_{6}}]}^{3-}}\] are
A)
1.73 and 1.73 BM
done
clear
B)
1.73 and 5.92 BM
done
clear
C)
0.0 and 1.73 BM
done
clear
D)
0.0 and 5.92 BM
done
clear
View Answer play_arrow
question_answer 90) The crystal field stabilization energy (CFSE) is the highest for
A)
\[{{[Co{{F}_{4}}]}^{2-}}\]
done
clear
B)
\[{{[Co{{(NHS)}_{4}}]}^{2-}}\]
done
clear
C)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
D)
\[{{[CoC{{l}_{4}}]}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 91) Which of the following reactions will not give the anhydrous\[AlC{{l}_{3}}\]?
A)
By heating \[AlC{{l}_{3}}\,\,6{{H}_{2}}O\]
done
clear
B)
By passing dry \[HCl\] gas on heated aluminium powder
done
clear
C)
By passing dry chlorine gas on heated aluminium powder
done
clear
D)
By passing dry chlorine gas over a heated mixture of alumina and coke
done
clear
View Answer play_arrow
question_answer 92) A metallic ion \[{{M}^{2+}}\]has an electronic configuration of 2, 8, 14 and the ionic weight is 56 u. The number of neutrons in its nucleus are
A)
30
done
clear
B)
32
done
clear
C)
34
done
clear
D)
42
done
clear
View Answer play_arrow
question_answer 93) Which of the following has the highest value of radioactivity?
A)
1 g of Ra
done
clear
B)
1 g of Ra \[S{{O}_{4}}\]
done
clear
C)
1 g of Ra \[B{{r}_{2}}\]
done
clear
D)
1 g of Ra \[(HP{{O}_{4}})\]
done
clear
View Answer play_arrow
question_answer 94) It is believed that atoms combine with each other such that the outermost shell acquires a stable configuration of 8 electrons. If stability were attained with 6 electrons rather than 8; what would be the formula of the stable fluoride ion?
A)
\[{{F}^{-}}\]
done
clear
B)
\[{{F}^{+}}\]
done
clear
C)
\[{{F}^{2+}}\]
done
clear
D)
\[{{F}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 95) When two ice cubes are pressed over each other, they unite to form one cube. Which of the following forces is responsible to hold them together?
A)
Dipole forces
done
clear
B)
van der Waals forces
done
clear
C)
Covalent forces
done
clear
D)
Hydrogen bond forces
done
clear
View Answer play_arrow
question_answer 96) In which of the following reactions, there is no change in valency?
A)
\[S{{O}_{2}}+2{{H}_{2}}S\xrightarrow{{}}2{{H}_{2}}O+3S\]
done
clear
B)
\[2Na+{{O}_{2}}\xrightarrow{{}}N{{a}_{2}}{{O}_{2}}\]
done
clear
C)
\[N{{a}_{2}}O+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}N{{a}_{2}}S{{O}_{4}}+{{H}_{2}}{{O}_{2}}\]
done
clear
D)
\[4KCl{{O}_{3}}\xrightarrow{{}}3KCl{{O}_{4}}+KCl\]
done
clear
View Answer play_arrow
question_answer 97) If helium is allowed to expand in vacuum, it liberates heat because
A)
helium is an inert gas
done
clear
B)
helium is an ideal gas
done
clear
C)
the critical temperature of helium is very low
done
clear
D)
helium is one of the lightest gases
done
clear
View Answer play_arrow
question_answer 98) Compound A undergoes Cannizaro reaction and B undergoes positive iodoform test. Therefore,
A)
A = acetaldehyde B = 1-pentanal
done
clear
B)
\[A={{C}_{6}}{{H}_{5}}C{{H}_{2}}CHO\] B = 3-pentanone
done
clear
C)
A = formaldehyde B = 2-pentanone
done
clear
D)
A = propionaldehyde B = 1-pentanol
done
clear
View Answer play_arrow
question_answer 99) Arrange the following free radicals in order of decreasing stability Methyl (I), Vinyl (II), Allyl (III), Benzyl (IV)
A)
\[I>II>III>IV\]
done
clear
B)
\[III>II>I>IV\]
done
clear
C)
\[II>I>IV>III\]
done
clear
D)
\[IV>III>I>II\]
done
clear
View Answer play_arrow
question_answer 100) Which isomer of hexane has only two different sets of structurally equivalent hydrogen atoms?
A)
2,2-dimethylbutane
done
clear
B)
2-methylpentane
done
clear
C)
3-methylpentane
done
clear
D)
2,3-dimethylbutane
done
clear
View Answer play_arrow
question_answer 101) An example for symbiotic bacteria
A)
Erwinia amylovora
done
clear
B)
Rhizobium leguminosarum
done
clear
C)
Xanthomonas campestris
done
clear
D)
Agrobacterium tumefaciens
done
clear
View Answer play_arrow
question_answer 102) In which plant, the fruit is a drupe, seed coat is thin, embryo is inconspicuous and endosperm is edible?
A)
Groundnut
done
clear
B)
Wheat
done
clear
C)
Apple
done
clear
D)
Coconut
done
clear
View Answer play_arrow
question_answer 103) Somaclonal variation appears in plants
A)
growing in polluted soil or water
done
clear
B)
exposed to gamma rays
done
clear
C)
raised in tissue culture
done
clear
D)
transformed by recombinant DNA technology
done
clear
View Answer play_arrow
question_answer 104) In a monoecious plant
A)
male and female sex organs are on different individuals
done
clear
B)
male and female gametes are of two morphologically distinct types
done
clear
C)
male and female sex organs are on the same individual
done
clear
D)
all the stamens are fused to form one unit
done
clear
View Answer play_arrow
question_answer 105) Which one of the following are intracellular obligate parasites?
A)
Bacteria
done
clear
B)
Viruses
done
clear
C)
Slime moulds
done
clear
D)
Blue-green algae
done
clear
View Answer play_arrow
question_answer 106) Pineapple fruit develops from
A)
unilocular polycarpellary flower
done
clear
B)
multipistillate syncarpus flower
done
clear
C)
multilocular monocarpellary flower
done
clear
D)
a cluster of compactly born flowers on an axis
done
clear
View Answer play_arrow
question_answer 107) A sewage treatment process in which a part of decomposer bacteria present in the waste is recycled into the starting of the process is called
A)
cyclic treatment
done
clear
B)
activated sludge treatment
done
clear
C)
primary treatment
done
clear
D)
tertiary treatment
done
clear
View Answer play_arrow
question_answer 108) Which of following mineral-nutrients plays an important role in biological nitrogen fixation?
A)
Zinc
done
clear
B)
Iron
done
clear
C)
Molybdenum
done
clear
D)
Magnesium
done
clear
View Answer play_arrow
question_answer 109) Which of the following is true?
A)
Vessels are unicellular and with narrow lumen
done
clear
B)
Vessels are multicellular and with wide lumen
done
clear
C)
Tracheids are unicellular and with wide lumen
done
clear
D)
Tracheids are multicellular and with arrow Lumen
done
clear
View Answer play_arrow
question_answer 110) In \[{{C}_{4}}\] plants, the bundle sheath cells
A)
have thin walls to facilitate gaseous exchange
done
clear
B)
have large intercellular spaces
done
clear
C)
are rich in PEP carboxylase
done
clear
D)
have a high density of chloroplasts
done
clear
View Answer play_arrow
question_answer 111) Potato spindle tuber disease is caused by
A)
a nematode
done
clear
B)
a virus
done
clear
C)
a bacterium
done
clear
D)
a viroid
done
clear
View Answer play_arrow
question_answer 112) In which of the following, all listed genera belong to the same class of algae?
A)
Chara, Fucus, Polysiphonia
done
clear
B)
Volvox, Spirogyra, Chlamydomonas
done
clear
C)
Porphyra, Ectocarpus, Ulothrix
done
clear
D)
Sargassum, Laminaria, Gracillaria
done
clear
View Answer play_arrow
question_answer 113) In root nodules of legumes, leg-haemoglobin is important because
A)
it transports oxygen to the root nodule
done
clear
B)
it acts as an oxygen scavenger
done
clear
C)
it provides energy to the nitrogen fixing bacterium
done
clear
D)
it acts as a catalyst in transamination
done
clear
View Answer play_arrow
question_answer 114) Darwin judged the fitness of an individual by
A)
ability to defend itself
done
clear
B)
strategy to obtain food
done
clear
C)
number of offsprings
done
clear
D)
dominance over other individuals
done
clear
View Answer play_arrow
question_answer 115) Edolation in plants is caused when
A)
they are grown in dark
done
clear
B)
they have mineral deficiency
done
clear
C)
they are grown in intense light
done
clear
D)
they are grown in blue light
done
clear
View Answer play_arrow
question_answer 116) Calorie is the unit of
A)
sound
done
clear
B)
temperature
done
clear
C)
light
done
clear
D)
heat
done
clear
View Answer play_arrow
question_answer 117) In an annual ring, the light coloured part is known as
A)
early wood
done
clear
B)
late wood
done
clear
C)
heartwood
done
clear
D)
sapwood
done
clear
View Answer play_arrow
question_answer 118) The chief component of the middle lamella in plant cell is
A)
potassium
done
clear
B)
calcium
done
clear
C)
magnesium
done
clear
D)
phosphorus
done
clear
View Answer play_arrow
question_answer 119) Tonoplast is a membrane surrounding the
A)
cytoplasm
done
clear
B)
vacuole
done
clear
C)
nucleus
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 120) Polyploidy can be produced artificially by
A)
colchicine
done
clear
B)
inbreeding
done
clear
C)
line breeding
done
clear
D)
self pollination
done
clear
View Answer play_arrow
question_answer 121) Recombination is involved in the process of
A)
cytokinesis
done
clear
B)
spindle formation
done
clear
C)
crossing over
done
clear
D)
chromosome duplication
done
clear
View Answer play_arrow
question_answer 122) A fibrous root system is excellent for
A)
food storage
done
clear
B)
nitrogen fixation
done
clear
C)
absorbing water from deeper layer of soil
done
clear
D)
providing good anchorage for the plant
done
clear
View Answer play_arrow
question_answer 123) If a primary root continues to grow, the type of root system will be known as
A)
secondary
done
clear
B)
fibrous
done
clear
C)
tap
done
clear
D)
stilt
done
clear
View Answer play_arrow
question_answer 124) A horizontal underground stem is a
A)
conn
done
clear
B)
phylloclade
done
clear
C)
rhizome
done
clear
D)
rhizoid
done
clear
View Answer play_arrow
question_answer 125) If global warming continues, the organism which may face more severe threat is
A)
cow
done
clear
B)
banana
done
clear
C)
snow leopard
done
clear
D)
dolphin
done
clear
View Answer play_arrow
question_answer 126) One advantage of cleistogamy is
A)
it leads to greater genetic diversity
done
clear
B)
seed dispersal is more efficient and widespread
done
clear
C)
seed set is not dependent on pollinators
done
clear
D)
each visit of a pollinator results in transfer of hundreds of pollen grains
done
clear
View Answer play_arrow
question_answer 127) Jute fibres are obtained from the
A)
secondary phloem
done
clear
B)
pith
done
clear
C)
xylem
done
clear
D)
endodermis
done
clear
View Answer play_arrow
question_answer 128) A chromosome in which the centromere is situated close to its end so that one arm is very short and the other very long is
A)
acrocentric
done
clear
B)
metacentric
done
clear
C)
sub-metacentric
done
clear
D)
telocentric
done
clear
View Answer play_arrow
question_answer 129) Resin and turpentine are products of
A)
teak
done
clear
B)
oak
done
clear
C)
Eucalyptus
done
clear
D)
pine
done
clear
View Answer play_arrow
question_answer 130) An inexhaustible non-conventional universal source of energy is
A)
wind energy
done
clear
B)
solar energy
done
clear
C)
hydrothermal energy
done
clear
D)
tidal energy
done
clear
View Answer play_arrow
question_answer 131) Which one of the following periods is largely associated with extinction of dinosaurs and the increase in flowering plants and reptiles?
A)
Jurassic
done
clear
B)
Triassic
done
clear
C)
Cretaceous
done
clear
D)
Permian
done
clear
View Answer play_arrow
question_answer 132) Lime is added to the soil which is too
A)
sandy
done
clear
B)
salty
done
clear
C)
alkaline
done
clear
D)
acidic
done
clear
View Answer play_arrow
question_answer 133) Which type of water is used by the plants?
A)
Gravitational water
done
clear
B)
Capillary water
done
clear
C)
Hygroscopic water
done
clear
D)
Bound water
done
clear
View Answer play_arrow
question_answer 134) Electroporation involves
A)
promotion of seed germination by induced imbibition of water with electric current
done
clear
B)
making transient pores in cell membrane to facilitate entry of gene constructs
done
clear
C)
purification of saline water with the help of an artificial membrane
done
clear
D)
passage of sucrose through sieve pores by electro-osmosis
done
clear
View Answer play_arrow
question_answer 135) One of the following acts as secondary pollutant
A)
\[B{{r}_{2}}\]
done
clear
B)
\[C{{l}_{2}}\]
done
clear
C)
\[N{{O}_{2}}\]
done
clear
D)
\[HN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 136) Cuticle is absent in
A)
mesophytes
done
clear
B)
young roots
done
clear
C)
mature stems
done
clear
D)
leaves
done
clear
View Answer play_arrow
question_answer 137) Sunflower belongs to the family
A)
Liliaceae
done
clear
B)
Asteraceae
done
clear
C)
Cruciferae
done
clear
D)
Fabaceac
done
clear
View Answer play_arrow
question_answer 138) The least porous soil among the following is a
A)
loamy soil
done
clear
B)
silty soil
done
clear
C)
clayey soil
done
clear
D)
peaty soil
done
clear
View Answer play_arrow
question_answer 139) In higher plants, the shape of the chloroplast is
A)
discoid
done
clear
B)
cup-shaped
done
clear
C)
girdle-shaped
done
clear
D)
reticulate
done
clear
View Answer play_arrow
question_answer 140) Which of the following statements is false?
A)
TMV has a double-stranded RNA molecule
done
clear
B)
Most plant viruses are RNA viruses
done
clear
C)
The bacteriophage has a double-stranded DNA molecule
done
clear
D)
Most animal viruses are DNA viruses
done
clear
View Answer play_arrow
question_answer 141) A kingdom common to unicellular animals and plants is
A)
Monera
done
clear
B)
Plantae
done
clear
C)
Fungi
done
clear
D)
Protista
done
clear
View Answer play_arrow
question_answer 142) Which of the following is a rootless aquatic plant, in which a portion of the leaf forms a tiny sac for trapping insects?
A)
Nepenthes
done
clear
B)
Drosera
done
clear
C)
Utricularia
done
clear
D)
Dionaea
done
clear
View Answer play_arrow
question_answer 143) The greatest problem of water conservation is to reduce the amount of
A)
precipitation
done
clear
B)
run-off water
done
clear
C)
groundwater
done
clear
D)
evaporation
done
clear
View Answer play_arrow
question_answer 144) Enzymes that catalyse inter-conversion of optical, geometrical or positional isomers are
A)
ligases
done
clear
B)
lyases
done
clear
C)
hydrolases
done
clear
D)
is omerases
done
clear
View Answer play_arrow
question_answer 145) According to abiogenesis life originate from
A)
non-living
done
clear
B)
pre-existing life
done
clear
C)
chemicals
done
clear
D)
extra-terrestrial matter
done
clear
View Answer play_arrow
question_answer 146) External fertilization occurs in majority of
A)
algae
done
clear
B)
fungi
done
clear
C)
liverworts
done
clear
D)
mosses
done
clear
View Answer play_arrow
question_answer 147) The final stable community in ecological succession is
A)
pioneers
done
clear
B)
sere
done
clear
C)
climax
done
clear
D)
carnivores
done
clear
View Answer play_arrow
question_answer 148) Which of the following combinations of characters is true for slime moulds?
A)
Parasitic, plasmodium with true walls, spores dispersed by air currents
done
clear
B)
Saprophytic, plasmodium without walls, spores dispersed by water
done
clear
C)
Parasitic, plasmodium without walls, spores dispersed by water
done
clear
D)
Saprophytic, plasmodium without walls, spores dispersed by air currents
done
clear
View Answer play_arrow
question_answer 149) Which is an organic compound found in most cells?
A)
Glucose
done
clear
B)
Water
done
clear
C)
Sodium chloride
done
clear
D)
Oxygen
done
clear
View Answer play_arrow
question_answer 150) Taxonomic hierarchy refers to
A)
stepwise arrangement of all tegories for classification of plants and animal:
done
clear
B)
a group of senior taxonomists who decide the nomenclature of plants and animals
done
clear
C)
a list of botanists or zoologists who have worked on taxonomy of a species or group
done
clear
D)
classification of a species based on fossil record
done
clear
View Answer play_arrow
question_answer 151) Reproductive isolation between segments of a single population is termed
A)
sympatry
done
clear
B)
allopatry
done
clear
C)
population divergence
done
clear
D)
disruptive divergence
done
clear
View Answer play_arrow
question_answer 152) Steroid hormones easily pass through the plasma membrane by simple diffusion because they
A)
are water soluble
done
clear
B)
contain carbon and hydrogen
done
clear
C)
enter through pores
done
clear
D)
are lipid soluble
done
clear
View Answer play_arrow
question_answer 153) Industrial melanism is an example of
A)
defensive adaptation of skin against UV radiations
done
clear
B)
drug resistance
done
clear
C)
protective resemblance with the surrounding
done
clear
D)
darkening of skin due to industries
done
clear
View Answer play_arrow
question_answer 154) The larva of Bombyx mori is known as
A)
nymph
done
clear
B)
trochophore
done
clear
C)
cocoon
done
clear
D)
caterpillar
done
clear
View Answer play_arrow
question_answer 155) Ampullae of Lorenzini are present in
A)
fish
done
clear
B)
lizard
done
clear
C)
frog
done
clear
D)
rabbit
done
clear
View Answer play_arrow
question_answer 156) Which of the following is a viviparous fish?
A)
Exocoetus
done
clear
B)
Gambusia
done
clear
C)
Clarias
done
clear
D)
Labeo
done
clear
View Answer play_arrow
question_answer 157) Fluidity of bio-membranes can be shown by
A)
electron microscope
done
clear
B)
tissue culture
done
clear
C)
phase-contrast microscope
done
clear
D)
fluorescence microscope
done
clear
View Answer play_arrow
question_answer 158) The cutaneous plexus and the papillary plexus consist of
A)
a network of nerves to provide dermal sensation
done
clear
B)
a network of arteries to provide dermal supply
done
clear
C)
specialized cells for cutaneous sensations
done
clear
D)
gland cells that release cutaneous secretions
done
clear
View Answer play_arrow
question_answer 159) The function of vagus nerve innervating the heart is to
A)
initiate the heart beat
done
clear
B)
reduce the heart beat
done
clear
C)
accelerate the heart beat
done
clear
D)
maintain constant heart beat
done
clear
View Answer play_arrow
question_answer 160) The size of pupil is controlled by the
A)
ciliary muscles
done
clear
B)
suspensory ligaments
done
clear
C)
cornea
done
clear
D)
iris muscles
done
clear
View Answer play_arrow
question_answer 161) Largest single mass of lymphatic tissue in the body is
A)
lung
done
clear
B)
spleen
done
clear
C)
liver
done
clear
D)
kidney
done
clear
View Answer play_arrow
question_answer 162) HIV is classified as a retrovirus because its genetic information is carried in
A)
DNA instead of RNA
done
clear
B)
DNA
done
clear
C)
R.NA instead of DNA
done
clear
D)
protein coat
done
clear
View Answer play_arrow
question_answer 163) Lung tuberculosis is caused by
A)
Pseudomonas aeruginosa
done
clear
B)
Mycobacterium tuberculosis
done
clear
C)
Streptococcus pneumoniae
done
clear
D)
Escherichia coli
done
clear
View Answer play_arrow
question_answer 164) Vomiting centre is located in the
A)
stomach and sometimes in duodenum
done
clear
B)
gastro-intestinal tract
done
clear
C)
hypothalamus
done
clear
D)
medulla oblongata
done
clear
View Answer play_arrow
question_answer 165) Pellagra is caused by deficiency of
A)
pyridoxine
done
clear
B)
niacin
done
clear
C)
folic acid
done
clear
D)
biotin
done
clear
View Answer play_arrow
question_answer 166) Sickle cell anaemia is
A)
autosomal dominant inheritance
done
clear
B)
X-linked recessive inheritance
done
clear
C)
autosomal recessive inheritance
done
clear
D)
X-linked dominant inheritance
done
clear
View Answer play_arrow
question_answer 167) Skeletal muscles are controlled by
A)
sympathetic nerves
done
clear
B)
parasympathetic nerves
done
clear
C)
somatic nerves
done
clear
D)
autonomic nerves
done
clear
View Answer play_arrow
question_answer 168) Niche is defined as the
A)
position of species in a community in relation to other species
done
clear
B)
place where organism lives
done
clear
C)
place where organism lives and performs its duty
done
clear
D)
place where population perform their duties
done
clear
View Answer play_arrow
question_answer 169) Erythropoiesis starts in
A)
kidney
done
clear
B)
liver
done
clear
C)
spleen
done
clear
D)
red bone marrow
done
clear
View Answer play_arrow
question_answer 170) In an aquatic ecosystem, the trophic level equivalent to cows in grasslands is
A)
phytoplankton
done
clear
B)
zooplankton
done
clear
C)
nekton
done
clear
D)
benthos
done
clear
View Answer play_arrow
question_answer 171) Oxidative phosphorylation refers to
A)
anaerobic production of ATP
done
clear
B)
the citric acid cycle production of ATP
done
clear
C)
production of ATP by chemiosmosis
done
clear
D)
alcoholic fermentation
done
clear
View Answer play_arrow
question_answer 172) Centrum of 8th vertebra of frog is
A)
procoelous
done
clear
B)
acoelous
done
clear
C)
amphicoelous
done
clear
D)
amphiplatyan
done
clear
View Answer play_arrow
question_answer 173) Downs syndrome is due to
A)
linkage
done
clear
B)
sex-linked inheritance
done
clear
C)
crossing over
done
clear
D)
non-disjunction of chromosome
done
clear
View Answer play_arrow
question_answer 174) Which one of the following mammals is not an odd-toed ungulate?
A)
Rhinoceros
done
clear
B)
Camel
done
clear
C)
Zebra
done
clear
D)
Horse
done
clear
View Answer play_arrow
question_answer 175) All flat worms differ from all round worms in having
A)
triploblastic body
done
clear
B)
solid mesodenn
done
clear
C)
bilateral symmetry
done
clear
D)
metamorphosis in the life history
done
clear
View Answer play_arrow
question_answer 176) Deserts, grasslands, forests and tundra are the examples of
A)
biomes
done
clear
B)
biogeographical regions
done
clear
C)
ecosystems
done
clear
D)
biospheres
done
clear
View Answer play_arrow
question_answer 177) Standing on tip toe is an example of
A)
elevation
done
clear
B)
flexion
done
clear
C)
extension
done
clear
D)
retraction
done
clear
View Answer play_arrow
question_answer 178) Which of the following is a free living nitrogen fixing bacterium present in the soil?
A)
Nitrosomonas
done
clear
B)
Rhizobium
done
clear
C)
Assotobacter
done
clear
D)
Pseudomonas
done
clear
View Answer play_arrow
question_answer 179) Aedes aegypti is a vector for
A)
both dengue and yellow fever
done
clear
B)
dengue fever
done
clear
C)
yellow fever
done
clear
D)
Japanese encephalitis
done
clear
View Answer play_arrow
question_answer 180) Inadequate protein intake leads to kwashiorkor. The subsequent edema is most closely related to inadequate synthesis of which protein?
A)
Gamma globulin
done
clear
B)
Glucagon
done
clear
C)
Insulin
done
clear
D)
Albumin
done
clear
View Answer play_arrow
question_answer 181) The lock and key model of enzyme action illustrates that a particular enzyme molecule
A)
may be destroyed and resynthesised several times
done
clear
B)
interacts with q specific type of substrate molecule
done
clear
C)
reacts at identical rates under all conditions
done
clear
D)
forms a permanent enzyme-substrate complex
done
clear
View Answer play_arrow
question_answer 182) If the pituitary gland of an adult rat is surgically removed, which of the following endocrine glands will be less affected?
A)
Adrenal cortex
done
clear
B)
Adrenal medulla
done
clear
C)
Thyroid
done
clear
D)
Gonads
done
clear
View Answer play_arrow
question_answer 183) If one litre of water is introduced in human blood, then
A)
BMR increases
done
clear
B)
RBC collapses and urine production increases
done
clear
C)
RBC collapses and urine production decreases
done
clear
D)
BMR decreases
done
clear
View Answer play_arrow
question_answer 184) Beadle and Tatum showed that each kind of mutant bread mould they studied lacked a specific enzyme. Their experiments demonstrated that
A)
cells needs specific enzymes in order to function
done
clear
B)
genes are made of DNA
done
clear
C)
enzymes are required to repair damage
done
clear
D)
genes carry information for making proteins
done
clear
View Answer play_arrow
question_answer 185) mRNA directs the building of proteins through a sequence of
A)
exons
done
clear
B)
introns
done
clear
C)
codons
done
clear
D)
anticodons
done
clear
View Answer play_arrow
question_answer 186) Carbon dioxide is called green-house gas because it is
A)
used in green-house to increase plant growth
done
clear
B)
transparent to heat but traps sunlight
done
clear
C)
transparent to sunlight but traps heat
done
clear
D)
transparent to both sunlight and heat
done
clear
View Answer play_arrow
question_answer 187) The hormone that increases the blood calcium level and decreases its excretion by kidney is
A)
parathormone
done
clear
B)
calcitonin
done
clear
C)
thyroxine
done
clear
D)
insulin
done
clear
View Answer play_arrow
question_answer 188) Signaling between cells usually results in the activation of protein
A)
lipases
done
clear
B)
kinases
done
clear
C)
proteases
done
clear
D)
nucleases
done
clear
View Answer play_arrow
question_answer 189) Estrogen and testosterone are steroid hormones, and are most likely bind to
A)
membrane ions channels
done
clear
B)
enzyme-linked membrane receptors
done
clear
C)
G-protein linked membrane receptors
done
clear
D)
cytoplasmic receptors
done
clear
View Answer play_arrow
question_answer 190) Which of the following is unique to mitosis and not a part of meiosis?
A)
Homologous chromosomes behave independently
done
clear
B)
Chromatids are separated during anaphase
done
clear
C)
Homologous chromosomes pair and form bivalents
done
clear
D)
Homologous chromosomes crossover
done
clear
View Answer play_arrow
question_answer 191) Heating milk at \[{{65}^{o}}C\] followed by sudden cooling is known as
A)
sterilization
done
clear
B)
preservation
done
clear
C)
pasteurization
done
clear
D)
fermentation
done
clear
View Answer play_arrow
question_answer 192) Osteomalacia is due to deficiency of
A)
vitamin-A
done
clear
B)
vitamin-C
done
clear
C)
vitamin-E
done
clear
D)
vitamin-D
done
clear
View Answer play_arrow
question_answer 193) Which of the following hormones regulates growth and metamorphosis in insect?
A)
Juvenile hormone
done
clear
B)
Brain hormone
done
clear
C)
Ecdyson
done
clear
D)
Prothoracicotropic hormone
done
clear
View Answer play_arrow
question_answer 194) Glycosuria is the condition, where a man
A)
eats more sugar
done
clear
B)
excretes sugar in urine
done
clear
C)
sugar is excreted in faeces
done
clear
D)
has low sugar level in blood
done
clear
View Answer play_arrow
question_answer 195) Diploid cells have
A)
two chromosomes
done
clear
B)
one set of chromosomes
done
clear
C)
two pairs of homologous chromosomes
done
clear
D)
two sets of chromosomes
done
clear
View Answer play_arrow
question_answer 196) The anti-parallel nature of DNA refers to
A)
its charged phosphate groups
done
clear
B)
the formation of hydrogen bonds between bases from opposite strands
done
clear
C)
the opposite direction of the two strands
done
clear
D)
the pairing of bases on one strand with bases on the other strand
done
clear
View Answer play_arrow
question_answer 197) Mass extinction at the end of mesozoic era was probably due to
A)
continental drift
done
clear
B)
the collision of earth with large meteorites
done
clear
C)
massive glaciations
done
clear
D)
change in earths orbit
done
clear
View Answer play_arrow
question_answer 198) In hurdle race, which of the following is accumulated in the leg muscle?
A)
Performed ATP
done
clear
B)
Glycolysis
done
clear
C)
Lactate
done
clear
D)
Oxidative metabolism
done
clear
View Answer play_arrow
question_answer 199) Tachyglossus is a connecting link between
A)
reptiles and birds
done
clear
B)
amphibians and reptiles
done
clear
C)
birds and mammals
done
clear
D)
reptiles and mammals
done
clear
View Answer play_arrow
question_answer 200) The effectiveness of an enzyme is affected least by
A)
temperature
done
clear
B)
concentration of the substrate
done
clear
C)
original activation energy of the system
done
clear
D)
concentration of the enzyme
done
clear
View Answer play_arrow