A) \[s{{p}^{2}}\]
B) \[s{{p}^{3}}{{d}^{2}}\]
C) \[s{{p}^{3}}\]
D) \[sp\]
Correct Answer: A
Solution :
\[\underset{(lps+\sigma -bps=1+2=3)}{\mathop{O=\overset{\centerdot \centerdot }{\mathop{S}}\,=O}}\,\]hence, hybridisation \[=s{{p}^{2}}\]S in 1st excited state =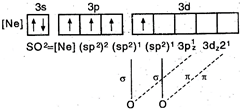 Hence, \[S{{O}_{2}}\] has angular geometry.
Hence, \[S{{O}_{2}}\] has angular geometry. You need to login to perform this action.
You will be redirected in
3 sec
