A) \[p-N{{O}_{2}}-{{C}_{6}}{{H}_{4}}-CH_{2}^{+}\]
B) \[{{C}_{6}}{{H}_{5}}CH_{2}^{+}\]
C) \[p-Cl-{{C}_{6}}{{H}_{4}}-CH_{2}^{+}\]
D) \[p-C{{H}_{3}}O-{{C}_{6}}{{H}_{4}}-CH_{2}^{+}\]
Correct Answer: D
Solution :
Presence of electron withdrawing group decreases the stability of carbonium ion and presence of electron donating group on benzene nucleus increases the stability of carbonium ion. Therefore, the order of stability is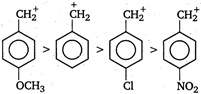
You need to login to perform this action.
You will be redirected in
3 sec
