question_answer 1) The efficiency of a Carnot engine operating with reservoir temperature of \[100{}^\circ C\] and \[-23{}^\circ C\] will be:
A)
\[\frac{373-250}{373}\]
done
clear
B)
\[\frac{275+250}{373}\]
done
clear
C)
\[\frac{100+23}{100}\]
done
clear
D)
\[\frac{373-123}{100}\]
done
clear
View Answer play_arrow
question_answer 2) Wavelength of light of frequency 100 Hz is :
A)
\[6\times {{10}^{6}}m\]
done
clear
B)
\[4\times {{10}^{6}}m\]
done
clear
C)
\[3\times {{10}^{6}}m\]
done
clear
D)
\[2\times {{10}^{6}}m\]
done
clear
View Answer play_arrow
question_answer 3) When a proton is accelerated through IV, then its kinetic energy will be :
A)
0.5 eV
done
clear
B)
1 eV
done
clear
C)
10.4 eV
done
clear
D)
1820 eV
done
clear
View Answer play_arrow
question_answer 4) The degree of freedom of triatomic gas is :
A)
8
done
clear
B)
6
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 5) The escape velocity of a sphere of mass m is given by (G = universal gravitational constant, M= mass of the earth and Re = radius of earth) :
A)
\[\sqrt{\frac{2GMm}{{{R}_{e}}}}\]
done
clear
B)
\[\sqrt{\frac{2GM+{{R}_{e}}}{2{{R}_{e}}}}\]
done
clear
C)
\[\sqrt{\frac{GM}{2{{R}_{e}}}}\]
done
clear
D)
\[\sqrt{\frac{2GM}{{{R}_{e}}}}\]
done
clear
View Answer play_arrow
question_answer 6) The waves of length 50 cm and 51 cm produced 12 beats per second. The velocity of sound is:
A)
380 m/s
done
clear
B)
2320 m/s
done
clear
C)
331 m/s
done
clear
D)
306 m/s
done
clear
View Answer play_arrow
question_answer 7) The dimensional formula of magnetic flux is :
A)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{\text{2}}}{{\text{T}}^{\text{-1}}}{{\text{A}}^{\text{3}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
B)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{0}}{{\text{L}}^{-2}}{{\text{T}}^{-2}}{{\text{A}}^{-2}}\text{ }\!\!]\!\!\text{ }\]
done
clear
C)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{\text{0}}}{{\text{T}}^{\text{-2}}}{{\text{A}}^{\text{-2}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
D)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{\text{2}}}{{\text{T}}^{\text{-2}}}{{\text{A}}^{\text{-1}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 8) In forward bias the width of potential barrier in a \[p-n\] junction diode :
A)
remains constant
done
clear
B)
increases
done
clear
C)
decreases
done
clear
D)
first (b) then (c)
done
clear
View Answer play_arrow
question_answer 9) Colours appear on a thin soap film and on soap bubbles because of the phenomenon of:
A)
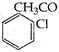
diffraction
done
clear
B)
interference
done
clear
C)
polarization
done
clear
D)
refraction
done
clear
View Answer play_arrow
question_answer 10) The photoelectric work function for a metal surface is 4.125 eV. The cut off wavelength for this surface is :
A)
\[3000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[4500\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[6000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[4125\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 11) In a \[p\]-type semiconductor the majority carriers of current are :
A)
neutrons
done
clear
B)
protons
done
clear
C)
electrons
done
clear
D)
holes
done
clear
View Answer play_arrow
question_answer 12) Ozone layer blocks the radiation of wavelength:
A)
more than \[3\times 10m\]
done
clear
B)
less than \[3\times {{10}^{-7}}m\]
done
clear
C)
equal to \[3\times {{10}^{-7}}m\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 13) The moment of inertia of a disc of mass M and radius R about an axis which is tangential to the circumference of the disc and parallel to its diameter is :
A)
\[\frac{4}{5}M{{R}^{2}}\]
done
clear
B)
\[\frac{5}{4}M{{R}^{2}}\]
done
clear
C)
\[\frac{2}{5}M{{R}^{2}}\]
done
clear
D)
\[\frac{5}{2}M{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 14) A force \[(3\hat{i}+2\hat{j})N\] displaces an object through a distance \[(2\hat{i}+3\hat{j})M\]The work done is:
A)
\[\text{13}\,\text{J}\]
done
clear
B)
\[15\,\text{J}\]
done
clear
C)
\[5\,\text{J}\]
done
clear
D)
\[0\,\text{J}\]
done
clear
View Answer play_arrow
question_answer 15) When air is replaced by a dielectric medium of constant K, the maximum force of attraction between two charges separated by a distance :
A)
increases \[\frac{1}{K}\] times
done
clear
B)
increases \[K\] times times
done
clear
C)
remains same
done
clear
D)
decreases \[K\] times
done
clear
View Answer play_arrow
question_answer 16) Which of following when added as an impurity into the silicon produces\[n\]-type semi conductor?
A)
Mg
done
clear
B)
B
done
clear
C)
Al
done
clear
D)
P
done
clear
View Answer play_arrow
question_answer 17) Two racing cars of masses \[{{m}_{1}}\] and \[{{m}_{2}}\] are moving in a circles of radii \[{{r}_{1}}\] and \[{{r}_{2}}\] respectively. Their speeds are such that each makes a complete round of circle in the same internal of time. The ratio of their angular speeds are :
A)
\[{{m}_{1}}{{m}_{2}}:{{r}_{1}}{{r}_{2}}\]
done
clear
B)
1:1
done
clear
C)
\[{{r}_{1}}:{{r}_{2}}\]
done
clear
D)
\[{{m}_{2}}:{{m}_{1}}\]
done
clear
View Answer play_arrow
question_answer 18) A thin circular ring of mass M and radius r is rotating about its axis with a constant angular velocity \[\omega \]. Two objects each of mass m are attached gently to the opposite ends of diameter of the ring. The ring will now rotate with an angular velocity of :
A)
\[\frac{\omega (M+2m)}{M}\]
done
clear
B)
\[\frac{\omega M}{(M-2m)}\]
done
clear
C)
\[\frac{\omega (M-2m)}{(M+2m)}\]
done
clear
D)
\[\frac{\omega M}{(M+2m)}\]
done
clear
View Answer play_arrow
question_answer 19) A black body is at temperature of 500 K, it emits energy at a rate which is proportional to :
A)
\[{{(500)}^{4}}\]
done
clear
B)
\[{{(500)}^{3}}\]
done
clear
C)
\[{{(500)}^{\frac{1}{2}}}\]
done
clear
D)
\[{{(500)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 20) If a magnetic dipole is rotated through an angle \[\text{ }\!\!\theta\!\!\text{ }\] with respect to the direction of the magnetic field of intensity H then work done is :
A)
MH sin\[\text{ }\!\!\theta\!\!\text{ }\]
done
clear
B)
MH (1 - sin \[\text{ }\!\!\theta\!\!\text{ }\])
done
clear
C)
\[\frac{MH}{2}\]
done
clear
D)
MH(1-cos\[\text{ }\!\!\theta\!\!\text{ }\])
done
clear
View Answer play_arrow
question_answer 21) A torque of a force \[\vec{F}=-3\hat{i}+5\hat{k}\] acting at a point \[\vec{r}=7\hat{i}+3\hat{j}+\hat{k}\]about the origin is :
A)
\[4\hat{i}-4\hat{j}+6\hat{k}\]
done
clear
B)
\[14\hat{i}+14\hat{j}-6\hat{k}\]
done
clear
C)
\[-21\hat{i}+\hat{j}-4\hat{k}\]
done
clear
D)
\[14\hat{j}-38\hat{j}+16\hat{k}\]
done
clear
View Answer play_arrow
question_answer 22) The momentum of a particle is increased by 100% then kinetic energy increases by :
A)
100%
done
clear
B)
300%
done
clear
C)
200%
done
clear
D)
150%
done
clear
View Answer play_arrow
question_answer 23) The internal resistance of a cell of emf \[12\,V\,is\,5\times 10{{-}^{2}}\Omega \]It is connected across an unknown resistance, the voltage across the cell when a current of 60 amp is drawn from it, is :
A)
10V
done
clear
B)
6V
done
clear
C)
12 V
done
clear
D)
9 V
done
clear
View Answer play_arrow
question_answer 24) Efficiency of a Carnot engine is 100% if :
A)
\[{{T}_{1}}=0K\]
done
clear
B)
\[{{T}_{1}}=273K\]
done
clear
C)
\[T_{1}^{2}=273K\]
done
clear
D)
\[{{T}_{2}}\]=0K
done
clear
View Answer play_arrow
question_answer 25) In a transverse progressive wave of amplitude A the maximum particle velocity is four times of its wave velocity. The wavelength of the wave is:
A)
\[\frac{\pi A}{4}\]
done
clear
B)
\[\frac{\pi A}{2}\]
done
clear
C)
\[2\pi A\]
done
clear
D)
\[4\pi A\]
done
clear
View Answer play_arrow
question_answer 26) A black body is heated from \[27{}^\circ C\] to \[927{}^\circ C\]. The ratio of radiation emitted will be :
A)
1:8
done
clear
B)
1:16
done
clear
C)
1:128
done
clear
D)
1:256
done
clear
View Answer play_arrow
question_answer 27) The potential at a point due to a positive charge of 100 \[\mu \]C at a distance of 9 m, is :
A)
\[{{10}^{5}}V\]
done
clear
B)
\[{{10}^{8}}V\]
done
clear
C)
\[{{10}^{6}}V\]
done
clear
D)
\[{{10}^{3}}V\]
done
clear
View Answer play_arrow
question_answer 28) Candela is a unit of :
A)
sound
done
clear
B)
illuminance
done
clear
C)
luminous flux
done
clear
D)
cuminous intensity
done
clear
View Answer play_arrow
question_answer 29) Which one of the following waves do not show the polarisation?
A)
Sinusoidal waves
done
clear
B)
Longitudinal waves in gas
done
clear
C)
Transverse waves in gas
done
clear
D)
Electromagnetic waves
done
clear
View Answer play_arrow
question_answer 30) A capacitor of 20 \[\mu \]F and charged up to 500 V, is connected in parallel to another capacitor of 10 \[\mu \] charged to 200 V. The common potential will be :
A)
600 V
done
clear
B)
400 V
done
clear
C)
100 V
done
clear
D)
200 V
done
clear
View Answer play_arrow
question_answer 31) Which is the correct expression for half-life?
A)
\[{{t}_{1/2}}=\lambda \log 2\]
done
clear
B)
\[{{t}_{1/2}}=\frac{\log 2}{\lambda }\]
done
clear
C)
\[{{t}_{1/2}}=\frac{2.303\log 2}{\lambda }\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 32) A man of height 6 feet stands in front of a plane mirror. The vertical height of the mirror, so that the man is able to see himself fully, is :
A)
8 feet
done
clear
B)
1.5 feet
done
clear
C)
4.5 feet
done
clear
D)
3 feet
done
clear
View Answer play_arrow
question_answer 33) The current gains of the semiconductor devices are \[\alpha \]\[=\left( \frac{{{i}_{c}}}{{{i}_{c}}} \right)\]and \[\beta \]=\[\left( \frac{{{i}_{c}}}{{{i}_{b}}} \right)\]The relation between \[\alpha \]and \[\beta \]are:
A)
\[\beta =\frac{\alpha +1}{\alpha }\]
done
clear
B)
\[\beta =\frac{\alpha }{1-\alpha }\]
done
clear
C)
\[\alpha =\frac{\beta }{1-\beta }\]
done
clear
D)
\[\alpha =\frac{1-\beta }{\beta }\]
done
clear
View Answer play_arrow
question_answer 34) A proton moving with a velocity of \[2.5\times {{10}^{7}}m/s,\] enters a magnetic field of intensity 2.5T making an angle 30° with the magnetic field. The force on the proton is :
A)
\[12.5\times {{10}^{-12}}N\]
done
clear
B)
\[5\times {{10}^{-12}}N\]
done
clear
C)
\[9\times {{10}^{-12}}N\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 35) A conductor of length 1m carrying current of 1 amp is placed parallel to a magnetic field of 1 gauss. The magnetic force acting on the conductor is :
A)
\[{{10}^{-4}}N\]
done
clear
B)
1 dyne
done
clear
C)
IN
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 36) Currents of 10 A and 2 A are passed through two parallel wires A and B respectively in opposite directions. If the wire is uniformly long and the length of wire B is 2m. The force on conductor B at separation 10 cm from A will be :
A)
\[40\times {{10}^{-7}}N\]
done
clear
B)
8\[\pi \] \[\times {{10}^{-7}}N\]
done
clear
C)
\[8\times {{10}^{-5}}N\]
done
clear
D)
\[4\times {{10}^{-5}}N\]
done
clear
View Answer play_arrow
question_answer 37) A man runs towards mirror at a speed of 4 m/s with what speed will his image move towards and relative to object?
A)
8 m/s
done
clear
B)
4 m/s
done
clear
C)
2 m/s
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 38) Two bulbs of rating 200 V, 40 W and 200V 100W are used in the house, which of the following statement is correct:
A)
Resistance of 100 watt is greater
done
clear
B)
Resistance of both bulbs are equal
done
clear
C)
Resistance of 40 watt is greater
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 39) The position \[x\] of a particle varying with time \[t\] as \[x=a{{t}^{2}}b{{t}^{3}}\] The acceleration of the particle at time t will be equal to :
A)
zero
done
clear
B)
\[\frac{a}{3b}\]
done
clear
C)
\[\frac{a}{b}\]
done
clear
D)
\[\frac{2a}{3b}\]
done
clear
View Answer play_arrow
question_answer 40) Two bodies of equal masses m1 and m2 moving along the same straight line with velocities + 3 m/s and - 5 m/s respectively collide elastically, their velocities after collision will be :
A)
- 5 m/s, 3 m/s
done
clear
B)
- 6 m/s, 4 m/s
done
clear
C)
- 4 m/s, both
done
clear
D)
3 m/s, - 5 m/s
done
clear
View Answer play_arrow
question_answer 41) A nucleus \[_{n}{{X}^{m-4}}\] emits one \[\alpha \] and two \[\beta \] particles. The resulting nucleus will be :
A)
\[_{n}{{X}^{m-4}}\]
done
clear
B)
\[_{n-2}X{{m}^{-4}}\]
done
clear
C)
\[_{n-1}X{{m}^{-2}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 42) In an isothermal change of an ideal gas \[\Delta \mathsf{U}=0.\]The change in heat energy \[\Delta Q\] is equal to:
A)
2 W
done
clear
B)
1.5 W
done
clear
C)
0.5 W
done
clear
D)
1 W
done
clear
View Answer play_arrow
question_answer 43) A point charge \[Q\] lies on the perpendicular bisector of an electrical dipole of dipole moment p. If the distance of \[Q\] from the dipole is r (much larger than the size of dipole), then the electric field at \[Q\] is proportional to :
A)
p and r-3
done
clear
B)
p2 and r3
done
clear
C)
p and r-2
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 44) Identify the vector quantity among the following :
A)
angular momentum
done
clear
B)
distance
done
clear
C)
energy
done
clear
D)
heat
done
clear
View Answer play_arrow
question_answer 45) The resistance of discharge tube is :
A)
non ohmic
done
clear
B)
zero
done
clear
C)
ohmic
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 46) After 1 a and 2 P emissions :
A)
mass number reduces by 1
done
clear
B)
atomic number remains same
done
clear
C)
mass number reduces by 2
done
clear
D)
atomic number reduces by 4
done
clear
View Answer play_arrow
question_answer 47) A parallel plate condenser with oil between plates (dielectric constant of oil K = 2) has a capacitance C. If the oil is removed, then capacitance of the capacitor becomes :
A)
C/2
done
clear
B)
\[C\sqrt{2}\]
done
clear
C)
C
done
clear
D)
\[\frac{\sqrt{2}}{C}\]
done
clear
View Answer play_arrow
question_answer 48) A long hollow copper pipe carries a current, the magnetic field produced will be :
A)
both inside and outside the pipe
done
clear
B)
neither inside nor outside the pipe
done
clear
C)
inside the pipe only
done
clear
D)
outside the pipe only
done
clear
View Answer play_arrow
question_answer 49) A transverse wave is represented by the equation \[y=yo\,\sin \,\frac{2\pi }{\lambda }\]\[(ut=x)\] for what value of \[\lambda \] is the particle velocity equal to two times the wave velocity?
A)
\[\lambda =\pi yo\]
done
clear
B)
\[\lambda =\frac{\pi yo}{4.}\]
done
clear
C)
\[\lambda =4\pi yo\]
done
clear
D)
\[\lambda =2\pi yo\]
done
clear
View Answer play_arrow
question_answer 50) Velocity of light is maximum in :
A)
vacuum
done
clear
B)
diamond
done
clear
C)
glass
done
clear
D)
water
done
clear
View Answer play_arrow
question_answer 51) The unit of illuminance is :
A)
watt
done
clear
B)
candela
done
clear
C)
lumen
done
clear
D)
lux
done
clear
View Answer play_arrow
question_answer 52) A isotropic point source of 100 candela is fastened to the ceiling of the room. Then the total luminous flux falling in the whole room is :
A)
1256 lumen
done
clear
B)
628 lumen
done
clear
C)
314 lumen
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 53) The distance between two coherent sours is 1 mm. The screen is placed at a distance of 1 m from the source. If the distance of third bright fringe is 1.2 mm from the central fringe. The wavelength of light used is :
A)
\[7200\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[6800\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[5500\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[4000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 54) The wave theory of light was given by :
A)
Planck
done
clear
B)
Thomas Young
done
clear
C)
Newton
done
clear
D)
Huygens
done
clear
View Answer play_arrow
question_answer 55) When a ray of light is refracted from one medium into another medium. The wave length changes from \[4000\overset{\text{o}}{\mathop{\text{A}}}\,\] to \[6000\overset{\text{o}}{\mathop{\text{A}}}\,\]. The critical angle for a ray from second medium to first medium will be :
A)
\[{{\sin }^{-1}}\left( \frac{2}{\sqrt{13}} \right)\]
done
clear
B)
\[{{\tan }^{-1}}\left( \frac{3}{2} \right)\]
done
clear
C)
\[{{\cos }^{-1}}\left( \frac{1}{7} \right)\]
done
clear
D)
\[{{\sin }^{-1}}\left( \frac{2}{3} \right)\]
done
clear
View Answer play_arrow
question_answer 56) A stone lies at the bottom of a stream. A boy wants to hit it with a stick. Taking aim the boy holds the stick in the air at an angle of \[45{}^\circ \]. At what distance from the stone will the stick hit the bottom. If the depth is 3; cm (given \[_{a}{{u}_{w}}\] 4/3) :
A)
12\[\sqrt{2}\] cm
done
clear
B)
16 cm
done
clear
C)
12 cm
done
clear
D)
8 cm
done
clear
View Answer play_arrow
question_answer 57) If \[{{\omega }_{1}}\]and \[{{\omega }_{2}}\] are dispersive powers of lenses of focal length\[{{f}_{1}}\] and\[{{f}_{2}}\] respectively. Then the conditions of chromatic aberration for two. lenses in contact is :
A)
\[\frac{{{\omega }_{1}}}{{{\omega }_{1}}}=\,\,\frac{{{f}_{2}}}{{{f}_{1}}}\]
done
clear
B)
\[\frac{{{\omega }_{2}}}{{{\omega }_{1}}}=\frac{{{f}_{1}}}{{{f}_{2}}}\]
done
clear
C)
\[\frac{{{\omega }_{1}}}{{{\omega }_{2}}}=\frac{{{f}_{1}}}{{{f}_{2}}}\]
done
clear
D)
\[\frac{{{\omega }_{1}}}{{{\omega }_{2}}}=\frac{{{f}_{1}}}{{{f}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 58) The electric field required to just balance a liquid drop of mass \[2.4\times {{10}^{-12}}kg\] and charge \[2.4\times {{10}^{-18}}\] coulomb, is:
A)
\[9.8\times {{10}^{6}}N/C\]
done
clear
B)
\[4.9\times {{10}^{6}}N/C\]
done
clear
C)
\[6.8\times {{10}^{6}}N/C\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 59) When the distance between two charged particles is halved. The coulomb force between them becomes :
A)
four times
done
clear
B)
double
done
clear
C)
one half
done
clear
D)
one fourth
done
clear
View Answer play_arrow
question_answer 60) The capacitance of a parallel plate air capacitor is 10 \[\mu \]F. If now the overlapping area of plates is doubled and separation between the plates is halved, the new capacitance will be :
A)
60\[\text{ }\!\!\mu\!\!\text{ F}\]
done
clear
B)
10\[\text{ }\!\!\mu\!\!\text{ F}\]
done
clear
C)
20 \[\text{ }\!\!\mu\!\!\text{ F}\]
done
clear
D)
40 \[\text{ }\!\!\mu\!\!\text{ F}\]
done
clear
View Answer play_arrow
question_answer 61) Three different capacitors are connected in series. On them there will be :
A)
equal charge and unequal potential
done
clear
B)
equal charge
done
clear
C)
equal potential
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 62) The plates of a parallel plate capacitor are charged with a battery so that the plates of the capacitor have acquired the potential- difference equal to emf of the battery. The ratio of the work done by the battery and energy stored in capacitor will be :
A)
4:1
done
clear
B)
2:1
done
clear
C)
1:2
done
clear
D)
1 :1
done
clear
View Answer play_arrow
question_answer 63) According to Joule, the heat produced in resistor of resistance R carrying current; is given by
A)
\[\frac{iRt}{J}kcal\]
done
clear
B)
\[{{i}^{2}}Rt\times 4.2\times {{10}^{3}}kcal\]
done
clear
C)
\[{{i}^{2}}Rt\,kcal\]
done
clear
D)
\[\frac{{{i}^{2}}Rt}{4.2\times {{10}^{3}}}kcal\]
done
clear
View Answer play_arrow
question_answer 64) A \[3{}^\circ C\] rise of temperature is observed in a conductor by passing a certain current, when the current is doubled, the rise. of temperature will be :
A)
\[3{}^\circ C\]
done
clear
B)
\[9{}^\circ C\]
done
clear
C)
\[12{}^\circ C\]
done
clear
D)
\[15{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 65) Water falls from a height 500 m. What is the raise in temperature of water at bottom. If the whole energy remains in the water? (Specific heat of water \[4200\text{ }J/kg\text{ }{}^\circ C\]):
A)
\[0.19{}^\circ C\]
done
clear
B)
\[2.3{}^\circ C\]
done
clear
C)
\[0.59{}^\circ C\]
done
clear
D)
\[1.17{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 66) Which of the following electromagnetic radiations have the smallest wave length?
A)
y-rays
done
clear
B)
X-rays
done
clear
C)
Microwave
done
clear
D)
U.V. waves
done
clear
View Answer play_arrow
question_answer 67) A neutron having mass of \[1.67\times {{10}^{-27}}\] and moving at.\[{{10}^{8}}m/s\] collides with a deuteron at rest and sticks to it. If the mass of the deuteron is \[3.34\times {{10}^{-27}}kg.\]The speed of the combination is :
A)
\[4.33\times {{10}^{7}}m/s\]
done
clear
B)
\[2.33\times {{10}^{7}}m/s\]
done
clear
C)
\[1.67\times {{10}^{7}}m/s\]
done
clear
D)
\[3.33\times {{10}^{7}}m/s\]
done
clear
View Answer play_arrow
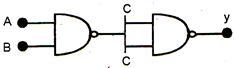
question_answer 68)
The following figure represents a logic symbol, this is equivalent to :
A)
NAND gate
done
clear
B)
NOT gate
done
clear
C)
OR gate
done
clear
D)
AND gate
done
clear
View Answer play_arrow
question_answer 69) 1 amu unit is equivalent to :
A)
931 Me V
done
clear
B)
931 keV
done
clear
C)
931 eV
done
clear
D)
1 eV
done
clear
View Answer play_arrow
question_answer 70) The radius of a nucleus, with nucleon number \[16is3\times {{10}^{-15}}m.\] The radius of other nucleus with nucleon number 128, will be :
A)
\[6\times {{10}^{-15}}m\]
done
clear
B)
\[3\times {{10}^{-15}}m\]
done
clear
C)
\[4.5\times {{10}^{-15}}m\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
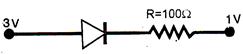
question_answer 71)
Assuming that the junctions diode is ideal the current through the diode is :
A)
200 mA
done
clear
B)
20 mA
done
clear
C)
2 mA
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 72) \[\beta \]-rays are :
A)
electromagnetic waves
done
clear
B)
fast moving electrons
done
clear
C)
singly ionised gas atoms
done
clear
D)
helium nuclei
done
clear
View Answer play_arrow
question_answer 73) Half-life of \[B{{i}^{210}}\] is 5 days. If we start with 50000 atoms of this isotope. The number of atoms left over after 10 days is :
A)
20000
done
clear
B)
12500
done
clear
C)
5000
done
clear
D)
25000
done
clear
View Answer play_arrow
question_answer 74) Hubbies law is expressed as \[u\]= speed of recession, r = distance of Galaxy and H= Hubbies constant) :
A)
\[\upsilon =\frac{H}{{{r}^{2}}}\]
done
clear
B)
\[\upsilon =\frac{H}{r}\]
done
clear
C)
\[\upsilon =Hr\]
done
clear
D)
\[\upsilon =H{{r}^{2}}\]
done
clear
View Answer play_arrow
question_answer 75) de Broglie wavelength vary with momentum of particle as :
A)
\[\lambda \propto \frac{1}{p}\]
done
clear
B)
\[\lambda \propto p\]
done
clear
C)
both of these
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 76) In which of the following the angle between the two covalent bonds is maximum?
A)
\[{{H}_{2}}O\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[C{{H}_{4}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 77) Which of the following is the correct relation of the first law of thermodynamics?
A)
\[\Delta E=Q-W\]
done
clear
B)
\[\Delta E=Q+W\]
done
clear
C)
\[\Delta E=\Delta Q+\Delta W\]
done
clear
D)
\[\Delta E=\Delta Q+W\]
done
clear
View Answer play_arrow
question_answer 78) The radium and uranium atoms in a sample of uranium mineral are in the ratio of \[1:2.8\times {{10}^{6}}\] If half-life period of radium is \[1620\] year, the half-life period of uranium will be:
A)
\[4.53\times {{10}^{9}}year\]
done
clear
B)
\[45.3\times {{10}^{9}}year\]
done
clear
C)
\[4.53\times {{10}^{10}}year\]
done
clear
D)
\[45.3\times {{10}^{10}}year\]
done
clear
View Answer play_arrow
question_answer 79) Specific conductance of \[0.1\text{ }NKCl\]solution at \[{{23}^{o}}C\]is\[0.012\text{ }oh{{m}^{-1}}c{{m}^{-1}}\]. Resistance of cell containing the solution at same temperature was found to be\[55ohm\]. The cell constant is:
A)
\[0.0616\text{ }c{{m}^{-1}}\]
done
clear
B)
\[0.616\text{ }c{{m}^{-1}}\]
done
clear
C)
\[6.16c{{m}^{-1}}\]
done
clear
D)
\[616\text{ }c{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 80) The solubility of a gas is directly proportional to the pressure of the gas. This statement is based on:
A)
Kohlrausch law
done
clear
B)
Raoults law
done
clear
C)
Henrys law
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 81) The powerful explosive RDX is formed during the nitration of:
A)
Glycerol
done
clear
B)
toluene
done
clear
C)
Urotropine
done
clear
D)
phenol
done
clear
View Answer play_arrow
question_answer 82) On dissolving 1 mole each of the following acids in 1 litre water, the acid which do not give a solution of 1 N strength is:
A)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
B)
\[HCl{{O}_{4}}\]
done
clear
C)
\[HN{{O}_{3}}~\]
done
clear
D)
\[HCl\]
done
clear
View Answer play_arrow
question_answer 83) The strongest reducing agent of the alkali metal is:
A)
\[Na\]
done
clear
B)
\[Li\]
done
clear
C)
\[K\]
done
clear
D)
\[Cs\]
done
clear
View Answer play_arrow
question_answer 84) Oxidation number of N in \[HN{{O}_{3}}\] is:
A)
\[+3.5\]
done
clear
B)
\[-3.5\]
done
clear
C)
\[+5\]
done
clear
D)
\[-5\]
done
clear
View Answer play_arrow
question_answer 85) In DNA, the complementary bases are:
A)
Adenine and thymine; .guanine and uracil
done
clear
B)
Uracil and adenine; cytosine and guanine
done
clear
C)
Adenine and guanine; thymine and cytosine
done
clear
D)
Adenine and thymine; guanine and cytosine
done
clear
View Answer play_arrow
question_answer 86) \[{{H}_{2}}{{O}_{2}}\] is used as:
A)
An acid only
done
clear
B)
An oxidant only
done
clear
C)
An oxidant, reductant and acid
done
clear
D)
A reductant only
done
clear
View Answer play_arrow
question_answer 87) At the critical micelle concentration, the surfactant molecules:
A)
Associate
done
clear
B)
Dissociate
done
clear
C)
Decompose
done
clear
D)
Become completely soluble
done
clear
View Answer play_arrow
question_answer 88) Aniline is usually purified by:
A)
Chromatographic
done
clear
B)
Fractional crystallization
done
clear
C)
By adding oxalic acid
done
clear
D)
Steam distillation
done
clear
View Answer play_arrow
question_answer 89) Bond formed in crystal by anion and cation is:
A)
Ionic
done
clear
B)
dipole
done
clear
C)
Covalent
done
clear
D)
metallic
done
clear
View Answer play_arrow
question_answer 90) Given, (i) \[C+{{O}_{2}}\xrightarrow{{}}C{{O}_{2}};\,\,\Delta {{H}^{o}}=-xkJ\] (ii) \[2C+{{O}_{2}}\xrightarrow{{}}2C{{O}_{2}};\Delta =-ykJ\] The enthalpy of formation of \[C{{O}_{2}}\] will be:
A)
\[2x-y\]
done
clear
B)
\[y-2x\]
done
clear
C)
\[\frac{(2x-y)}{2}\]
done
clear
D)
\[\frac{(y-2x)}{2}\]
done
clear
View Answer play_arrow
question_answer 91) When enthalpy and entropy change for a chemical reaction are \[-2.5\times {{10}^{3}}\]joules and \[7.4\text{ }J\text{ }de{{g}^{-1}}\]respectively. The reaction at \[298K\]is:
A)
Spontaneous
done
clear
B)
Non-spontaneous
done
clear
C)
reversible
done
clear
D)
Irreversible
done
clear
View Answer play_arrow
question_answer 92) Acetylene and \[HCHO\]react in presence of copper acetylide catalyst to form:
A)
\[1-butyne-1,4,\text{ }diol\]
done
clear
B)
\[2-butyne-1,2,\text{ }diol\]
done
clear
C)
\[2-butyne-1,4,\text{ }diol\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 93) During the titration of \[KMn{{O}_{4}}\]with oxalic acid in acid medium, oxidation number of \[M{{n}^{+1}}\] change to:
A)
\[0\]
done
clear
B)
\[+2\]
done
clear
C)
\[+3\]
done
clear
D)
\[+5\]
done
clear
View Answer play_arrow
question_answer 94) The orbitals of same energy level providing the most efficient overlapping are:
A)
\[sp-sp\]
done
clear
B)
\[s{{p}^{2}}-s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{3}}-s{{p}^{3}}\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 95) Hydrogen peroxide is reduced by:
A)
Ozone
done
clear
B)
Barium peroxide
done
clear
C)
Acidic solution of \[~KMn{{O}_{4}}\]
done
clear
D)
Lead sulphide suspension
done
clear
View Answer play_arrow
question_answer 96) Red phosphorus is less reactive than yellow phosphorus because:
A)
It is highly polymerized
done
clear
B)
It is insoluble in \[CC{{l}_{4}}\]
done
clear
C)
It is red in colour
done
clear
D)
It is hard
done
clear
View Answer play_arrow
question_answer 97) \[C{{F}_{2}}=C{{F}_{2}}\]is a unit of:
A)
Teflon
done
clear
B)
Polyethene
done
clear
C)
Backelite
done
clear
D)
buha-S
done
clear
View Answer play_arrow
question_answer 98) In Williamsons synthesis:
A)
An alcohol is heated with cone. \[{{H}_{2}}S{{O}_{4}}\]at \[{{140}^{o}}C\]
done
clear
B)
An alkyl halide is treated with sodium
done
clear
C)
An alkyl halide is treated with sodium alkoxide
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 99) Which of the following oxides is the most acidic?
A)
\[S{{b}_{2}}{{O}_{5}}\]
done
clear
B)
\[A{{s}_{2}}{{O}_{5}}\]
done
clear
C)
\[{{P}_{2}}{{O}_{5}}\]
done
clear
D)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 100) The solubility product \[({{K}_{sp}})\] of \[Ca{{F}_{2}}\] is \[4\times {{10}^{-11}}\]. The solubility of \[CaF\] is:
A)
\[0.017g\text{ }litr{{e}^{-1}}~~~\]
done
clear
B)
\[0.071\text{ }g\text{ }litr{{e}^{-1}}\]
done
clear
C)
\[0.17g\text{ }litr{{e}^{-1}}\]
done
clear
D)
\[1.7\text{ }g\text{ }litr{{e}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 101) Identify the product D in the following series of reaction: \[C{{H}_{3}}COOH\xrightarrow{LiAl{{H}_{4}}}A\xrightarrow{{{H}^{+}},443K}\]\[B\xrightarrow{B{{r}_{2}}}C\xrightarrow{alc.\,KOH}D\]Acetic acid:
A)
acetylene
done
clear
B)
methane
done
clear
C)
benzaldehyde
done
clear
D)
alcohol
done
clear
View Answer play_arrow
question_answer 102) In the following radioactive decay, \[_{92}{{X}^{232}}{{\xrightarrow{{}}}_{89}}{{Y}^{220}}\], how many a- and (3-particles are ejected from X to Y?
A)
\[5\alpha \] and \[5\beta \]
done
clear
B)
\[5\alpha \] and \[4\beta \]
done
clear
C)
\[5\alpha \] and \[3\beta \]
done
clear
D)
\[3\alpha \] and \[3\beta \]
done
clear
View Answer play_arrow
question_answer 103) Which of the following element has the maximum electron affinity?
A)
\[F\]
done
clear
B)
\[Cl\]
done
clear
C)
\[Br\]
done
clear
D)
\[I\]
done
clear
View Answer play_arrow
question_answer 104) Oppenauer oxidation is the reverse process of:
A)
Merwein Pondrof verley reduction
done
clear
B)
Clemmensens reduction
done
clear
C)
Rosemund reduction
done
clear
D)
Wolf Kishner reduction
done
clear
View Answer play_arrow
question_answer 105) Purification of aluminium by electrolyte refining, is known as:
A)
Bayers process
done
clear
B)
Halls process
done
clear
C)
Serpecks process
done
clear
D)
Hoopes process
done
clear
View Answer play_arrow
question_answer 106) The atomic weight of helium is 4. The number of atoms in 1 g of helium is:
A)
\[6.02\times {{10}^{23}}\times 4\]
done
clear
B)
\[6.02\times {{10}^{23}}\]
done
clear
C)
\[\frac{6.02\times {{10}^{23}}}{2}\]
done
clear
D)
\[\frac{6.02\times {{10}^{23}}}{4}\]
done
clear
View Answer play_arrow
question_answer 107) Bell metal is an alloy of:
A)
\[Cu+Ni\]
done
clear
B)
\[Cu+Pb\]
done
clear
C)
\[Cu+Sn\]
done
clear
D)
\[Cu+Zn\]
done
clear
View Answer play_arrow
question_answer 108) Hoffmann rearrangement during the conversion of an amide to amine is:
A)
Intermolecular
done
clear
B)
Intra molecular
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 109) In the reaction\[C{{H}_{3}}N+2H\xrightarrow[Ether]{HCl}X\xrightarrow{Boiling\,{{H}_{2}}O}Y\] The product Y Is:
A)
Acetaldehyde
done
clear
B)
Acetone
done
clear
C)
Dimethylamine
done
clear
D)
Ethanamine
done
clear
View Answer play_arrow
question_answer 110) Natural rubber is a polymer of:
A)
cis-isoprene
done
clear
B)
trans isoprene
done
clear
C)
Cis and trans isoprene
done
clear
D)
None of these above
done
clear
View Answer play_arrow
question_answer 111) Cassiterite is an ore of:
A)
\[Sn\]
done
clear
B)
\[Ni\]
done
clear
C)
\[Sb\]
done
clear
D)
\[Mn\]
done
clear
View Answer play_arrow
question_answer 112) lodoform gives a precipitate with \[AgN{{O}_{3}}\] on heating but chloroform does not because:
A)
\[C-I\]bond in iodoform is weak and \[C-Cl\] bond in chloroform is strong
done
clear
B)
Chloroform is covalent
done
clear
C)
Iodoform is ionic
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 113) Which of the following is the sweetest sugar?
A)
Glucose
done
clear
B)
Maltose
done
clear
C)
Fructose
done
clear
D)
Sucrose
done
clear
View Answer play_arrow
question_answer 114) Rate of esterification of \[{{C}_{2}}{{H}_{5}}OH\] is maximum in case of:
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}COOH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
C)
\[HCOOH\]
done
clear
D)
\[C{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 115) Percentage of lead in lead pencil is:
A)
\[30\]
done
clear
B)
\[20\]
done
clear
C)
\[10\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 116) Deficiency of the following metal ion causes anaemia:
A)
\[Na\]
done
clear
B)
\[Fe\]
done
clear
C)
\[Zn\]
done
clear
D)
\[Mg\]
done
clear
View Answer play_arrow
question_answer 117) Which of the following compounds will be most easily attacked by an electrophile?
A)
\[{{C}_{6}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}Cl\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}OH\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 118) A 5% solution of cane sugar (molecular weight 342) is isotonic with 1% solution of substance X. The molecular weight of X is:
A)
\[34.2\]
done
clear
B)
\[136.2\]
done
clear
C)
\[68.4\]
done
clear
D)
\[171.2\]
done
clear
View Answer play_arrow
question_answer 119) Terylene is a condensation polymer of ethylene glycol and:
A)
Salicylic acid
done
clear
B)
Phthallic acid
done
clear
C)
Terephthalic acid
done
clear
D)
benzoic acid
done
clear
View Answer play_arrow
question_answer 120) Electric refining is used for refining of:
A)
\[Fe\]
done
clear
B)
\[Cu\]
done
clear
C)
\[Pb\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 121) The correct order of the \[O-O\] bond length in \[{{O}_{2}}\], \[{{H}_{2}}O\] and \[{{O}_{3}}\] is:
A)
\[{{H}_{2}}{{O}_{2}}>{{O}_{3}}>{{O}_{2}}\]
done
clear
B)
\[{{O}_{2}}>{{H}_{2}}{{O}_{2}}>{{O}_{3}}\]
done
clear
C)
\[{{O}_{3}}>{{H}_{2}}{{O}_{2}}>{{O}_{2}}\]
done
clear
D)
\[{{O}_{2}}>{{O}_{3}}>{{H}_{2}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 122) Silver metal is obtained by:
A)
Magnetic separation
done
clear
B)
Carbon reduction process
done
clear
C)
Froth floatation process
done
clear
D)
Parkes process
done
clear
View Answer play_arrow
question_answer 123) The concentration units, independent of temperature, would be:
A)
Weight volume per cent
done
clear
B)
Normality
done
clear
C)
Molality
done
clear
D)
Molarity
done
clear
View Answer play_arrow
question_answer 124) What is the half-life of a radioactive substance is 87.5% of a given amount of the substance disintegrates in 40 minutes?
A)
\[10\text{ }min\]
done
clear
B)
\[13.58\text{ }min\]
done
clear
C)
\[20\text{ }min\]
done
clear
D)
\[160\text{ }min\]
done
clear
View Answer play_arrow
question_answer 125) Oxidation state of \[Fe\] in \[F{{e}_{2}}{{O}_{4}}\]is:
A)
\[8/3\]
done
clear
B)
\[3/2\]
done
clear
C)
\[5/4\]
done
clear
D)
\[4/5\]
done
clear
View Answer play_arrow
question_answer 126) In the reaction\[X+Y\xrightarrow{{}}XY\], if the concentration of X and Y are doubled, the rate of reaction will be:
A)
Increased two times
done
clear
B)
Increased four times
done
clear
C)
Decreased one times
done
clear
D)
Decreased two times
done
clear
View Answer play_arrow
question_answer 127) Who modified Bohrs theory by Introducing elliptical orbits for electron path?
A)
Sommer field
done
clear
B)
Rutherford
done
clear
C)
Hund
done
clear
D)
Thomson
done
clear
View Answer play_arrow
question_answer 128) On increasing the pressure three fold, the rate of reaction of \[2{{H}_{2}}S\text{ }+{{O}_{2}}\xrightarrow{{}}products\] would increase:
A)
\[3times\]
done
clear
B)
\[9\text{ }times\]
done
clear
C)
\[12\text{ }times\]
done
clear
D)
\[27\text{ }times\]
done
clear
View Answer play_arrow
question_answer 129) Which of the following has more unpaired d-electrons?
A)
\[C{{u}^{+}}\]
done
clear
B)
\[{{N}^{3+}}\]
done
clear
C)
\[Z{{n}^{+}}\]
done
clear
D)
\[F{{e}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 130) On adding A to\[AB(s)A(g)+B(g)\], the new equilibrium concentration of .A becomes double. The equilibrium concentration of B would become:
A)
Twice of its original value
done
clear
B)
Half of its original value
done
clear
C)
one fourth of its original value
done
clear
D)
One eight of its original value
done
clear
View Answer play_arrow
question_answer 131) The pH of blood is:
A)
\[<7\]
done
clear
B)
\[>10\]
done
clear
C)
\[>8\] but \[<9\]
done
clear
D)
\[>7\] but \[<8\]
done
clear
View Answer play_arrow
question_answer 132) The conjugate base of \[{{H}_{3}}B{{O}_{3}}\]is:
A)
\[HB{{O}_{3}}^{2-}\]
done
clear
B)
\[{{H}_{4}}B{{O}_{3}}^{+}\]
done
clear
C)
\[{{H}_{2}}B{{O}_{3}}^{-}\]
done
clear
D)
\[B{{(OH)}_{4}}^{-}\]
done
clear
View Answer play_arrow
question_answer 133) For a reaction to occur spontaneously :
A)
\[\Delta S\] must be negative
done
clear
B)
\[\Delta H\] must be negative
done
clear
C)
\[(\Delta H+T\Delta S)\] must be negative
done
clear
D)
\[(\Delta H-T\Delta S)\] must be negative
done
clear
View Answer play_arrow
question_answer 134) Energy required to dissociate \[4g\] of gaseous hydrogen in to free gaseous atoms is \[208kcal\] at \[{{25}^{o}}C\]. The bond energy of \[H-H\] bond will be:
A)
\[104kcal\]
done
clear
B)
\[104kcal\]
done
clear
C)
\[10.4\text{ }kcal\]
done
clear
D)
\[1040\text{ }kcal\]
done
clear
View Answer play_arrow
question_answer 135) For a first-order reaction, the half-life period is independent of:
A)
First power of final concentration
done
clear
B)
Square root of final concentration
done
clear
C)
Cube root of initial concentration
done
clear
D)
Initial concentration
done
clear
View Answer play_arrow
question_answer 136) Which one of the following is not the characteristics of alkali metals?
A)
Their ions are isoeleccronic with noble gases
done
clear
B)
High ionisation energies
done
clear
C)
Low electronegathity
done
clear
D)
Low molecular print
done
clear
View Answer play_arrow
question_answer 137) What is the correct sequence of bond order?
A)
\[{{O}_{2}}^{+}>{{O}_{2}}>{{O}_{2}}^{-}\]
done
clear
B)
\[{{O}_{2}}^{-}>{{O}_{2}}^{+}>{{O}_{2}}\]
done
clear
C)
\[{{O}_{2}}^{+}>{{O}_{2}}^{-}>{{O}_{2}}\]
done
clear
D)
\[{{O}_{2}}>{{O}_{2}}^{-}>{{O}_{2}}^{+}\]
done
clear
View Answer play_arrow
question_answer 138) Coal gas is a mixture of:
A)
\[C{{H}_{4}}+CO\]
done
clear
B)
\[{{H}_{2}}O+CO\]
done
clear
C)
\[{{H}_{2}}+CO\]
done
clear
D)
\[{{H}_{2}}+CO+C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 139) Element with electronic configuration Is , \[2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}4{{p}^{6}}4{{d}^{10}},5{{s}^{2}}4{{p}^{3}}\] belongs to the following group of the periodic table:
A)
2nd
done
clear
B)
3rd
done
clear
C)
5th
done
clear
D)
7th
done
clear
View Answer play_arrow
question_answer 140) The number of geometrical isomers in case of \[C{{H}_{3}}CH=CH-CH=CH-{{C}_{2}}{{H}_{5}}\] is:
A)
\[6\]
done
clear
B)
\[5\]
done
clear
C)
\[4\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 141) The atomic radius of elements of which of the following series would be nearly the same?
A)
\[F,Cl,Br,I\]
done
clear
B)
\[Fe,Co,Ni,Cu\]
done
clear
C)
\[Na,K,Rb,Cs\]
done
clear
D)
\[Li,Be,B,C\]
done
clear
View Answer play_arrow
question_answer 142) The structure of \[CsCl\]is:
A)
Octahedral lattice
done
clear
B)
Face centred cubic lattice
done
clear
C)
Body centred cubic lattice
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 143) The concentration units independent of temperature would be:
A)
Mass volume percent
done
clear
B)
Normality
done
clear
C)
Molarity
done
clear
D)
Molality
done
clear
View Answer play_arrow
question_answer 144) An aqueous solution containing \[1g\]urea I boils at\[{{100.25}^{o}}C\]. The aqueous solution containing \[3g\]of glucose in the same volume will boil at:
A)
\[{{100}^{o}}C\]
done
clear
B)
\[{{100.5}^{o}}C\]
done
clear
C)
\[{{100.25}^{o}}C\]
done
clear
D)
\[{{100.75}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 145) The standard heats of formation of \[N{{O}_{2}}(g)\] and \[{{N}_{2}}{{O}_{4}}(g)\] are \[8.0\] and \[2.0kcal\,\,mol{{e}^{-1}}\]respectively. The heat of dimerisation of \[N{{O}_{2}}\] in kcal is:
A)
\[-14.0\]
done
clear
B)
\[-12.0\]
done
clear
C)
\[-6.0\]
done
clear
D)
\[-10.0\]
done
clear
View Answer play_arrow
question_answer 146) o-chloro toluene on further nitration gives:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 147) EMF of cell, \[Ni|N{{i}^{2+}}(1.0M)||A{{u}^{3+}}(1.0M)|Au\] where \[{{E}^{o}}\] for \[N{{i}^{2+}}|Ni\] is \[-0.25V\]and \[{{E}^{o}}\] for \[A{{u}^{3+}}|Au\]is \[1.50V\]is:
A)
\[-1.75V\]
done
clear
B)
\[+1.25V\]
done
clear
C)
\[+\text{ }1.75V\]
done
clear
D)
\[+4.0V\]
done
clear
View Answer play_arrow
question_answer 148) The reaction of \[C{{H}_{3}}MgI\]with acetone and hydrolysis of the resulting product gives:
A)
\[{{(C{{H}_{3}})}_{3}}COH\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CHOH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 149) If \[C{{H}_{3}}COOH+O{{H}^{-}}\to C{{H}_{3}}CO{{O}^{-}}+{{H}_{2}}O\]\[+{{q}_{1}}\] and \[{{H}^{+}}+O{{H}^{-}}\to {{H}_{2}}O+{{q}_{2}}\] then the enthalpy change for the reaction, \[C{{H}_{3}}COOH\xrightarrow{{}}C{{H}_{3}}CO{{O}^{-}}+{{H}^{+}}\] is equal to:
A)
\[{{q}_{1}}-{{q}_{2}}\]
done
clear
B)
\[{{q}_{2}}-{{q}_{1}}\]
done
clear
C)
\[{{q}_{1}}+{{q}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 150) The colour of \[FeS{{O}_{4}}{{(N{{H}_{4}})}_{2}}S{{O}_{4}}.6{{H}_{2}}O\]
A)
Blue
done
clear
B)
green
done
clear
C)
White
done
clear
D)
red
done
clear
View Answer play_arrow
question_answer 151) The transition zone between two different communities is called as :
A)
ecad
done
clear
B)
ecotype
done
clear
C)
ecotone
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 152) The pyramid of biomass in a parasitic ecosystem is:
A)
upright
done
clear
B)
inverted
done
clear
C)
rhomboidal
done
clear
D)
linear
done
clear
View Answer play_arrow
question_answer 153) A Nobel prize was awarded for proposing the mechanism of ATP formation in the light reaction to :
A)
Mitchell
done
clear
B)
Salisbury
done
clear
C)
Bassham
done
clear
D)
Calvin
done
clear
View Answer play_arrow
question_answer 154) The enzyme libase brings about :
A)
condensation of glycerol and fatty acids into fats
done
clear
B)
hydrolysis of triglycerides
done
clear
C)
both a and b
done
clear
D)
conversion of glyoxalate to glycolate
done
clear
View Answer play_arrow
question_answer 155) Which will show both alternation of generation and alternation of host ?
A)
Taenia
done
clear
B)
Ascaris
done
clear
C)
Fasciola
done
clear
D)
Cyclostoma
done
clear
View Answer play_arrow
question_answer 156) Wuchereria is transmitted by :
A)
Culex
done
clear
B)
Female Anopheles
done
clear
C)
Tse-tsefly
done
clear
D)
phlebotomous
done
clear
View Answer play_arrow
question_answer 157) Lac is :
A)
epidermal secretion of body in insects
done
clear
B)
excretory product
done
clear
C)
plant product
done
clear
D)
a dead rat insect
done
clear
View Answer play_arrow
question_answer 158) Larvae bilateral and adults are radial in :
A)
Arthropoda
done
clear
B)
Annelida
done
clear
C)
Echinodermata
done
clear
D)
Mollusca
done
clear
View Answer play_arrow
question_answer 159) The ratio of xanthophyll to carotene in young leaves in nature is :
A)
3 : 1
done
clear
B)
1 : 3
done
clear
C)
1 : 2
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 160) The primitive atmosphere on the earth largely had :
A)
\[C{{H}_{4}}\] and \[N{{H}_{3}}\]
done
clear
B)
\[C{{O}_{2}}\] and \[N{{H}_{3}}\]
done
clear
C)
\[C{{O}_{2}}\] and He
done
clear
D)
\[{{H}_{2}}\] and He
done
clear
View Answer play_arrow
question_answer 161) Guayule rubber comes from :
A)
Taraxacum sp
done
clear
B)
Landophila sp
done
clear
C)
Funtumia sp
done
clear
D)
Parthenium sp
done
clear
View Answer play_arrow
question_answer 162) Which of the following plants is generally described as a living fossil ?
A)
Cycas
done
clear
B)
Cupressus
done
clear
C)
Taxus
done
clear
D)
Ephedra
done
clear
View Answer play_arrow
question_answer 163) In snakes the poisonous glands are modification of :
A)
parotid glands
done
clear
B)
mascitters glands
done
clear
C)
sublingual glands
done
clear
D)
lingual glands
done
clear
View Answer play_arrow
question_answer 164) In snakes eyelids are :
A)
immovable
done
clear
B)
no eyelids
done
clear
C)
movable
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 165) Mouth parts of Housefly are of :
A)
sponging and sucking type
done
clear
B)
piercing type
done
clear
C)
laping and chewing type.
done
clear
D)
siphoning type
done
clear
View Answer play_arrow
question_answer 166) Entaimoeba histolytica is :
A)
found in intestine
done
clear
B)
found in liver
done
clear
C)
found in coelom
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 167) The base of oxysome is also called as :
A)
\[{{F}_{5}}\] particle
done
clear
B)
\[{{F}_{6}}\] particle
done
clear
C)
\[{{F}_{1}}\]particle
done
clear
D)
\[{{F}_{0}}\]particle
done
clear
View Answer play_arrow
question_answer 168) Temin was awarded Nobel prize in the year :
A)
1968
done
clear
B)
1973
done
clear
C)
1975
done
clear
D)
1978
done
clear
View Answer play_arrow
question_answer 169) Circnotropous ovules are found in :
A)
Butomus
done
clear
B)
Brassica
done
clear
C)
Opuntia
done
clear
D)
Polygonum
done
clear
View Answer play_arrow
question_answer 170) In Potato the bud dormancy is due to :
A)
ABA
done
clear
B)
inhibor B
done
clear
C)
ethylene
done
clear
D)
cyanides
done
clear
View Answer play_arrow
question_answer 171) Amphioxus belongs to :
A)
Cephalochordata
done
clear
B)
Urochordata
done
clear
C)
Vertebrata
done
clear
D)
Hemichordata
done
clear
View Answer play_arrow
question_answer 172) Inorganic ions help in blood clotting :
A)
\[M{{g}^{++}}\]
done
clear
B)
\[C{{a}^{++}}\]
done
clear
C)
\[N{{a}^{+}}\]
done
clear
D)
\[{{K}^{+}}\]
done
clear
View Answer play_arrow
question_answer 173) Natural selection theory is proposed by Darwin along with :
A)
Wallace
done
clear
B)
Mendel
done
clear
C)
Morgan
done
clear
D)
Lamarck
done
clear
View Answer play_arrow
question_answer 174) Biodegradable pollutant is :
A)
sewage
done
clear
B)
asbestos
done
clear
C)
plastic
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 175) Which of the following originates from mesoderm ?
A)
Eye ball and retina
done
clear
B)
Dermis and heart
done
clear
C)
Intestine
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 176) In Frog the ear has :
A)
pinna
done
clear
B)
cochlea and pinna
done
clear
C)
pinna and drum
done
clear
D)
ear drum and internal ear
done
clear
View Answer play_arrow
question_answer 177) Without exception, all birds are :
A)
omnivorous
done
clear
B)
have feathers and fly
done
clear
C)
form nests and care them
done
clear
D)
have calcareous shelled egg
done
clear
View Answer play_arrow
question_answer 178) In honey which of the following sugar is present?
A)
Glucose
done
clear
B)
Glycogen
done
clear
C)
Sucrose
done
clear
D)
Cellulose
done
clear
View Answer play_arrow
question_answer 179) Phosphate pollution is caused by :
A)
phosphate rock only
done
clear
B)
agriculture fertilizers only
done
clear
C)
sewage and phosphate rock
done
clear
D)
sewage and agriculture fertilizers
done
clear
View Answer play_arrow
question_answer 180) Angiosperms, to which the largest flower belongs, is :
A)
total stem parasite
done
clear
B)
partial stem parasite
done
clear
C)
total root parasite
done
clear
D)
partial root parasite
done
clear
View Answer play_arrow
question_answer 181) Which of the following is free living aerobic non-photo-synthetic nitrogen-fixing bacterium ?
A)
Rhizobium
done
clear
B)
Azotobacter
done
clear
C)
Nostoc
done
clear
D)
Azospirillum
done
clear
View Answer play_arrow
question_answer 182) Which of the following ecosystem has the highest gross primary productivity?
A)
Grassland
done
clear
B)
Coral reef
done
clear
C)
Mangroves
done
clear
D)
Rain forest
done
clear
View Answer play_arrow
question_answer 183) Net gain of ATP molecules, during aerobic respiration, is :
A)
36 molecules
done
clear
B)
38 molecules
done
clear
C)
40 molecules
done
clear
D)
48 molecules
done
clear
View Answer play_arrow
question_answer 184) The antherozoids of Funaria are :
A)
aciliated
done
clear
B)
biciliated
done
clear
C)
multiciliated
done
clear
D)
monociliated
done
clear
View Answer play_arrow
question_answer 185) Which of the following organ has single membrane ?
A)
nucleus
done
clear
B)
cell wall
done
clear
C)
nucleolus
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 186) Centromere is a part of :
A)
ribosomes
done
clear
B)
mitochondria
done
clear
C)
chromosomes
done
clear
D)
endoplasmic reticulum
done
clear
View Answer play_arrow
question_answer 187) \[NADP{{H}_{2}}\]is generated through :
A)
glycolysis
done
clear
B)
photosystem-I
done
clear
C)
photosystem-II
done
clear
D)
anaerobic respiration
done
clear
View Answer play_arrow
question_answer 188) Heterospory, seed habit is often exhibited by a plant possessing :
A)
bract
done
clear
B)
spathe
done
clear
C)
petiole
done
clear
D)
ligule
done
clear
View Answer play_arrow
question_answer 189) Columella is a specialized structure found in the sporangium of :
A)
Ulothrix
done
clear
B)
Rhizopus
done
clear
C)
Spirogyra
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 190) The maximum bio-magnification would be in which of the following case of aquatic ecosystem ?
A)
Fishes
done
clear
B)
Birds
done
clear
C)
Zooplanktons
done
clear
D)
Phytoplanktons
done
clear
View Answer play_arrow
question_answer 191) The codons causing chain termination are :
A)
TAG, TAA, TGA
done
clear
B)
GAT, AAT, ACT
done
clear
C)
AGT, TAG, UGA
done
clear
D)
UAG, UGA, UAA
done
clear
View Answer play_arrow
question_answer 192) Which of the following is the key intermediate compound linking glycolysis to the Krebs cycle ?
A)
NADH
done
clear
B)
ATP
done
clear
C)
Malic acid
done
clear
D)
Acetyl Co-A
done
clear
View Answer play_arrow
question_answer 193) Land mass occupied by forest is about :
A)
11 %
done
clear
B)
22%
done
clear
C)
30%
done
clear
D)
60%
done
clear
View Answer play_arrow
question_answer 194) In soil, water available for plants is :
A)
capillary water
done
clear
B)
hygroscopic water
done
clear
C)
gravitational water
done
clear
D)
chemically bound water
done
clear
View Answer play_arrow
question_answer 195) The RNA that pick up specific amino acid from amino acid pool in the cytoplasm to ribosome during protein synthesis is called :
A)
m-RNA
done
clear
B)
t-RNA
done
clear
C)
r-RNA
done
clear
D)
RNA
done
clear
View Answer play_arrow
question_answer 196) Which of the following is a living fossil ?
A)
pinns longifolia
done
clear
B)
Delbergia sissoo
done
clear
C)
Mirabilis jalapa
done
clear
D)
Ginkgo biloba
done
clear
View Answer play_arrow
question_answer 197) Edible part in Litchi is :
A)
mesocarp
done
clear
B)
fleshy aril
done
clear
C)
endosperm
done
clear
D)
pericarp
done
clear
View Answer play_arrow
question_answer 198) Initiation codon in eukaryotes is :
A)
AUG
done
clear
B)
UAG
done
clear
C)
GAU
done
clear
D)
AGU
done
clear
View Answer play_arrow
question_answer 199) Gobar gas contains mainly :
A)
\[C{{H}_{4}}+C{{O}_{2}}\]
done
clear
B)
\[~C{{H}_{4}}+C{{O}_{2}}\]
done
clear
C)
\[C{{O}_{2}}+\text{ }{{H}_{2}}\]
done
clear
D)
\[C{{O}_{2}}+\text{ }{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 200) With an increase in the turgidity of cell, the wall pressure will :
A)
increase
done
clear
B)
decrease
done
clear
C)
fluctuate
done
clear
D)
remain unchanged
done
clear
View Answer play_arrow
question_answer 201) The aquatic fern, which is an excellent biofertilizer, is :
A)
Azolla
done
clear
B)
Saluinia
done
clear
C)
Marsilea
done
clear
D)
Pteridium
done
clear
View Answer play_arrow
question_answer 202) The joint between atlas and axis is called :
A)
pivot joint
done
clear
B)
saddle joint
done
clear
C)
angular joint
done
clear
D)
hinge joint
done
clear
View Answer play_arrow
question_answer 203) Cholecystokinin and duocrimin are secreted by :
A)
intestine
done
clear
B)
pancrease
done
clear
C)
adrenal cortex
done
clear
D)
thyroid gland
done
clear
View Answer play_arrow
question_answer 204) A person with the sex chromosomes XXY suffer from :
A)
Downs syndrome
done
clear
B)
Turners syndrome
done
clear
C)
Gynandromorphism
done
clear
D)
Klinefelters syndrome
done
clear
View Answer play_arrow
question_answer 205) Genetic identity of a human male is determined by :
A)
autosome
done
clear
B)
nucleolus
done
clear
C)
sex-chromosome
done
clear
D)
cell organelles
done
clear
View Answer play_arrow
question_answer 206) Which of the following is pollution related disorder ?
A)
Flurosis
done
clear
B)
Leprosis
done
clear
C)
Silicosis
done
clear
D)
Pneumonicosis
done
clear
View Answer play_arrow
question_answer 207) To which of the following family do folk acid and pentothenic acid belong?
A)
Vitamin K
done
clear
B)
Vitamin A
done
clear
C)
Vitamin C
done
clear
D)
Vitamin B complex
done
clear
View Answer play_arrow
question_answer 208) Rate of heartbeat is determined by :
A)
AV node
done
clear
B)
SA node
done
clear
C)
Purkinje fibres
done
clear
D)
papillary muscles
done
clear
View Answer play_arrow
question_answer 209) Which of the following are homologous organs?
A)
Wings of bird and wings of insect
done
clear
B)
Wings of bat and wings of Cockroach
done
clear
C)
Wings of bird and hand of human
done
clear
D)
Nails of human being and calws in animals
done
clear
View Answer play_arrow
question_answer 210) The problem, due to Rh‑ factor arises when the blood of two ( Rh+ and Rh-) mix up :
A)
in test tube
done
clear
B)
through transfusion
done
clear
C)
during pregnancy
done
clear
D)
both a and c
done
clear
View Answer play_arrow
question_answer 211) DDT is :
A)
a non-degradable pollutant
done
clear
B)
a biodegradable pollutant
done
clear
C)
not a pollutant
done
clear
D)
an antibiotic
done
clear
View Answer play_arrow
question_answer 212) In human beings, multiple genes are involved in the inheritance of :
A)
colour blindness
done
clear
B)
phenylketonuria
done
clear
C)
sickle cell anaemia
done
clear
D)
skin colour
done
clear
View Answer play_arrow
question_answer 213) What is common among Silver fish, Scorpion, Crab and Honey bee?
A)
Compound eyes
done
clear
B)
Poison glands
done
clear
C)
Jointed legs
done
clear
D)
Metamorphosis
done
clear
View Answer play_arrow
question_answer 214) Plague (black death) is caused by :
A)
bacteria
done
clear
B)
virus
done
clear
C)
protozoan
done
clear
D)
fungi
done
clear
View Answer play_arrow
question_answer 215) Viruses possess :
A)
organelle for its vital mechanism
done
clear
B)
either DNA or RNA
done
clear
C)
ribosomes to synthesize protein
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 216) The roof of cranium of Frog is formed by :
A)
parasphenoid
done
clear
B)
alisphenoid
done
clear
C)
frontoparietal
done
clear
D)
orbitosphenoid
done
clear
View Answer play_arrow
question_answer 217) The embryonated egg of Ascciris represents :
A)
an egg with an egg
done
clear
B)
an egg with gastrula
done
clear
C)
an egg with blastula
done
clear
D)
an egg with a juvenile
done
clear
View Answer play_arrow
question_answer 218) Diabetes is due to :
A)
\[N{{a}^{+}}\] deficiency
done
clear
B)
hormonal deficiency
done
clear
C)
enzyme deficiency
done
clear
D)
iodine deficiency
done
clear
View Answer play_arrow
question_answer 219) The 10% energy transfer law of food chain was given by :
A)
Stanley
done
clear
B)
Weismann
done
clear
C)
Lindemann
done
clear
D)
Tansley
done
clear
View Answer play_arrow
question_answer 220) Which of the following plant kingdom is called amphibians?
A)
Tracheophyta
done
clear
B)
Bryophyta
done
clear
C)
Pteridophyta
done
clear
D)
Thallophyta
done
clear
View Answer play_arrow
question_answer 221) The rate at which light energy is converted into chemical energy of organic molecules is the ecosystems ?
A)
Net primary productivity
done
clear
B)
Gross secondary productivity
done
clear
C)
Net secondary productivity
done
clear
D)
Gross primary productivity
done
clear
View Answer play_arrow
question_answer 222) Typhoid fever is caused by:
A)
Giardia
done
clear
B)
Salmonella
done
clear
C)
Shigella
done
clear
D)
Escherichia
done
clear
View Answer play_arrow
question_answer 223) A bacterium divides every 35 minutes. If a culture containing 10 cell per ml is grown for 175 minutes, what will be the cell concentration per ml after 175 minutes?
A)
\[175\times {{10}^{5}}\] cells
done
clear
B)
\[85\times {{10}^{5}}\] cells
done
clear
C)
\[53\times {{10}^{5}}\] cells
done
clear
D)
\[32\times {{10}^{5}}\] cells
done
clear
View Answer play_arrow
question_answer 224) Taxon is the unit of a group of :
A)
species
done
clear
B)
genus
done
clear
C)
order
done
clear
D)
taxonomy
done
clear
View Answer play_arrow
question_answer 225) When water enters in roots due to diffusion, is termed as :
A)
endocytosis
done
clear
B)
active absorption
done
clear
C)
osmosis
done
clear
D)
passive absorption
done
clear
View Answer play_arrow
question_answer 226) 2, 4-D is an effective :
A)
fungicide
done
clear
B)
weedicide
done
clear
C)
rodenticide
done
clear
D)
wormicide
done
clear
View Answer play_arrow
question_answer 227) The term meiosis was given by :
A)
Johansson
done
clear
B)
Knoll and Ruska
done
clear
C)
Flemming
done
clear
D)
Farmer and Moore
done
clear
View Answer play_arrow
question_answer 228) Puccinia forms uredia and :
A)
pycnia on Barberry leaves
done
clear
B)
aecia on Wheat leaves
done
clear
C)
telia on Wheat leaves
done
clear
D)
aecia on Barberry leaves
done
clear
View Answer play_arrow
question_answer 229) Two bacteria found to be very useful in genetic engineering experiments are :
A)
Nitrosomonas and Kliebsiella
done
clear
B)
Escherichia and Agrobacterium
done
clear
C)
Nitrobacter .and Azotobacter
done
clear
D)
Rhizobium and Diplococcus
done
clear
View Answer play_arrow
question_answer 230) Which of the following meristems is responsible for extrastelar secondary growth in dicotyledonous stem ?
A)
Phellogen
done
clear
B)
Intrafascicular cambium
done
clear
C)
Interfascicular cambium
done
clear
D)
Intercalary meristem
done
clear
View Answer play_arrow
question_answer 231) The periderm includes :
A)
cork
done
clear
B)
cambium
done
clear
C)
secondary phloem
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 232) Genetic drift operates only in :
A)
island populations
done
clear
B)
smaller populations
done
clear
C)
larger populations
done
clear
D)
Mendelian populations
done
clear
View Answer play_arrow
question_answer 233) Which one of the following statement is correct?
A)
Homo erectus is the ancestor of man
done
clear
B)
Cro-magnon mans fossil has been found in Ethiopia
done
clear
C)
Australopitheciis is the real ancestor of modern man
done
clear
D)
Neanderthal man is the direct ancestor of Homo sapiens
done
clear
View Answer play_arrow
question_answer 234) When a single gene influences more than one trait it is called :
A)
pleiotrophy
done
clear
B)
epistasis
done
clear
C)
pseudo-dominance
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 235) The exchanges of gases in the alveoli of the lungs takes place by :
A)
osmosis
done
clear
B)
simple diffusion
done
clear
C)
passive transport
done
clear
D)
active transport
done
clear
View Answer play_arrow
question_answer 236) Phytochrome becomes active in :
A)
green light
done
clear
B)
blue light
done
clear
C)
red light
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 237) Which one among the following chemicals is used for causing defoliation of forest trees ?
A)
amo-1618
done
clear
B)
phosphon-D
done
clear
C)
malic hydrazide
done
clear
D)
2, 4-D
done
clear
View Answer play_arrow
question_answer 238) Two opposite forces operate in the growth and development of every population. One of them related to the ability to reproduce at a given rate. The force opposing to it is called :
A)
biotic control
done
clear
B)
mortality
done
clear
C)
fecundity
done
clear
D)
environmental resistances
done
clear
View Answer play_arrow
question_answer 239) Colchicine is an inhibitory chemical, which:
A)
prevents the spindle formation in mitosis
done
clear
B)
prevents the formation of equatorial plane
done
clear
C)
stops the functioning of centriole
done
clear
D)
prevents attaching of centromeres with rays
done
clear
View Answer play_arrow
question_answer 240) The polygenic genes show :
A)
different genotypes
done
clear
B)
different phenotypes
done
clear
C)
different karyotypes
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 241) Maximum absorption of light occurs in the region of :
A)
400 - 700 nm
done
clear
B)
700 - 900 nm
done
clear
C)
1000 - 1200 nm
done
clear
D)
1500 - 2000 nm
done
clear
View Answer play_arrow
question_answer 242) Which one of the following fish is introduced in India by foreigners ?
A)
Labeo rohita
done
clear
B)
Pomphret
done
clear
C)
Mystus singhala
done
clear
D)
Calsius batrachus
done
clear
View Answer play_arrow
question_answer 243) What is the major cause of diminishing wild life number ?
A)
Cannibalism
done
clear
B)
Habitat destruction
done
clear
C)
Falling of trees
done
clear
D)
Paucity of drinking water
done
clear
View Answer play_arrow
question_answer 244) Lactose is composed of :
A)
glucose + fructose
done
clear
B)
glucose + glucose
done
clear
C)
glucose + galactose
done
clear
D)
fructose+ galactose
done
clear
View Answer play_arrow
question_answer 245) H.J. Muller had received Nobel prize for :
A)
discovering the linkage of genes
done
clear
B)
discovering the induced mutations by X-rays
done
clear
C)
his studies on Drosophila for genetic study
done
clear
D)
proving that the DNA is a genetic material
done
clear
View Answer play_arrow
question_answer 246) Pasteurisation is a process, which means heating of drinks. It is carried out, at what temperature and for how much duration?
A)
\[120{}^\circ C\text{ }and\text{ }60\text{ }minutes\]
done
clear
B)
\[60-70{}^\circ C\text{ }and\text{ }30\text{ }minutes\]
done
clear
C)
\[70{}^\circ C\text{ }and\text{ }60\text{ }minutes\]
done
clear
D)
\[80{}^\circ C\text{ }and\text{ }30\text{ }minutes\]
done
clear
View Answer play_arrow
question_answer 247) Which hormones stimulates the secretion of milk from female?
A)
LH
done
clear
B)
Prolactin
done
clear
C)
Oxytocin
done
clear
D)
Progesterone
done
clear
View Answer play_arrow
question_answer 248) Coelom is found between the cavity of :
A)
ectoderm and endoderm
done
clear
B)
mesoderm and ectoderm
done
clear
C)
endoderm and ectoderm
done
clear
D)
mesoderm and endoderm
done
clear
View Answer play_arrow
question_answer 249) When 1500 ml air is present in the lungs, it is called :
A)
vital capacity
done
clear
B)
tidal volume
done
clear
C)
residua volume
done
clear
D)
inspiratory reserve volume
done
clear
View Answer play_arrow
question_answer 250) AIDS disease was first reported in :
A)
Russia
done
clear
B)
Germany
done
clear
C)
USA
done
clear
D)
France
done
clear
View Answer play_arrow
question_answer 251) In \[{{C}_{4}}\] plants, \[C{{O}_{2}}\] fixation is done by :
A)
mesophyll cells
done
clear
B)
guard cells
done
clear
C)
sclerenchyma
done
clear
D)
chlorenchyma and hypodermis
done
clear
View Answer play_arrow
question_answer 252) The prokaryotic flagella possess :
A)
unit membrane enclosed fibre
done
clear
B)
protein membrane enclosed fibre
done
clear
C)
helically arranged protein molecule
done
clear
D)
9 + 1 membrane enclosed structure
done
clear
View Answer play_arrow
question_answer 253) The black rust of Wheat is a fungal disease caused by :
A)
Melanipsora lini
done
clear
B)
Clauiceps purpurea
done
clear
C)
Albugo Candida
done
clear
D)
Puccinia graminis tritici
done
clear
View Answer play_arrow
question_answer 254) Two zoogeographical regions, separated by high mountain ranges, are :
A)
oriental and Australian
done
clear
B)
palaearctic and oriental
done
clear
C)
nearctic and palaearctic
done
clear
D)
neotropical and Ethiopian
done
clear
View Answer play_arrow
question_answer 255) The twinning of tendrils around a support is a good example of :
A)
nastic movements
done
clear
B)
thigmotropism
done
clear
C)
phototropism
done
clear
D)
chemotropism
done
clear
View Answer play_arrow
question_answer 256) When a cell is fully turgid which of the following will be zero ?
A)
Wall pressure
done
clear
B)
Osmotic pressure
done
clear
C)
Turgor pressure
done
clear
D)
Water potential
done
clear
View Answer play_arrow
question_answer 257) DNA synthesis can be specifically measured by estimating the incorporation of radio-labeled :
A)
uracil
done
clear
B)
adenine
done
clear
C)
thymidine
done
clear
D)
deoxyribose sugar
done
clear
View Answer play_arrow
question_answer 258) How many mitotic divisions are needed for a single cell to make 128 cells ?
A)
7
done
clear
B)
14
done
clear
C)
28
done
clear
D)
32
done
clear
View Answer play_arrow
question_answer 259) Co-factor (prosthetic group) is a part of holoenzyme. It is :
A)
loosely attached inorganic part
done
clear
B)
accessory non-protein substance attached firmly
done
clear
C)
loosely attached organic part
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 260) Bryophytes can be separated from algae, because they :
A)
are thalloid forms
done
clear
B)
have no conducting tissue
done
clear
C)
possess archegonia
done
clear
D)
contain chloroplast
done
clear
View Answer play_arrow
question_answer 261) The core metal of chlorophyll is :
A)
Fe
done
clear
B)
Mg
done
clear
C)
Ni
done
clear
D)
Cu
done
clear
View Answer play_arrow
question_answer 262) Which of the following symptoms indicate red-sickness?
A)
Red and luncerated skin
done
clear
B)
Nausea and anaemia
done
clear
C)
Nausea and loss of hair
done
clear
D)
Uncerated skin, nausea and loss of hair
done
clear
View Answer play_arrow
question_answer 263) The end product of fermentation are :
A)
\[C{{O}_{2}}\] and \[{{O}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\] and \[{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[{{O}_{2}}\]and \[{{C}_{2}}{{H}_{5}}OH\]
done
clear
D)
\[C{{O}_{2}}\] and acetaldehyde
done
clear
View Answer play_arrow
question_answer 264) The desmosomes are concerned with :
A)
cytolysis
done
clear
B)
cellular excretion
done
clear
C)
cell division
done
clear
D)
cell adherence
done
clear
View Answer play_arrow
question_answer 265) The lymph serves to :
A)
transport \[C{{O}_{2}}\] to the lungs
done
clear
B)
transport \[{{O}_{2}}\]to the brain
done
clear
C)
return the interstitial fluid to the blood
done
clear
D)
return the WBCs and. RBCs to the lymph nodes
done
clear
View Answer play_arrow
question_answer 266) The carbon dioxide is transported via blood to lungs mostly :
A)
in the form of carbonic acid only
done
clear
B)
as carbaminohaemoglobin and as carbonic acid
done
clear
C)
in combination with haemoglobin only
done
clear
D)
dissolved in blood plasma
done
clear
View Answer play_arrow
question_answer 267) Which one of the following pairs is correctly matched ?
A)
Excessive perspiration-xeric adaptation
done
clear
B)
Streamlined body-aquatic adaptation
done
clear
C)
Parasitism-intra-specific relationship
done
clear
D)
Uricotelism-aquatic habitat
done
clear
View Answer play_arrow
question_answer 268) Which one of the following isotopes is most dangerous to Homo sapiens ?
A)
Caesium - 137
done
clear
B)
Iodine - 131
done
clear
C)
Phosphorus - 32
done
clear
D)
Strontium - 90
done
clear
View Answer play_arrow
question_answer 269) Identify the correct match between Tiger reserve and its state :
A)
Bandipur - Tamil Nadu
done
clear
B)
Palau- Orissa
done
clear
C)
Manas - Assam
done
clear
D)
Corbett - Madhya Pradesh
done
clear
View Answer play_arrow
question_answer 270) In abiotic community, the primary consumers are :
A)
carnivores
done
clear
B)
omnivores
done
clear
C)
detritivores
done
clear
D)
herbivores
done
clear
View Answer play_arrow
question_answer 271) Barr body in mammals represents :
A)
all the heterochromatin in female cells
done
clear
B)
one of the two X - chromosomes in somatic cells of females
done
clear
C)
all the heterochromatin in male and female cells
done
clear
D)
the Y - chromosome in somatic cells of male
done
clear
View Answer play_arrow
question_answer 272) An environment agent, which triggers transcription from an operon, is a :
A)
regulator
done
clear
B)
inducer
done
clear
C)
depressor
done
clear
D)
controlling element
done
clear
View Answer play_arrow
question_answer 273) The concept that population tends to increase geometrically while food supply increases arithmetically was put forward by :
A)
Stuart Mill
done
clear
B)
Adam Smith
done
clear
C)
Thomas Malthus
done
clear
D)
Charles Darwin
done
clear
View Answer play_arrow
question_answer 274) The blood cancer is known as :
A)
leukaemia
done
clear
B)
thrombosis
done
clear
C)
haemolysis
done
clear
D)
haemophilia
done
clear
View Answer play_arrow
question_answer 275) How many chromosomes are present in ovum of man ?
A)
22 + X
done
clear
B)
22 + Y -
done
clear
C)
44 + X
done
clear
D)
44 + Y
done
clear
View Answer play_arrow
question_answer 276) Stratum germinativum is an example of which kind of epithelium ?
A)
Cuboidal
done
clear
B)
Ciliated
done
clear
C)
Columnar
done
clear
D)
Squamous
done
clear
View Answer play_arrow
question_answer 277) If pancreas is removed, the compund which remain undigested is :
A)
carbohydrates
done
clear
B)
fats
done
clear
C)
proteins
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 278) The life span of human WBC is approximately :
A)
less than 10 days
done
clear
B)
between 20 to 30 days
done
clear
C)
between 2 to 3 months
done
clear
D)
more than 4 months
done
clear
View Answer play_arrow
question_answer 279) T. Schwann and M Schleiden were :
A)
Dutch biologists
done
clear
B)
Austrian biologists
done
clear
C)
German biologists
done
clear
D)
English biologists
done
clear
View Answer play_arrow
question_answer 280) Lysosomes are the store house of :
A)
hydrolytic enzymes
done
clear
B)
proteins
done
clear
C)
sugar
done
clear
D)
ATP
done
clear
View Answer play_arrow
question_answer 281) Which of the following is responsible for the mechanical support, protein synthesis and enzyme transport ?
A)
Endoplasmic reticulum
done
clear
B)
Mitochondria
done
clear
C)
Cell membrane
done
clear
D)
Dictyosome
done
clear
View Answer play_arrow
question_answer 282) The kingdoms-Monera, Protista, Fungi, Plantae and Animalia are distinguished on the basis of :
A)
type of nutrition
done
clear
B)
type of cell
done
clear
C)
type of reproduction
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 283) The protists have :
A)
membrane bound nucleoproteins lying embedded in the cytoplasm
done
clear
B)
gene containing nuclepproteins condensed together in loss mass
done
clear
C)
nucleoproteins in direct contact with the rest of the cell substance
done
clear
D)
only free nucleic acid aggregates
done
clear
View Answer play_arrow
question_answer 284) Which of the following is obtained from algae ?
A)
Carragenin
done
clear
B)
Chocolate
done
clear
C)
Butter
done
clear
D)
Wax
done
clear
View Answer play_arrow
question_answer 285) Which of the following plant kingdom is called Amphibians ?
A)
Thallophyta
done
clear
B)
Pteridophyta
done
clear
C)
Tracheophyta
done
clear
D)
Bryophyta
done
clear
View Answer play_arrow
question_answer 286) Enzymes are not found in :
A)
cyanobacteria
done
clear
B)
viruses
done
clear
C)
algae
done
clear
D)
fungi
done
clear
View Answer play_arrow
question_answer 287) For formation of one glucose molecule, a Calvin cycle circulates :
A)
8 times
done
clear
B)
6 times
done
clear
C)
4 times
done
clear
D)
2 times
done
clear
View Answer play_arrow
question_answer 288) Which of the following is grouped under phanerogams ?
A)
Pteridophyta
done
clear
B)
Angiosperms
done
clear
C)
Gymnosperms
done
clear
D)
both b and c
done
clear
View Answer play_arrow
question_answer 289) Iponmoea batata belongs to family :
A)
Ranunculaceae
done
clear
B)
Rutaceae
done
clear
C)
Gramineae
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 290) Buttress roots are found in :
A)
Pandanus
done
clear
B)
Terminalia
done
clear
C)
Banyan
done
clear
D)
Sorghum
done
clear
View Answer play_arrow
question_answer 291) Which of the following causes white rust of crucifers ?
A)
Aspergillus
done
clear
B)
Puccinia
done
clear
C)
Mucor
done
clear
D)
Albugo
done
clear
View Answer play_arrow
question_answer 292) Treeless terrestrial biome of cold climates is :
A)
plankton
done
clear
B)
tundra
done
clear
C)
savanaha
done
clear
D)
taiga
done
clear
View Answer play_arrow
question_answer 293) Rhododendron is the characteristic vegetation of :
A)
mangrove belt
done
clear
B)
gangetic plains
done
clear
C)
alpine zone
done
clear
D)
tropical zone
done
clear
View Answer play_arrow
question_answer 294) The pyramid of energy is always :
A)
inverted
done
clear
B)
horizontal
done
clear
C)
both a and b
done
clear
D)
upright
done
clear
View Answer play_arrow
question_answer 295) Loss of relative biological equilibrium is due to :
A)
low temperature
done
clear
B)
high temperature
done
clear
C)
radiation
done
clear
D)
pollution
done
clear
View Answer play_arrow
question_answer 296) The healing of wounds in plants takes place by the activity of :
A)
intercalary meristem
done
clear
B)
secondary meristem
done
clear
C)
lateral meristem
done
clear
D)
apical meristem
done
clear
View Answer play_arrow
question_answer 297) In which of the following form, sugar is transported within the body of plant?
A)
Maltose
done
clear
B)
Glucose
done
clear
C)
Lactose
done
clear
D)
Sucrose
done
clear
View Answer play_arrow
question_answer 298) In angiosperm, endosperm is formed by :
A)
division of fused synergids and male gamete
done
clear
B)
division of fused polar nuclei
done
clear
C)
division of fused polar nuclei and male gamete
done
clear
D)
free nuclear divisions of megaspore
done
clear
View Answer play_arrow
question_answer 299) Increase in the carbon-dioxide concentration of micro environment of leaf will cause :
A)
wider opening of stomata
done
clear
B)
no effect on stomata
done
clear
C)
opening of stomata
done
clear
D)
closure of stomata
done
clear
View Answer play_arrow
question_answer 300) The first reaction in photorespiration is :
A)
phosphorylation
done
clear
B)
decarboxylation
done
clear
C)
oxygenation
done
clear
D)
carboxylation
done
clear
View Answer play_arrow