A) \[\frac{a\sqrt{2}}{3}\]
B) \[\frac{a\sqrt{3}}{2}\]
C) \[a\sqrt{3}\]
D) \[\frac{a}{\sqrt{2}}\]
Correct Answer: B
Solution :
In a bcc lattice, the atoms touch one another along the body diagonal. For cube of length a and atomic radius r, we have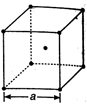 \[r=\frac{\sqrt{3}}{4}a\] \[\therefore \]Distance between two atoms \[=\frac{2\times \sqrt{3}a}{4}\] \[=\frac{\sqrt{3}}{2}a\]
\[r=\frac{\sqrt{3}}{4}a\] \[\therefore \]Distance between two atoms \[=\frac{2\times \sqrt{3}a}{4}\] \[=\frac{\sqrt{3}}{2}a\]
You need to login to perform this action.
You will be redirected in
3 sec
