A) 2
B) 1
C) 0
D) 3
Correct Answer: C
Solution :
The electronic configuration of \[Pt=[Xe]4{{f}^{14}},5{{d}^{9}},6{{s}^{1}}\] \[\therefore \] \[P{{t}^{2+}}=[Xe]\,4{{f}^{14}},5{{d}^{8}},6{{s}^{0}}\] \[{{[Pt{{(CN)}_{4}}]}^{2-}}=[Xe]\,4{{f}^{14}}\]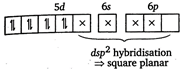 \[\therefore \]No unpaired electron is present in \[{{[Pt{{(CN)}_{4}}]}^{2-}}\]ion.
\[\therefore \]No unpaired electron is present in \[{{[Pt{{(CN)}_{4}}]}^{2-}}\]ion.
You need to login to perform this action.
You will be redirected in
3 sec
