A) sea saw
B) V-shaped
C) trigonal planar
D) T-shaped
Correct Answer: D
Solution :
In \[\text{XeO}{{\text{F}}_{\text{2}}}\text{,}\]the central atom\[\text{Xe}\]has 3 bond pairs of electrons and 2 lone pairs of electrons. Thus, the central atom is \[s{{p}^{3}}d\]hybridised and its geometry should be trigonal bipyramidal. But due to the presence of two lone pairs, geometry becomes T-shaped (according to VSEPR theory).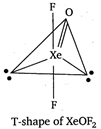
You need to login to perform this action.
You will be redirected in
3 sec
