A) \[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
B) \[{{[Fe{{(CN)}_{6}}]}^{3-}}\]
C) \[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
D) \[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{2+}}\]
Correct Answer: A
Solution :
Higher the number of unpaired electrons, higher is the magnetic moment. \[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}},Cr\]is present as \[C{{r}^{3+}}.\]Thus,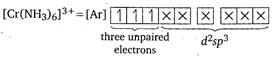 In\[{{[Fe{{(CN)}_{6}}]}^{3-}},\]one unpaired electron is present and in \[{{[Fe{{(CN)}_{6}}]}^{4-}}\] and\[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{2+}}\] both unpaired electrons are absent. Hence,\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]has the highest magnetic moment among the given complex ions. \[[\mu =\sqrt{3(3+2)}=3.87BM].\](\[C{{N}^{-}}\] is a strong field ligand, thus causes pairing.)
In\[{{[Fe{{(CN)}_{6}}]}^{3-}},\]one unpaired electron is present and in \[{{[Fe{{(CN)}_{6}}]}^{4-}}\] and\[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{2+}}\] both unpaired electrons are absent. Hence,\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]has the highest magnetic moment among the given complex ions. \[[\mu =\sqrt{3(3+2)}=3.87BM].\](\[C{{N}^{-}}\] is a strong field ligand, thus causes pairing.)
You need to login to perform this action.
You will be redirected in
3 sec
