A) p-nitrobenzoic acid
B) p-methylbenzoic acid
C) p-chlprobenzoic acid
D) p-methoxybenzoic acid
Correct Answer: D
Solution :
Higher the tendency of an acid to release a proton, higher is the acidic strength. Presence of electron releasing group such as\[-OC{{H}_{3}},C{{H}_{3}}\]etc (\[-OC{{H}_{3}}\] is more electron releasing than \[C{{H}_{3}}\]group) increases the electron density of the carboxylate anion. Consequently, the anion becomes unstable and the release of proton becomes difficult. Thus, weaker is the acid. Hence, \[p-\]methoxy benzoic acid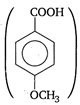 is the least acidic among the given acids.
is the least acidic among the given acids.
You need to login to perform this action.
You will be redirected in
3 sec
