A) \[+\,3,\text{ }3d\]and 4
B) \[~+\,3,\text{ }4d\] and 6
C) \[+\,3,\text{ }3d\]and 6
D) \[~+\,2,\text{ }3d\] and 6
Correct Answer: C
Solution :
Let the oxidation number of Cr is \[x.\] \[cis[Cr{{(en)}_{2}}C{{l}_{2}}]Cl\] \[x+(0)\times 2+(-1)\times 2-1=0\] \[x-3=0\] \[x=+\,3\] In \[[Cr{{(en)}_{2}}C{{l}_{2}}]Cl,\] the Cr atom is present as \[C{{r}^{3+}}.\] \[C{{r}^{3+}}=[Ar]\,3{{d}^{3}}4{{s}^{0}}\] \[cis[Cr{{(en)}_{2}}C{{l}_{2}}]Cl=[Ar]\]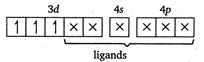 (en being a strong field ligand, can cause pairing.) Thus,3d orbitals are occupied. Since, two \[Cl\]atoms (monodentate) and two ettiylene diammine, ie, en molecules (didentate) are linked two the central atom, the coordination number of central atom \[=2+\underbrace{2\times 2}_{(due\,to\,en)}=6\]
(en being a strong field ligand, can cause pairing.) Thus,3d orbitals are occupied. Since, two \[Cl\]atoms (monodentate) and two ettiylene diammine, ie, en molecules (didentate) are linked two the central atom, the coordination number of central atom \[=2+\underbrace{2\times 2}_{(due\,to\,en)}=6\]
You need to login to perform this action.
You will be redirected in
3 sec
