A) allyl iodide
B) allyl alcohol
C) glycericacid
D) acrolein
Correct Answer: D
Solution :
Potassium hydrogen sulphate, \[\text{KHS}{{\text{O}}_{\text{4}}}\]works as a dehydrating agent at \[473\text{ }K-503\text{ }K\]and thus, removes water.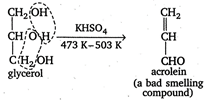
You need to login to perform this action.
You will be redirected in
3 sec
