A) \[m-\]bromophenol
B) mixture of ortho and para bromophenol
C) \[~p-\] bromophenol
D) 2, 4, 6-tribromophenol
Correct Answer: B
Solution :
In the presence of non-polar solvent like \[\text{CHC}{{\text{l}}_{\text{3}}}\]and at low temperature, phenoxide ion is not generated. Now, itis the phenol that takes part in the electrophilic substitution reaction and gives a mixture ofortho and para bromophenol.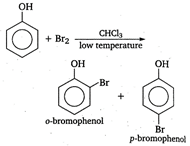
You need to login to perform this action.
You will be redirected in
3 sec
