A) \[s{{p}^{3}}d\]
B) \[s{{p}^{3}}{{d}^{2}}\]
C) \[{{d}^{2}}s{{p}^{2}}\]
D) \[s{{p}^{3}}\]
Correct Answer: A
Solution :
In \[\text{PC}{{\text{l}}_{\text{5}}}\]five chlorine atoms directly attached to central atom and it has no lone pairs and no single electrons hence\[\text{PC}{{\text{l}}_{\text{5}}}\]has\[\text{s}{{\text{p}}^{\text{3}}}\text{d}\] hybridization.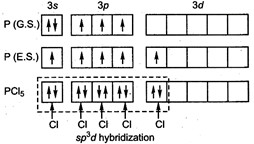
You need to login to perform this action.
You will be redirected in
3 sec
