A) there is no attractive or repulsive forces between molecules
B) attractive and repulsive forces between molecules are equal
C) attractive forces between molecules are predominant
D) repulsive forces between molecules are predominant
Correct Answer: C
Solution :
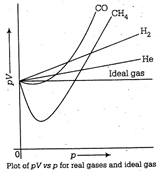 It can be seen easily that at constant temperature, pV vs p plot for real gases is not a straight line. There is a significant deviation from ideal behaviour. Two types of curves are seen. In the curves for dihydrogen and helium, as the pressure increases the value of pV also increases. The second type of plot is seen in the case of other gases like carbon monoxide and methane: In these plots, first there is a negative deviation from ideal behaviour, then pV value decreases with increase in pressure and reaches to a minimum value characteristic of gas. In second case, attractive forces between molecules are predominant. Compressibility factor (Z) is less than one.
It can be seen easily that at constant temperature, pV vs p plot for real gases is not a straight line. There is a significant deviation from ideal behaviour. Two types of curves are seen. In the curves for dihydrogen and helium, as the pressure increases the value of pV also increases. The second type of plot is seen in the case of other gases like carbon monoxide and methane: In these plots, first there is a negative deviation from ideal behaviour, then pV value decreases with increase in pressure and reaches to a minimum value characteristic of gas. In second case, attractive forces between molecules are predominant. Compressibility factor (Z) is less than one.
You need to login to perform this action.
You will be redirected in
3 sec
