A) \[PtC{{l}_{4}}\]
B) \[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}\]
C) \[Pt{{(N{{H}_{3}})}_{3}}Cl\]
D) \[Ni{{(N{{H}_{3}})}_{3}}Cl\]
Correct Answer: B
Solution :
Heteroleptic complexes with coordination numbers 4 and 6 show this type of isomerism. It arises due to the difference in the geometrical arrangement of the ligands around the central metal ion. When same ligands occupies the adjacent position in the polyhedra are known as cis-isomers, whereas when same ligands occupy the opposite position in the polyhedra are known as trans-isomers.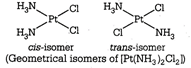 (Geometrical isomers of \[[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]
(Geometrical isomers of \[[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]
You need to login to perform this action.
You will be redirected in
3 sec
