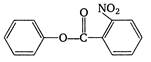
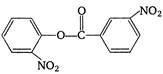
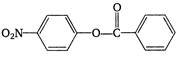
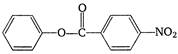
question_answer 1) The sound wave produced in a gas is always :
A)
longitudinal
done
clear
B)
transverse
done
clear
C)
stationary
done
clear
D)
electromagnetic
done
clear
View Answer play_arrow
question_answer 2) Source of sound and the observer are mutually at rest. If the speed of sound is changed, then the frequency of sound heard by the observer will appear to be:
A)
increased
done
clear
B)
decreased
done
clear
C)
unchanged
done
clear
D)
decreasing exponentially
done
clear
View Answer play_arrow
question_answer 3) With what velocity should an observer approach stationary sound source, so that the apparent frequency of sound appear to be double of the initial frequency? (given velocity of sound = v)
A)
\[{{v}_{0}}=\frac{v}{2}\]
done
clear
B)
\[{{v}_{0}}=3v\]
done
clear
C)
\[{{v}_{0}}=2v\]
done
clear
D)
\[{{v}_{0}}=v\]
done
clear
View Answer play_arrow
question_answer 4) A charge q is lying at mid-point of the line joining the two similar charges Q. The system will be in equilibrium, if the value of q is:
A)
\[\frac{Q}{2}\]
done
clear
B)
\[-\frac{Q}{2}\]
done
clear
C)
\[\frac{Q}{4}\]
done
clear
D)
\[-\frac{Q}{4}\]
done
clear
View Answer play_arrow
question_answer 5) Charges 2q, -q and - q lie at the vertices of a triangle. The value of E and V at the centroid of equilateral triangle will be :
A)
\[E\ne 0andV\ne 0\]
done
clear
B)
\[E=0andV=0\]
done
clear
C)
\[E\ne 0andV=0\]
done
clear
D)
\[E=0andV\ne 0\]
done
clear
View Answer play_arrow
question_answer 6) Infinite charges of magnitude q each are lying at x = 1, 2, 4, 8, ... metre on X-axis. The value of intensity of electric field at point x = 0 due to these charges will be :
A)
\[12\times {{10}^{9}}q\text{ }N/C\]
done
clear
B)
zero
done
clear
C)
\[6\times {{10}^{9}}q\text{ }N/C\]
done
clear
D)
\[4\times {{10}^{9}}q\text{ }N/C\]
done
clear
View Answer play_arrow
question_answer 7) The capacity of parallel plate capacitor in air and on immersing it into oil is 50\[\mu \]F and 110\[\mu \]F respectively. The dielectric constant of oil is :
A)
0.45
done
clear
B)
0.55
done
clear
C)
1.10
done
clear
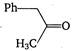
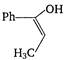
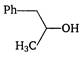
D)
2.20
done
clear
View Answer play_arrow
question_answer 8) The energy stored in a condenser is in the form of:
A)
kinetic energy
done
clear
B)
potential energy
done
clear
C)
elastic energy
done
clear
D)
magnetic energy
done
clear
View Answer play_arrow
question_answer 9) On increasing the plate separation of a charged condenser, the energy :
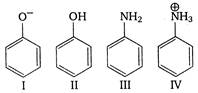
A)
increases
done
clear
B)
decreases
done
clear
C)
remains unchanged
done
clear
D)
becomes zero
done
clear
View Answer play_arrow
question_answer 10) The ratio of electric fields on the axis and at equator of an electric dipole will be :
A)
1 : 1
done
clear
B)
2 : 1
done
clear
C)
4 : 1
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 11) Some electric bulbs are connected in series across a 220 V supply in a room. If one bulb is fused, then remaining bulbs are connected again in series across the same supply. The illumination in the room will be :
A)
increase
done
clear
B)
decrease
done
clear
C)
remain the same
done
clear
D)
not continuous
done
clear
View Answer play_arrow
question_answer 12) If one junction of thermocouple is kept at 0°C and its emf is given by \[e=at+b{{t}^{2}},\] then the neutral temperature will be :
A)
\[\frac{a}{b}\]
done
clear
B)
\[-\frac{a}{b}\]b
done
clear
C)
\[\frac{a}{2b}\]
done
clear
D)
\[-\frac{a}{2b}\]
done
clear
View Answer play_arrow
question_answer 13) A cube is constructed from 12 identical wires. Current enters one corner of the cube and it leaves the opposite corner. If the resistance of each wire is r, then equivalent resistance will be :
A)
\[\frac{6r}{5}\]
done
clear
B)
\[\frac{5r}{6}\]
done
clear
C)
\[\frac{5r}{12}\]
done
clear
D)
\[\frac{12r}{5}\]
done
clear
View Answer play_arrow
question_answer 14) A source of emf E = 15 V and having negligible internal resistance, is connected to a variable resistance, so that the current in the circuit increases with time as 1 = 1.2t + 3. Then, the total charge that will flow in first 5 s will be:
A)
10C
done
clear
B)
20 C
done
clear
C)
30 C
done
clear
D)
40 C
done
clear
View Answer play_arrow
question_answer 15) The magnetic induction at the centre of a current carrying circular of radius r, is :
A)
directly proportional to r
done
clear
B)
inversely proportional to r
done
clear
C)
directly proportional to r2
done
clear
D)
inversely proportional to r2
done
clear
View Answer play_arrow
question_answer 16) A current carrying conductor produces :
A)
only electric field
done
clear
B)
only magnetic field
done
clear
C)
both electric and magnetic fields
done
clear
D)
neither electric nor magnetic field
done
clear
View Answer play_arrow
question_answer 17) A proton of energy 8 eV is moving in a circular path in a uniform magnetic field. The energy of an alpha particle moving in the same magnetic field and along the same path will be :
A)
4 eV
done
clear
B)
2 eV
done
clear
C)
8 eV
done
clear
D)
6 eV
done
clear
View Answer play_arrow
question_answer 18) Aim long wire is lying at right angles to the magnetic field. A force of 1 kg wt. is acting on it in a magnetic field of 0.98 T. The current flowing in it will be :
A)
100 A
done
clear
B)
10 A
done
clear
C)
1 A
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 19) The resultant magnetic moment of neon atom will be :
A)
infinity
done
clear
B)
\[{{\mu }_{B}}\]
done
clear
C)
zero
done
clear
D)
\[\frac{{{\mu }_{B}}}{2}\]
done
clear
View Answer play_arrow
question_answer 20) The temperature at which ferromagnetic material becomes paramagnetic is called a :
A)
neutral temperature
done
clear
B)
Curie temperature
done
clear
C)
inversion temperature
done
clear
D)
critical temperature
done
clear
View Answer play_arrow
question_answer 21) The ultimate individual unit of magnetism in any magnet is called :
A)
north pole
done
clear
B)
south pole
done
clear
C)
dipole
done
clear
D)
quadruple
done
clear
View Answer play_arrow
question_answer 22) The power loss in AC circuit will be minimum when :
A)
resistance is high, inductance is high
done
clear
B)
resistance is high, inductance is low
done
clear
C)
resistance is low, inductance is low
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 23) At high frequency, the capacitor offer :
A)
more reactance
done
clear
B)
less reactance
done
clear
C)
zero reactance
done
clear
D)
infinite reactance
done
clear
View Answer play_arrow
question_answer 24) The quantity that remain unchanged in transformer is :
A)
voltage
done
clear
B)
current
done
clear
C)
frequency
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 25) A piece of plane glass is placed on a word with letters of different colours. The letters which appear minimum raised are :
A)
red
done
clear
B)
green
done
clear
C)
yellow
done
clear
D)
violet
done
clear
View Answer play_arrow
question_answer 26) When light waves suffer reflection at the interface between air and glass, the change of phase of the reflected wave is equal to :
A)
zero
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\pi \]
done
clear
D)
\[2\pi \]
done
clear
View Answer play_arrow
question_answer 27) If the wavelength of light is \[4000\overset{\text{o}}{\mathop{\text{A}}}\,\], then the number of waves in 1 mm length will be :
A)
25
done
clear
B)
0.25
done
clear
C)
\[0.25\times 104\]
done
clear
D)
\[25\times 104\]
done
clear
View Answer play_arrow
question_answer 28) The wave theory of light was given by :
A)
Maxwell
done
clear
B)
Planck
done
clear
C)
Huygen
done
clear
D)
Young
done
clear
View Answer play_arrow
question_answer 29) In Young?s double slit experiment the amplitudes of two sources are 3a and a respectively. The ratio of intensities of bright and dark fringes will be :
A)
3 : 1
done
clear
B)
4 : 1
done
clear
C)
2 : 1
done
clear
D)
9:1
done
clear
View Answer play_arrow
question_answer 30) The diffraction effect can be observed in :
A)
only sound waves
done
clear
B)
only light waves
done
clear
C)
only ultrasonic waves
done
clear
D)
sound as well as light waves
done
clear
View Answer play_arrow
question_answer 31) At what distance from a convex lens of focal length 30 cm, an object should be placed, so that the size of the image both of the object?
A)
30 cm
done
clear
B)
60 cm
done
clear
C)
15 cm
done
clear
D)
90 cm
done
clear
View Answer play_arrow
question_answer 32) Two coherent sources of intensity ratio 1 : 4 produce an interference pattern. The fringe visibility will be :
A)
1
done
clear
B)
0.8
done
clear
C)
0.4
done
clear
D)
0.6
done
clear
View Answer play_arrow
question_answer 33) The specific charge of an electron is :
A)
\[1.6\times {{10}^{-19}}C\]
done
clear
B)
\[4.8\times {{10}^{-19}}stat-C\]
done
clear
C)
\[1.76\times {{10}^{-11}}C/kg\]
done
clear
D)
\[1.76\times {{10}^{11}}C/kg\]
done
clear
View Answer play_arrow
question_answer 34) The colour of the second line of Balmer series is :
A)
blue
done
clear
B)
yellow
done
clear
C)
red
done
clear
D)
violet
done
clear
View Answer play_arrow
question_answer 35) If elements with principal quantum number n > 4 were not allowed in nature, the number of possible elements would be :
A)
60
done
clear
B)
32
done
clear
C)
4
done
clear
D)
64
done
clear
View Answer play_arrow
question_answer 36) The energy of incident photons corresponding to maximum wavelength of visible light is :
A)
3.2 eV
done
clear
B)
7 eV
done
clear
C)
1.55 eV
done
clear
D)
1 eV
done
clear
View Answer play_arrow
question_answer 37) If the work function of potassium is 2 eV, then its photoelectric threshold wavelength is :
A)
310 nm
done
clear
B)
620 nm
done
clear
C)
6200 nm
done
clear
D)
3100 nm
done
clear
View Answer play_arrow
question_answer 38) Threshold wavelength for a metal 5200 A. The photo electrons will be ejected, if it is irradiated by light from:
A)
50 W infrared lamp
done
clear
B)
1 W infrared lamp
done
clear
C)
50 W ultraviolet lamp
done
clear
D)
0.5 W infrared lamp
done
clear
View Answer play_arrow
question_answer 39) An electron and a proton are accelerated through the same potential difference. The ratio of their de-Broglie wavelength will be :
A)
\[{{\left( \frac{{{m}_{p}}}{{{m}_{e}}} \right)}^{1/2}}\]
done
clear
B)
\[\frac{{{m}_{e}}}{{{m}_{p}}}\]
done
clear
C)
\[\frac{{{m}_{p}}}{{{m}_{e}}}\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 40) A particle with rest mass zero is moving with speed c. The de-Broglie wavelength associated with it:
A)
zero
done
clear
B)
infinity
done
clear
C)
\[\frac{hv}{c}\]
done
clear
D)
\[\frac{{{m}_{0}}c}{h}\]
done
clear
View Answer play_arrow
question_answer 41) The nearest distance between two atoms in case of a bcc lattice is equal to :
A)
\[\frac{a\sqrt{2}}{3}\]
done
clear
B)
\[\frac{a\sqrt{3}}{2}\]
done
clear
C)
\[a\sqrt{3}\]
done
clear
D)
\[\frac{a}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 42) Which of the following is an amorphous substance?
A)
Gold
done
clear
B)
Silver
done
clear
C)
Copper
done
clear
D)
Glass
done
clear
View Answer play_arrow
question_answer 43) The maximum efficiency of full wave rectifier is :
A)
100%
done
clear
B)
25.20%
done
clear
C)
40.6%
done
clear
D)
81.2%
done
clear
View Answer play_arrow
question_answer 44) In a npn-transistor, the collector current is 10 mA. If 90% of the electrons emitted reach the collector, then the emitter current will be :
A)
9 mA
done
clear
B)
11 mA
done
clear
C)
1 mA
done
clear
D)
0.1 mA
done
clear
View Answer play_arrow
question_answer 45)
The given truth table is of : A B X 0 0 0 0 1 1 1 0 1 1 1 1
A)
OR gate
done
clear
B)
AND gate
done
clear
C)
NOT gate
done
clear
D)
XOR gate
done
clear
View Answer play_arrow
question_answer 46) If force (F), length (I) and time (T) are assumed to be the fundamental units, then the dimensional formula of the mass will be :
A)
\[[F{{L}^{-1}}{{T}^{2}}]\]
done
clear
B)
\[[F{{L}^{-1}}{{T}^{-2}}]\]
done
clear
C)
\[[F{{L}^{-1}}{{T}^{-1}}]\]
done
clear
D)
\[[F{{L}^{2}}{{T}^{2}}]\]
done
clear
View Answer play_arrow
question_answer 47) The horizontal range of a projectile is \[4\sqrt{3}\]times its maximum height. Its angle of projection will be:
A)
\[45{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 48) Two balls of same size but the density of one is greater than that of the other are dropped from the same height, then which ball will reach the earth first (air resistance is negligible)?
A)
Heavy ball
done
clear
B)
Light ball
done
clear
C)
Both simultaneously
done
clear
D)
Will depend upon the density of the balls
done
clear
View Answer play_arrow
question_answer 49) A person moves 30 m north and then 20 m towards east and finally \[4\text{ }m/{{s}^{2}}\]in south-west direction. The displacement of the person from the origin will be :
A)
10 m along north
done
clear
B)
10 m along south
done
clear
C)
10 m along west
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 50) If the velocity of a particle is given by \[v={{(180-16\times )}^{1/2}}m/s,\] then its acceleration will be :
A)
zero
done
clear
B)
\[8m/{{s}^{2}}\]
done
clear
C)
\[-8\text{ }m/{{s}^{2}}\]
done
clear
D)
\[4\text{ }m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 51) Neglecting the air resistance, the time of flight of a projectile is determined by :
A)
\[{{U}_{vertical}}\]
done
clear
B)
\[{{U}_{horizontal}}\]
done
clear
C)
\[U=\mathsf{U}_{vertical}^{2}+\mathsf{U}_{horizontal}^{2}\]
done
clear
D)
\[U={{(\mathsf{U}_{vertical}^{2}+\mathsf{U}_{horizontal}^{2})}^{{}^{1}/{}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 52) A train is moving towards east and a car is along north, both with same speed. The observed direction of car to the passenger in the train is :
A)
east-north direction
done
clear
B)
west-north direction
done
clear
C)
south-east direction
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 53) A body moving with velocity v has momentum and kinetic energy numerically equal. What is the value of v?
A)
2m/s
done
clear
B)
42 m/s
done
clear
C)
1 m/s
done
clear
D)
0.2 m/s
done
clear
View Answer play_arrow
question_answer 54) If the kinetic energy of a body becomes four times, then its momentum will be :
A)
\[{{p}_{new}}=3{{p}_{initial}}\]
done
clear
B)
\[{{p}_{new}}=4{{p}_{initial}}\]
done
clear
C)
\[{{p}_{new}}=2{{p}_{initial}}\]
done
clear
D)
\[{{p}_{new}}={{p}_{initial}}\]
done
clear
View Answer play_arrow
question_answer 55) A block of mass 2 kg is lying on an inclined plane, inclined to the horizontal at 30°. If the coefficient of friction between the block and the plane is 0.7, then magnitude of frictional force acting on the block will be :
A)
11.9 N
done
clear
B)
1.19 N
done
clear
C)
0.19 N
done
clear
D)
1109 N
done
clear
View Answer play_arrow
question_answer 56) A ring of mass m and radius r is melted and then moulded into a sphere. The moment of inertia of the sphere will be :
A)
more than that of the ring
done
clear
B)
less than that of the ring
done
clear
C)
equal to that of the ring
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 57) A solid sphere and a hollow sphere of the same material and of a same size can be distinguished without weighing :
A)
by determining their moments of inertia about their coaxial axes
done
clear
B)
by rolling them simultaneously on an inclined plane
done
clear
C)
by rotating them about a common axis of rotation
done
clear
D)
by applying equal torque on them
done
clear
View Answer play_arrow
question_answer 58) Point masses 1, 2, 3 and 4 kg are lying at the point (0, 0, 0), (2, 0, 0), (0, 3, 0) and (-2, - 2, 0) respectively. The moment of inertia of this system about x-axis will be :
A)
\[43kg-{{m}^{2}}\]
done
clear
B)
\[34kg-{{m}^{2}}\]
done
clear
C)
\[27\text{ }kg-{{m}^{2}}\]
done
clear
D)
\[72kg-{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 59) The radius of gyration of a body about an axis at a distance 6 cm from its centre of mass is 10 cm. Then its radius of gyration about a parallel axis through its centre of mass will be:
A)
80 cm
done
clear
B)
8 cm
done
clear
C)
0.8 cm
done
clear
D)
80 m
done
clear
View Answer play_arrow
question_answer 60) Two planets of radii in the ratio 2 : 3 are made from the material of density in the ratio 3 : 2. Then, the ratio of acceleration due to gravity\[\frac{{{g}_{1}}}{{{g}_{2}}}\] at the surface of the two planets will be :
A)
1
done
clear
B)
2.25
done
clear
C)
4/9
done
clear
D)
0.12
done
clear
View Answer play_arrow
question_answer 61) If the radius of the earth contracts to half of its present day value without change in mass, then the length of the day will be :
A)
24 h
done
clear
B)
48 h
done
clear
C)
6 h
done
clear
D)
12 h
done
clear
View Answer play_arrow
question_answer 62) A person will get more quantity of matter in kg-wt at:
A)
poles
done
clear
B)
at latitude of 60°
done
clear
C)
equator
done
clear
D)
satellite
done
clear
View Answer play_arrow
question_answer 63) The unit of the coefficient of viscosity in SI system is :
A)
\[m/kg-s\]
done
clear
B)
\[m-s/k{{g}^{2}}\]
done
clear
C)
\[kg/m-{{s}^{2}}\]
done
clear
D)
\[kg/m-s\]
done
clear
View Answer play_arrow
question_answer 64) If the excess pressure inside a soap bubble is balanced by oil column of height 2 mm, then the surface tension of soap solution will be : (r = 1 cm and density d = 0.8 g/ cc)
A)
\[3.9\text{ }N/m\]
done
clear
B)
\[3.9\times {{10}^{-1}}N/m\]
done
clear
C)
\[3.9\times {{10}^{-2}}N/m\]
done
clear
D)
\[3.9\text{ }dyne/m\]
done
clear
View Answer play_arrow
question_answer 65) 50. A vessel, whose bottom has round holes with diameter of 1 mm is filled with water. Assuming that surface tension acts only at holes. Then, the maximum height to which the water can be filled in vessel without leakage is : (surface tension of water is \[75\times {{10}^{-3}}N/m\]and \[g=10\text{ }m/{{s}^{2}}\])
A)
3 cm
done
clear
B)
0.3 cm
done
clear
C)
3 mm
done
clear
D)
3 m
done
clear
View Answer play_arrow
question_answer 66) For which of the two pairs, the angle of contact is same?
A)
Water and glass, glass and mercury
done
clear
B)
Pure water and glass, glass and alcohol
done
clear
C)
Silver and water, mercury and glass
done
clear
D)
Silver and chromium, water and chromium
done
clear
View Answer play_arrow
question_answer 67) At what temperature the rms velocity of helium molecules will be equal to that of hydrogen moelcules at NTP?
A)
844 K
done
clear
B)
64 K
done
clear
C)
\[273{}^\circ C\]
done
clear
D)
273 K
done
clear
View Answer play_arrow
question_answer 68) Which of the following is unique function of initial and final states?
A)
dQ
done
clear
B)
dW
done
clear
C)
dU
done
clear
D)
AQ and AW
done
clear
View Answer play_arrow
question_answer 69) If the initial temperatures of metallic sphere and disc of same radius and nature are equal, then the ratio of their rate of cooling will be :
A)
1 : 4
done
clear
B)
4 : 1
done
clear
C)
1 : 2
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 70) What will be the ratio of temperatures of sun and moon, if the wavelengths of their maximum emission radiations rates are 140 A and \[4200\overset{\text{o}}{\mathop{\text{A}}}\,\] respectively?
A)
1 : 30
done
clear
B)
30 : 1
done
clear
C)
42 : 14
done
clear
D)
14 : 42
done
clear
View Answer play_arrow
question_answer 71) A bar magnet is oscillating in the earth?s magnetic field with time period T. If its mass is increased four times, then its time period will be :
A)
4T
done
clear
B)
2T
done
clear
C)
T
done
clear
D)
\[\frac{T}{2}\]
done
clear
View Answer play_arrow
question_answer 72) The time period of a simple pendulum, when it is made to oscillate on the surface of moon :
A)
increases
done
clear
B)
decreases
done
clear
C)
remains unchanged
done
clear
D)
becomes infinite
done
clear
View Answer play_arrow
question_answer 73) A condenser of capacity 20\[\mu \]F is first charged and then discharged through a 10 mH inductance. Neglecting the resistance of the coil, the frequency of the resulting vibrations will be :
A)
\[356\text{ }cycle/h\]
done
clear
B)
\[356\text{ }cycle/s\]
done
clear
C)
\[356\times {{10}^{3}}cycle/s\]
done
clear
D)
\[3.56\text{ }cycle/s\]
done
clear
View Answer play_arrow
question_answer 74) Infinite springs with force constants k, 2k, 4/c and 8 k ... respectively are connected in series. The effective force constant of the spring will be :
A)
2k
done
clear
B)
k
done
clear
C)
\[\frac{k}{2}\]
done
clear
D)
2048
done
clear
View Answer play_arrow
question_answer 75) The intensity of sound gets reduced by 10% on passing through a slab. The reduction in intensity on passage through three consecutive slabs is :
A)
30%
done
clear
B)
27.1%
done
clear
C)
20%
done
clear
D)
36%
done
clear
View Answer play_arrow
question_answer 76) The hydrogen electrode is dipped in a solution of\[pH=3\]at\[{{25}^{o}}C\]. The potential of the cell would be (the value of 2.303 RT/E is 0.059 V)
A)
0.177V
done
clear
B)
0.087V
done
clear
C)
\[-0.177V\]
done
clear
D)
0.059V
done
clear
View Answer play_arrow
question_answer 77) Specific conductivity of a solution:
A)
increases with dilution
done
clear
B)
decreases with dilution
done
clear
C)
remains unchanged with dilution
done
clear
D)
depends on mass of electrolyte
done
clear
View Answer play_arrow
question_answer 78) 1 mol of\[{{H}_{2}}S{{O}_{4}}\]is mixed with 2 moles of\[NaOH\]. The heat evolved will be:
A)
57.3 kJ
done
clear
B)
\[2\times 57.3kJ\]
done
clear
C)
57.3/2kJ
done
clear
D)
cannot be predicted
done
clear
View Answer play_arrow
question_answer 79) In a reversible process,\[\Delta {{S}_{system}}+\Delta {{S}_{surrounding}}\]is:
A)
\[>0\]
done
clear
B)
\[<0\]
done
clear
C)
\[\ge 0\]
done
clear
D)
\[=0\]
done
clear
View Answer play_arrow
question_answer 80) For the reaction,\[{{N}_{2}}+3{{H}_{2}}2N{{H}_{3}};\Delta H=?\]
A)
\[\Delta E+2RT\]
done
clear
B)
\[\Delta E-2RT\]
done
clear
C)
\[\Delta E+RT\]
done
clear
D)
\[\Delta E-RT\]
done
clear
View Answer play_arrow
question_answer 81) One mole of a perfect gas expands isothermally to ten times its original volume. The change in entropy is:
A)
0.1 R
done
clear
B)
2.303 R
done
clear
C)
10.0 R
done
clear
D)
100.0 R
done
clear
View Answer play_arrow
question_answer 82) Which of the following solutions will have the highest boiling point?
A)
\[0.1\text{ }M\text{ }FeC{{l}_{3}}\]
done
clear
B)
\[0.1\text{ }M\text{ }BaC{{l}_{2}}\]
done
clear
C)
\[0.1\text{ }M\text{ }NaCl\]
done
clear
D)
\[0.1\text{ }M\text{ }urea\]
done
clear
View Answer play_arrow
question_answer 83) Maximum freezing point falls in:
A)
camphor
done
clear
B)
naphthalene
done
clear
C)
benzene
done
clear
D)
water
done
clear
View Answer play_arrow
question_answer 84) Azeotropic mixture of\[HCl\]and water has:
A)
48% \[HCl\]
done
clear
B)
22.2%\[HCl\]
done
clear
C)
36% \[HCl\]
done
clear
D)
20.2%\[HCl\]
done
clear
View Answer play_arrow
question_answer 85) Vapour pressure of dilute aqueous solution of glucose is 750 mm of mercury at 373 K. The mole fraction of solute is:
A)
\[\frac{1}{76}\]
done
clear
B)
\[\frac{1}{7.6}\]
done
clear
C)
\[\frac{1}{38}\]
done
clear
D)
\[\frac{1}{10}\]
done
clear
View Answer play_arrow
question_answer 86) Volume of\[0.1\text{ }M\text{ }{{\text{K}}_{2}}C{{r}_{2}}{{O}_{7}}\] required to oxidize 35 mL of\[0.5\text{ }M\text{ }FeS{{O}_{4}}\]solution is:
A)
29.2 mL
done
clear
B)
17.5 mL
done
clear
C)
175 mL
done
clear
D)
145 mL
done
clear
View Answer play_arrow
question_answer 87) 100 cc of\[0.6\text{ }N\text{ }{{H}_{2}}S{{O}_{4}}\]and 200 cc of\[0.3\text{ }N\text{ }HCl\]were mixed together. The normality of the -solution will be:
A)
0.2 N
done
clear
B)
0.4 N
done
clear
C)
0.8 N
done
clear
D)
0.6 N
done
clear
View Answer play_arrow
question_answer 88) The rate of diffusion of a gas is proportional to:
A)
\[\frac{P}{\sqrt{d}}\]
done
clear
B)
\[\sqrt{\frac{P}{d}}\]
done
clear
C)
\[\frac{P}{d}\]
done
clear
D)
\[\frac{\sqrt{P}}{d}\]
done
clear
View Answer play_arrow
question_answer 89) Molar volume of\[C{{O}_{3}}\]is maximum at:
A)
NTP
done
clear
B)
\[{{0}^{o}}C\]and 2.0 arm
done
clear
C)
\[{{127}^{o}}C\]and 1 atm
done
clear
D)
\[{{273}^{o}}C\]and 2.0 atm
done
clear
View Answer play_arrow
question_answer 90) Number of atoms of oxygen present in 10.6 g of\[N{{a}_{2}}C{{O}_{3}}\]will be:
A)
\[6.02\times {{10}^{23}}\]
done
clear
B)
\[12.04\times {{10}^{22}}\]
done
clear
C)
\[1.806\times {{10}^{23}}\]
done
clear
D)
\[31.80\times {{10}^{28}}\]
done
clear
View Answer play_arrow
question_answer 91) The equilibrium \[{{P}_{4}}(s)+6C{{l}_{2}}(g)4PC{{l}_{3}}(g)\]is attained by mixing equal moles of\[{{P}_{4}}\]and\[C{{l}_{2}}\]in an evacuated vessel. Then at equilibrium is:
A)
\[[C{{l}_{2}}]>[PC{{l}_{3}}]\]
done
clear
B)
\[[C{{l}_{2}}]>[{{P}_{4}}]\]
done
clear
C)
\[[{{P}_{4}}]>[C{{l}_{2}}]\]
done
clear
D)
\[[PC{{l}_{3}}]>[{{P}_{4}}]\]
done
clear
View Answer play_arrow
question_answer 92) The activation energy for most of the reactions is approximately\[50\,kJ\,mo{{l}^{-1}}\]. The value of temperature coefficient for such reactions is:
A)
> 2
done
clear
B)
> 3
done
clear
C)
< 1
done
clear
D)
> 4
done
clear
View Answer play_arrow
question_answer 93) If the mass defect of\[_{4}^{9}X\]is 0.090 amu, then binding energy per nucleon is: (1 amu =931.5 MeV)
A)
9.315 MeV
done
clear
B)
931.5 MeV
done
clear
C)
83.0 MeV
done
clear
D)
8.38 MeV
done
clear
View Answer play_arrow
question_answer 94) 50 mL of\[0.1\text{ }M\text{ }HCl\]and 50 mL of\[0.2\text{ }M\text{ }NaOH\]are mixed. The pH of the resulting solution is:
A)
1.30
done
clear
B)
4.2
done
clear
C)
12.70
done
clear
D)
11.70
done
clear
View Answer play_arrow
question_answer 95) A substance\[{{A}_{x}}{{B}_{y}}\]crystallises in a face centred cubic lattice in which A atom occupies each corner of cube and atom B occupies the centres of each face of the cube. Identify the correct composition of the substance\[{{A}_{x}}{{B}_{y}}\]
A)
\[A{{B}_{3}}\]
done
clear
B)
\[{{A}_{4}}{{B}_{3}}\]
done
clear
C)
\[{{A}_{3}}B\]
done
clear
D)
composition cannot be specified
done
clear
View Answer play_arrow
question_answer 96) In coagulating the colloidal solution of\[A{{s}_{2}}{{S}_{3}}\]which has the maximum coagulating value?
A)
\[NaCl\]
done
clear
B)
\[KCl\]
done
clear
C)
\[BaC{{l}_{2}}\]
done
clear
D)
\[AlC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 97) Which of the following is the strongest oxidising agent?
A)
\[HOCl\]
done
clear
B)
\[HCl{{O}_{2}}\]
done
clear
C)
\[HCl{{O}_{3}}\]
done
clear
D)
\[HCl{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 98) In the equation \[4M+8C{{N}^{-}}+2{{H}_{2}}O+{{O}_{2}}\xrightarrow{{}}4{{[M{{(CN)}_{2}}]}^{-}}\] \[+4O{{H}^{-}}\]Identify the metal M. It is
A)
copper
done
clear
B)
iron
done
clear
C)
silver
done
clear
D)
zinc
done
clear
View Answer play_arrow
question_answer 99) The formula of azurite is:
A)
\[CuC{{O}_{3}}-Cu{{(OH)}_{2}}\]
done
clear
B)
\[2CuC{{O}_{3}}-Cu{{(OH)}_{2}}\]
done
clear
C)
\[CuC{{O}_{3}}-2Cu{{(OH)}_{2}}\]
done
clear
D)
\[CuS{{O}_{4}}-Cu{{(OH)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 100) The decreasing order of bond angle is:
A)
\[N{{O}_{2}}>NO_{2}^{+}>NO_{2}^{-}\]
done
clear
B)
\[NO_{2}^{-}>N{{O}_{2}}>NO_{2}^{+}\]
done
clear
C)
\[NO_{2}^{+}>N{{O}_{2}}>NO_{2}^{-}\]
done
clear
D)
\[NO_{2}^{+}>NO_{2}^{-}>N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 101) The fresh precipitate can be transformed in colloidal state by:
A)
peptization
done
clear
B)
coagulation
done
clear
C)
diffusion
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 102) Milk is:
A)
fat dispersed in water
done
clear
B)
fat dispersed in milk
done
clear
C)
fat dispersed in fat
done
clear
D)
water dispersed in milk
done
clear
View Answer play_arrow
question_answer 103) Purest form of iron is:
A)
cast iron
done
clear
B)
pig iron
done
clear
C)
wrought iron
done
clear
D)
steel
done
clear
View Answer play_arrow
question_answer 104) Most unstable hydride is:
A)
\[N{{H}_{3}}\]
done
clear
B)
\[P{{H}_{3}}\]
done
clear
C)
\[As{{H}_{3}}\]
done
clear
D)
\[Bi{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 105) Out of the following metals that cannot be obtained by electrolysis of the aqueous solution of their salts are:
A)
Ag
done
clear
B)
Cr
done
clear
C)
Cu
done
clear
D)
Mg
done
clear
View Answer play_arrow
question_answer 106) \[KI\]and\[CuS{{O}_{4}}\]solution when mixed gives:
A)
\[Cu{{l}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[C{{u}_{2}}{{l}_{2}}+{{K}_{2}}S{{O}_{4}}\]
done
clear
C)
\[K{{ }_{2}}S{{O}_{4}}+C{{u}_{2}}{{I}_{2}}+{{I}_{2}}\]
done
clear
D)
\[{{K}_{2}}S{{O}_{4}}+Cu{{I}_{2}}+{{I}_{2}}\]
done
clear
View Answer play_arrow
question_answer 107) The strongest reducing agent among the following is:
A)
\[{{F}^{-}}\]
done
clear
B)
\[C{{l}^{-}}\]
done
clear
C)
\[B{{r}^{-}}\]
done
clear
D)
\[{{I}^{-}}\]
done
clear
View Answer play_arrow
question_answer 108) \[Xe{{F}_{6}}\]on complete hydrolysis gives:
A)
\[Xe\]
done
clear
B)
\[Xe{{O}_{2}}\]
done
clear
C)
\[Xe{{O}_{3}}\]
done
clear
D)
\[Xe{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 109) The correct name of the compound \[[Cu{{(N{{H}_{3}})}_{4}}]{{(N{{O}_{3}})}_{2}},\]according to IUPAC system is:
A)
cuprammonium nitrate
done
clear
B)
tetrammine copper (II) dinitrate
done
clear
C)
tetrammine copper (II) nitrate
done
clear
D)
tetrammine copper (II) dinitrite
done
clear
View Answer play_arrow
question_answer 110) Which of the following complex species does not involve inner orbital hybridisation?
A)
\[{{[Co{{F}_{6}}]}^{3-}}\]
done
clear
B)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
C)
\[{{[Fe{{(CN)}_{6}}]}^{3-}}\]
done
clear
D)
\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 111) \[_{27}C{{o}^{60}}\]is radioactive because:
A)
its atomic number is high
done
clear
B)
it has high\[\frac{p}{n}\]ratio
done
clear
C)
it has high\[\frac{n}{p}\]ratio
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 112) The correct order of solubility of the sulphates of alkaline earth metals in water is:
A)
\[Be>Ca>Mg>Ba>Sr\]
done
clear
B)
\[Mg>Be>Ba>Ca>Sr\]
done
clear
C)
\[Be>Mg>Ca>Sr>Ba\]
done
clear
D)
\[Mg>Ca>Ba>Be>Sr\]
done
clear
View Answer play_arrow
question_answer 113) Correct order of radii is:
A)
\[N<Be<B\]
done
clear
B)
\[{{F}^{-}}<{{O}^{2-}}<{{N}^{3-}}\]
done
clear
C)
\[Na<Li<K\]
done
clear
D)
\[F{{e}^{3+}}<F{{e}^{2+}}<F{{e}^{4+}}\]
done
clear
View Answer play_arrow
question_answer 114) A sudden large jump between the values of first and second ionisation energies of elements would be associated with which of the following electronic configurations?
A)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}\]
done
clear
B)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{1}}\]
done
clear
C)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}3{{p}^{2}}\]
done
clear
D)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 115) Which one shows most pronounced inert pair effect?
A)
\[Si\]
done
clear
B)
\[Sn\]
done
clear
C)
\[Pb\]
done
clear
D)
\[C\]
done
clear
View Answer play_arrow
question_answer 116) Which of the following will form a colorless complex?
A)
\[N{{i}^{2+}}\]
done
clear
B)
\[C{{u}^{+}}\]
done
clear
C)
\[T{{i}^{2+}}\]
done
clear
D)
\[F{{e}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 117) Silver containing lead as impurity is purified by:
A)
poling
done
clear
B)
cupellation
done
clear
C)
lavigation
done
clear
D)
distillation
done
clear
View Answer play_arrow
question_answer 118) The metal extracted by cyanide process is:
A)
silver
done
clear
B)
copper
done
clear
C)
iron
done
clear
D)
sodium
done
clear
View Answer play_arrow
question_answer 119) On the extraction of iron, the slag produced is:
A)
\[CO\]
done
clear
B)
\[FeSi{{O}_{3}}\]
done
clear
C)
\[MgSi{{O}_{3}}\]
done
clear
D)
\[CaSi{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 120) Complex forming tendency is more for:
A)
\[N{{a}^{+}}\]
done
clear
B)
\[{{K}^{+}}\]
done
clear
C)
\[L{{i}^{+}}\]
done
clear
D)
\[R{{b}^{+}}\]
done
clear
View Answer play_arrow
question_answer 121)
In the reaction:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 122)
The IUPAC name of the following compound is:
A)
propionic anhydride
done
clear
B)
dipropanoic anhydride
done
clear
C)
ethoxy propanoic acid
done
clear
D)
propanoic anhydride
done
clear
View Answer play_arrow
question_answer 123) Which of the following compounds is not aromatic?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 124) Which of the following is the most stable cation?
A)
\[{{F}_{3}}C-CH_{2}^{\oplus }\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}C{{H}^{\oplus }}\]
done
clear
C)
\[CH_{3}^{\oplus }\]
done
clear
D)
\[CF_{3}^{\oplus }\]
done
clear
View Answer play_arrow
question_answer 125)
The product A is:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 126) Tautomerism is not exhibited by:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 127)
In the compound
A)
S, S
done
clear
B)
R, S
done
clear
C)
S, R
done
clear
D)
R, R
done
clear
View Answer play_arrow
question_answer 128)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 129) Hinsberg reagent is:
A)
\[{{C}_{6}}{{H}_{5}}S{{O}_{3}}H\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}NO\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}{{N}_{2}}Cl\]
done
clear
View Answer play_arrow
question_answer 130) Acetaldehyde cannot show:
A)
iodoform test
done
clear
B)
Lucas test
done
clear
C)
Benedict's test
done
clear
D)
Tollen's test
done
clear
View Answer play_arrow
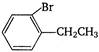
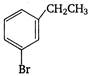
question_answer 131) Ethylbenzene with bromine in presence of \[FeB{{r}_{3}},\]predominantly gives:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
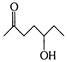
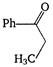
question_answer 132) Which of the following will be most readily dehydrated under acidic conditions?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 133) Which of the following cannot reduce Fehling solution?
A)
\[HCOOH\]
done
clear
B)
\[{{H}_{3}}CCOOH\]
done
clear
C)
\[HCHO\]
done
clear
D)
\[{{H}_{3}}CCHO\]
done
clear
View Answer play_arrow
question_answer 134) Absolute alcohol is prepared by:
A)
vacuum distillation
done
clear
B)
azeotropic distillation
done
clear
C)
steam distillation
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 135) Which of the following compounds is resistant to nucleophilic attack by hydroxyl ion?
A)
Methylacetate
done
clear
B)
Acetonitrile
done
clear
C)
Acetamide
done
clear
D)
Diethylether
done
clear
View Answer play_arrow
question_answer 136) Hydrogenation of benzoyl chloride in presence of Pd on\[BaS{{O}_{4}}\]gives:
A)
benzyl alcohol
done
clear
B)
benzaldehyde
done
clear
C)
benzoic acid
done
clear
D)
phenol
done
clear
View Answer play_arrow
question_answer 137) \[Ph-C\equiv C-C{{H}_{3}}\xrightarrow[{}]{H{{g}^{2+}}{{H}^{+}}}A\] The product A is:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 138) Ethylamine reacts with nitrous acid to form:
A)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}OH,{{N}_{2}},{{H}_{2}}O\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}N_{2}^{+}C{{l}^{-}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}NHOH,N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 139) Rice is deficient in:
A)
lysine
done
clear
B)
alanine
done
clear
C)
glycine
done
clear
D)
leucine
done
clear
View Answer play_arrow
question_answer 140) Mutarotation does not occur in:
A)
sucrose
done
clear
B)
D-glucose
done
clear
C)
L-glucose
done
clear
D)
none of-these
done
clear
View Answer play_arrow
question_answer 141) Aldehyde which is formed during photo synthesis of plants is:
A)
methanol
done
clear
B)
acetaldehyde
done
clear
C)
propanal
done
clear
D)
phenylmethanal
done
clear
View Answer play_arrow
question_answer 142)
Coupling of diazonium salts of following takes place in the order:
A)
\[IV\text{ }<\text{ }II\text{ }<\text{ }III\text{ }<\text{ }I\]
done
clear
B)
\[IV\text{ }>\text{ }III\text{ }<\text{ }II\text{ }<\text{ }I\]
done
clear
C)
\[II\text{ }<\text{ }IV\text{ }<\text{ }I\text{ }<\text{ }III\]
done
clear
D)
\[I\text{ }<\text{ }II\text{ }<\text{ }III\text{ }<\text{ }IV\]
done
clear
View Answer play_arrow
question_answer 143) Which is decreasing order of strength of bases? \[\overline{O}H.\overline{N}{{H}_{2}},HC\equiv {{C}^{-}}\]and \[C{{H}_{3}}CH_{2}^{-}\]
A)
\[{{H}_{3}}CCH_{2}^{-}>NH_{2}^{-}>HC\equiv {{C}^{-}}>O{{H}^{-}}\]
done
clear
B)
\[HC\equiv {{C}^{-}}>C{{H}_{3}}CH_{2}^{-}>NH_{2}^{-}>O{{H}^{-}}\]
done
clear
C)
\[O{{H}^{-}}>NH_{2}^{-}>CH\equiv {{C}^{-}}>{{H}_{3}}CCH_{2}^{-}\]
done
clear
D)
\[NH_{2}^{-}>HC\equiv {{C}^{-}}>O{{H}^{-}}>{{H}_{3}}CCH_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 144) The reagent that reacts with nitromethane to form methyl hydroxylamine is:
A)
\[Zn/HCl\]
done
clear
B)
\[Zn/N{{H}_{4}}Cl\]
done
clear
C)
\[Zn/NaOH\]
done
clear
D)
\[Sn/HCl\]
done
clear
View Answer play_arrow
question_answer 145) Which is most basic?
A)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
B)
\[{{({{C}_{6}}{{H}_{5}})}_{2}}NH\]
done
clear
C)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}NH\]
done
clear
View Answer play_arrow
question_answer 146) For d-electron, the orbital angular momentum
A)
\[\frac{\sqrt{6}h}{2\pi }\]
done
clear
B)
\[\frac{\sqrt{2}h}{2\pi }\]
done
clear
C)
\[\frac{h}{2\pi }\]
done
clear
D)
\[\frac{2h}{\pi }\]
done
clear
View Answer play_arrow
question_answer 147) Two nodal planes are present in:
A)
\[{{\pi }^{*}}2{{p}_{x}}\]
done
clear
B)
\[\sigma 2{{p}_{z}}\]
done
clear
C)
\[\pi \,2{{p}_{x}}\]
done
clear
D)
\[\pi \,2{{p}_{y}}\]
done
clear
View Answer play_arrow
question_answer 148) One gram mole of a gas at NTP occupies 22.4 L. This fact was derived from:
A)
law of gaseous volumes
done
clear
B)
Avogadro's hypothesis
done
clear
C)
Berzelius hypothesis
done
clear
D)
Dalton's atomic theory
done
clear
View Answer play_arrow
question_answer 149) At\[{{90}^{o}}C,\]pure water has\[[{{H}_{3}}{{O}^{+}}]={{10}^{-6}}mol/L.\] The value of\[{{K}_{w}}\]at\[{{90}^{o}}C\]is:
A)
\[{{10}^{-6}}\]
done
clear
B)
\[{{10}^{-8}}\]
done
clear
C)
\[{{10}^{-12}}\]
done
clear
D)
\[{{10}^{-14}}\] In pure water: \[[{{H}^{+}}]=[O{{H}^{-}}]\] \[{{K}_{w}}=[{{H}^{+}}][O{{H}^{-}}]\] \[{{K}_{w}}={{10}^{-6}}\times {{10}^{-6}}={{10}^{-12}}\]
done
clear
View Answer play_arrow
question_answer 150) The pH of a buffer solution of\[0.1\text{ }M\text{ }N{{H}_{4}}OH\] \[[p{{K}_{b}}=5.0]\]and\[0.01\text{ }M\text{ }N{{H}_{4}}Cl\]is:
A)
1
done
clear
B)
4
done
clear
C)
10
done
clear
D)
13
done
clear
View Answer play_arrow
question_answer 151) In prokaryotic cells:
A)
internal compartments are absent
done
clear
B)
nucleus is absent
done
clear
C)
ribosomes are 70S
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 152) Plant cell may be without:
A)
plastids
done
clear
B)
vacuoles
done
clear
C)
centrioles
done
clear
D)
cell wall
done
clear
View Answer play_arrow
question_answer 153) Middle lamella mainly contains :
A)
Ca
done
clear
B)
Mg
done
clear
C)
Na
done
clear
D)
K
done
clear
View Answer play_arrow
question_answer 154) Which is a component of chlorophyll?
A)
Mg
done
clear
B)
Mn
done
clear
C)
Fe
done
clear
D)
Zn
done
clear
View Answer play_arrow
question_answer 155) Chemical nature of cellulose is :
A)
polypeptide
done
clear
B)
polysaccharide
done
clear
C)
polynucleotide
done
clear
D)
disaccharide
done
clear
View Answer play_arrow
question_answer 156) Cilia are :
A)
short (5-10 \[\mu \]m) hair like narrow protoplasmic processes
done
clear
B)
with sweeping or pendular movements
done
clear
C)
more numerous
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 157) Which is not properly paired?
A)
Golgi apparatus-breaking of complex macromolecules
done
clear
B)
Endoplasmic reticulum-protein synthesis
done
clear
C)
Chloroplast-photosynthesis
done
clear
D)
Mitochondria-oxidative phosphorylation
done
clear
View Answer play_arrow
question_answer 158) What is true about fluid mosaic model?
A)
Phospholipid layer is sandwiched between two protein layers
done
clear
B)
Phospholipid monolayer is present on the top of a protein layer
done
clear
C)
Phospholipid bilayer is present on the top of a protein layer
done
clear
D)
Proteins as embedded at places in the phospholipid bilayer
done
clear
View Answer play_arrow
question_answer 159) Besides proteins ribosomes contain :
A)
DNA
done
clear
B)
RNA
done
clear
C)
both (a) and (b)
done
clear
D)
lipids
done
clear
View Answer play_arrow
question_answer 160) Which of the following is a correct statement?
A)
Orchids has palmate fleshy roots
done
clear
B)
Pandanus has stilt roots
done
clear
C)
Sweet potato has root tubers
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 161) Phylloclades are :
A)
green, photosynthetic, succulent stems of indefinite growth
done
clear
B)
one internode long stems
done
clear
C)
leaf modifications
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 162) The bladders of Utricularia and pitchers of Nepenthes are modification of :
A)
stems
done
clear
B)
leaves
done
clear
C)
roots
done
clear
D)
flowers
done
clear
View Answer play_arrow
question_answer 163) Intercalary meristem occurs in :
A)
mint
done
clear
B)
grasses
done
clear
C)
both (a) & (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 164) Vascular cambium of stem is :
A)
partly primary and partly secondary meristem
done
clear
B)
primary meristem
done
clear
C)
secondary meristem
done
clear
D)
intercalary meristem
done
clear
View Answer play_arrow
question_answer 165) A tree grows at the rate of 0.5 m per year. What will be the height of the board fixed at 1.5 m above the base five years ago?
A)
4.0 m
done
clear
B)
3.5 m
done
clear
C)
1.5 m
done
clear
D)
4.5 m
done
clear
View Answer play_arrow
question_answer 166) Fibres associated with phloem are fibres.
A)
hard
done
clear
B)
wood
done
clear
C)
surface
done
clear
D)
bast
done
clear
View Answer play_arrow
question_answer 167) Which of the following statements is correct?
A)
DPD = OP ? WP
done
clear
B)
DPD = OP + WP
done
clear
C)
DPD = WP ? OP
done
clear
D)
DPD = TP + OP
done
clear
View Answer play_arrow
question_answer 168) Which of the following are responsible for opening and closing of stomata?
A)
Rise in pH of guard cells causes hydrolysis of starch
done
clear
B)
Cytokinin and cyclic AMP are required
done
clear
C)
Abscisic acid promotes closure
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 169) Passive absorption of mineral salts is not dependent of:
A)
diffusion
done
clear
B)
osmosis
done
clear
C)
donnan equilibrium
done
clear
D)
ionic exchange
done
clear
View Answer play_arrow
question_answer 170) In \[{{C}_{4}}\] plants synthesis of sugars/final \[C{{O}_{2}}\] fixation occurs in :
A)
epidermis cells
done
clear
B)
spongy cells
done
clear
C)
undifferentiated mesophyll cells
done
clear
D)
bundle sheath cells
done
clear
View Answer play_arrow
question_answer 171) Dichlorophenyl dimethyl urea (DCMU) :
A)
inhibits \[{{O}_{2}}\] evolution and non-cyclic photophosphyorylation
done
clear
B)
promotes \[{{O}_{2}}\]evolution and non-cyclic photophosphorylation
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 172) Auxins promote :
A)
cell growth and enlargement
done
clear
B)
cambial activity
done
clear
C)
apical dominance
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 173) Tendrils exhibit:
A)
thigmotropism
done
clear
B)
seismonasty
done
clear
C)
heliotropism
done
clear
D)
diageotropism
done
clear
View Answer play_arrow
question_answer 174) Chlamydomonas nivalis is responsible for :
A)
red snow
done
clear
B)
red rust of tea
done
clear
C)
yellow snow
done
clear
D)
brown snow
done
clear
View Answer play_arrow
question_answer 175) The hyphae of Rhizopus are :
A)
unbranched, aseptate and uninucleate
done
clear
B)
branched, aseptate and muitinucleate
done
clear
C)
branched, septate and uninucleate
done
clear
D)
unbranched, septate and coenocytic
done
clear
View Answer play_arrow
question_answer 176) Dryopteris differs from Funaria in having :
A)
an independent gametophyte
done
clear
B)
an independent sporophyte
done
clear
C)
swimming antherozoids
done
clear
D)
archegonia
done
clear
View Answer play_arrow
question_answer 177) The number of integuments in the seeds of Pinus is :
A)
one
done
clear
B)
two
done
clear
C)
three
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 178) A flower which can be divided into equal vertical halves by more than one plane of division is :
A)
actinomorphic
done
clear
B)
zygomorphic
done
clear
C)
heteromorphic
done
clear
D)
cyclic
done
clear
View Answer play_arrow
question_answer 179) Which of the following pairs is not correct ?
A)
Corymb-candytuft
done
clear
B)
Capitulum-sunflower
done
clear
C)
Catkin-mulberry
done
clear
D)
Raceme-wheat
done
clear
View Answer play_arrow
question_answer 180) Devices for self-pollination are :
A)
dicliny or unisexuality
done
clear
B)
dichogamy
done
clear
C)
heterostyly
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 181) In mango-(Mangifera indica) the edible portion of fruit is :
A)
mesocarp
done
clear
B)
epicarp
done
clear
C)
endocarp
done
clear
D)
thalamus
done
clear
View Answer play_arrow
question_answer 182) In pea, castor and maize the number of cotyledons are :
A)
2, 2 and 1 respectively
done
clear
B)
1, 2 and 2 respectively
done
clear
C)
2, 1 and 2 respectively
done
clear
D)
1,2 and 1 respectively
done
clear
View Answer play_arrow
question_answer 183) Colchicine brings about :
A)
chromosome aberrations
done
clear
B)
duplication of chromosomes
done
clear
C)
gene mutations
done
clear
D)
quick replication
done
clear
View Answer play_arrow
question_answer 184) Which one of the following is correct?
A)
Herbicides kill .paint mostly by blocking PS-11 (photolysis of water) and occasionally phloem transport
done
clear
B)
Insecticides kill insects mostly through impairment of nerve conduction and sometimes through respiratory arrest
done
clear
C)
both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 185) Which of the following is free living aerobic and non-photosynthetic nitrogen fixing bacterium ?
A)
Azotobacter
done
clear
B)
Rhizobium
done
clear
C)
Anabaena
done
clear
D)
Clostridium
done
clear
View Answer play_arrow
question_answer 186) Leu. ca. ena leucocephala is :
A)
called subabul
done
clear
B)
a small leguminous tree with edible fruits and seeds
done
clear
C)
a fodder plant as its pods and leaves are consumed by cattle
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 187) Which of the following is not paired correctly?
A)
Winged bean-wood used for timber purposes
done
clear
B)
Jojoba-seeds yields liquid wax
done
clear
C)
Guayule-yields latex, which can be converted to rubber
done
clear
D)
Leucaena-wood is a source of timber, paper pulp and rayon
done
clear
View Answer play_arrow
question_answer 188) When number of chromosomes is already reduced to half in the first reductional division of meiosis, where is the neccessity of second meiotic division?
A)
The division is required for the formation of four gametes
done
clear
B)
Division ensures equal distribution of halpoid chromosomes
done
clear
C)
Division ensures equal distribution of genes on chromosomes
done
clear
D)
Division is required for segregation of replicated chromosomes
done
clear
View Answer play_arrow
question_answer 189) Human Immunodeficiency Virus (HIV) is comprised of a protein coat and genetic material, which is :
A)
single stranded DNA
done
clear
B)
single stranded RNA
done
clear
C)
double stranded RNA
done
clear
D)
double stranded DNA
done
clear
View Answer play_arrow
question_answer 190) Plasmids are extra-chromosomal circular DNA molecules :
A)
which have their own point of replication and can replicate independently
done
clear
B)
which have their own point of replicant but cannot replicate independently
done
clear
C)
which do not have their own point of replication and cannot replicate indepen dent of bacterial chromosomal DNA
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 191) Which one of the following codons is not a termination codon ?
A)
UAG
done
clear
B)
UGA
done
clear
C)
UAA
done
clear
D)
AUG
done
clear
View Answer play_arrow
question_answer 192) Operon model for regulation of transcription was proposed by :
A)
Meselon and Stahl in 1998
done
clear
B)
Jacob and Monod in 1961
done
clear
C)
Watson and Crick in 1951
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 193) Clover leaf secondary structure of t-RNA has anticodon arm, which :
A)
contains in us loop three nucleotides of the codon
done
clear
B)
contains in its loop three nucleotides of the anticodon
done
clear
C)
contains in its no nucleotides
done
clear
D)
both (a) and (b)
done
clear
View Answer play_arrow
question_answer 194) A person with XXY sex chromosomes is diagonosed as suffering from :
A)
Turner?s syndrome
done
clear
B)
Down?s syndrome
done
clear
C)
Klinefelter?s syndrome
done
clear
D)
Addison?s syndrome
done
clear
View Answer play_arrow
question_answer 195) Bacteriophages are :
A)
viruses that infect bacteria
done
clear
B)
bacteria that infect virus
done
clear
C)
bacteria that infect other bacteria
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 196) Schleiden and Schwann proposed :
A)
phenomenon of Brownian movement
done
clear
B)
Cell theory or cell doctrine
done
clear
C)
protoplasm as a physical basis of life
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 197) Inner lining of stomach and intestine is formed of:
A)
squamous epithelium
done
clear
B)
columnar epithelium
done
clear
C)
cuboidal epithelium
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 198) One molecule of haemoglobin can carry as much as :
A)
just one molecule of oxygen
done
clear
B)
two molecules of oxygen
done
clear
C)
three molecules of oxygen
done
clear
D)
four molecules of oxygen
done
clear
View Answer play_arrow
question_answer 199) Coagulation of blood in blood vessels in living body is prevented by :
A)
prothrombin
done
clear
B)
heparin
done
clear
C)
prothrombin and calcium together
done
clear
D)
plasminogen and calcium together
done
clear
View Answer play_arrow
question_answer 200) Ornithine cycle removes :
A)
\[C{{O}_{2}}\] and ammonia from blood in liver
done
clear
B)
ammonia and urea from blood in liver
done
clear
C)
\[C{{O}_{2}}\]and urea from blood in liver
done
clear
D)
ammonia and uric acid from blood in liver
done
clear
View Answer play_arrow
question_answer 201) Human cranium has ?? bones.
A)
8
done
clear
B)
14
done
clear
C)
20
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 202) Functional unit of skeletal muscle is called :
A)
sarcomere
done
clear
B)
twitch
done
clear
C)
Z-band
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 203) Choroid plexus functions to produce :
A)
lymph
done
clear
B)
endolymph
done
clear
C)
cerebrospinal fluid
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 204) Which is the largest endocrine gland ?
A)
Thyroid gland
done
clear
B)
Adrenal gland
done
clear
C)
Thymus
done
clear
D)
Pituitary gland
done
clear
View Answer play_arrow
question_answer 205) Which one of the following pituitary hormones does not have a target organ to act upon?
A)
Thyrotrophin
done
clear
B)
Gonadotrophin
done
clear
C)
Adrenocorticotrophin
done
clear
D)
Somatotrophin
done
clear
View Answer play_arrow
question_answer 206) Oral contraceptives are prescribed in females to check :
A)
entry of sperms in vagina
done
clear
B)
implantation
done
clear
C)
ovulation
done
clear
D)
fertilization
done
clear
View Answer play_arrow
question_answer 207) Which one of the following systems is not mesodermal in origin?
A)
Circulatory system
done
clear
B)
Muscular system
done
clear
C)
Nervous system
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 208) Cerebellum of brain is responsible for:
A)
the maintenance of equilibrium and posture
done
clear
B)
olfactory functions
done
clear
C)
controlling optic functions
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 209) Malarial parasite is introduced into the blood of man as a :
A)
metacryptozoite
done
clear
B)
schizont'
done
clear
C)
oocyte
done
clear
D)
sporozoite
done
clear
View Answer play_arrow
question_answer 210) Coelom is a space between :
A)
ectoderm and endoderm
done
clear
B)
mesoderm and ectoderm
done
clear
C)
splitted mesoderms
done
clear
D)
mesoderm and body wall
done
clear
View Answer play_arrow
question_answer 211) Match the correct combination :
A)
Leishmania donovam-Sleeping sickness
done
clear
B)
Wuchereria bancrofti-Filariasis
done
clear
C)
Amoeba proteus-Kala azar
done
clear
D)
Anopoheles maculipennis-Malaria
done
clear
View Answer play_arrow
question_answer 212) Silk is obtained from :
A)
Bombyx mori
done
clear
B)
Laccifera lacca
done
clear
C)
Apis melifera
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 213) Bat belongs to order
A)
chiroptera
done
clear
B)
lagmorpha
done
clear
C)
urodela
done
clear
D)
hymenoptera
done
clear
View Answer play_arrow
question_answer 214) Which one of the following is not a bird?
A)
Magpie
done
clear
B)
Albastross
done
clear
C)
Himalayan quail
done
clear
D)
Bufo
done
clear
View Answer play_arrow
question_answer 215) Earth originated approximately :
A)
4,500 million years ago
done
clear
B)
3,600 million years ago
done
clear
C)
between 1,600-2,600 million years ago
done
clear
D)
2.5 million years ago
done
clear
View Answer play_arrow
question_answer 216) Organs having similar functions, but different origin and development are known as :
A)
vestigial organs
done
clear
B)
analogous organs
done
clear
C)
homologous organs
done
clear
D)
metalogous organs
done
clear
View Answer play_arrow
question_answer 217) Dinosaurs were abundant during :
A)
Jurassic period
done
clear
B)
Pleistocene period
done
clear
C)
Devonian period
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 218) Archaeopteryx is a connecting link between :
A)
reptiles and amphibians
done
clear
B)
mammals and birds
done
clear
C)
reptiles and birds
done
clear
D)
reptiles and mammals
done
clear
View Answer play_arrow
question_answer 219) Who developed vaccine against smallpox?
A)
Louis Pasture
done
clear
B)
Selman Waksman
done
clear
C)
Edward Jenner
done
clear
D)
Alexander Flemming
done
clear
View Answer play_arrow
question_answer 220) Hybridoma technology has been successfully used in :
A)
production of somatic hybrids
done
clear
B)
synthesis of monoclonal antibodies
done
clear
C)
synthesis of haemoglobin
done
clear
D)
production of alcohol in bulk
done
clear
View Answer play_arrow
question_answer 221) Which of the following is a bacterial disease?
A)
Measles
done
clear
B)
Chicken pox
done
clear
C)
Rabies
done
clear
D)
Tuberculosis
done
clear
View Answer play_arrow
question_answer 222) Insecticides usually act upon :
A)
digestive system
done
clear
B)
nervous system
done
clear
C)
circulatory system
done
clear
D)
muscular system
done
clear
View Answer play_arrow
question_answer 223) Which of the following is not an air pollutant ?
A)
\[N{{O}_{3}}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
Hydrocarbons
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 224) Which one of the following diseases is a sexually transmitted disease ?
A)
Cancer
done
clear
B)
Syphilis
done
clear
C)
Diphtheria
done
clear
D)
Myocarditis
done
clear
View Answer play_arrow
question_answer 225) Malaria is a common disease caused by :
A)
bacterium
done
clear
B)
virion
done
clear
C)
sporozoan
done
clear
D)
helminth
done
clear
View Answer play_arrow
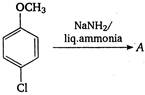 The major product A is:
The major product A is:
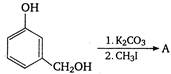
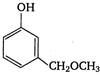
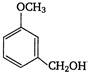
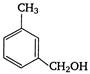
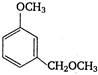
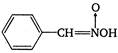
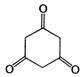
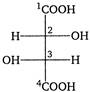 Configuration at\[{{C}_{2}}\]and\[{{C}_{3}}\]atoms are:
Configuration at\[{{C}_{2}}\]and\[{{C}_{3}}\]atoms are:
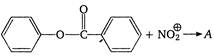 The product A is:
The product A is: