question_answer 1) Vector which is perpendicular to a cos\[\theta \hat{i}\]+ b sin \[\theta \hat{j}\] is:
A)
\[b\sin \theta \hat{i}-a\cos \theta \hat{j}\]
done
clear
B)
\[\frac{1}{2}\sin \theta \hat{i}-\frac{1}{b}\cos \theta \hat{j}\]
done
clear
C)
\[5k\]
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 2) Which of the following statements is true?
A)
When the co-ordinate axes are translated the component of a vector in a plane changes
done
clear
B)
When the co-ordinate axes are rotated through some angle components of the vector change but the vector?s magnitude remains constant
done
clear
C)
Sum of \[\vec{a}\] and \[\vec{b}\] is \[\vec{R}\] If the magnitude of\[\vec{a}\] alone is increased angle between \[\vec{b}\] and\[\vec{R}\] decreases
done
clear
D)
The cross product of 3 \[\vec{i}\] and 4\[\vec{j}\] is 12
done
clear
View Answer play_arrow
question_answer 3) From a balloon rising vertically upwards as 5 m/s a stone is thrown up at 10 m/s relative to the balloon. Its velocity with respect to ground after 2 s is (assume\[g=10\text{ }m/{{s}^{2}}\]) :
A)
zero
done
clear
B)
5 m/s
done
clear
C)
10 m/s
done
clear
D)
20 m/s
done
clear
View Answer play_arrow
question_answer 4) Two bodies arc projected from ground with equal speed 20 m/s from the same position in the same vertical plane to have equal range but at different angles above the horizontal. If one of the angle is 30° the sum of their maximum heights is (assume \[g=10\text{ }m/{{s}^{2}}\]) :
A)
400 m
done
clear
B)
20 m
done
clear
C)
30 m
done
clear
D)
40 m
done
clear
View Answer play_arrow
question_answer 5) Which of die following quantities measured from different inertial reference frames are same?
A)
Force
done
clear
B)
Velocity
done
clear
C)
Displacement
done
clear
D)
Kinetic energy
done
clear
View Answer play_arrow
question_answer 6) Two particles of equal mass arc connected to a rope AB of negligible mass, such that one is at end A and the other dividing the length of the rope in the ratio 1 : 2 from B. The rope is rotated about end B in a horizontal plane. Ratio of the tensions in the smallei part to the other is (ignore effect of gravity) :
A)
4:3
done
clear
B)
1 : 4
done
clear
C)
1: 2
done
clear
D)
1 : 3
done
clear
View Answer play_arrow
question_answer 7) Angle of banking tor a vehicle speed of 10 m/s for a radius of curvature 10 m is (assume\[g=10\text{ }m/{{s}^{2}}\]) :
A)
\[30{}^\circ \]
done
clear
B)
\[{{\tan }^{-1}}\left( \frac{1}{2} \right)\]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
\[45{}^\circ \]
done
clear
View Answer play_arrow
question_answer 8) A body of mass 2 kg is projected at 20 m/s at an angle \[60{}^\circ \] above the horizontal. Power due to the gravitational force at its highest point is :
A)
200 W
done
clear
B)
100\[\sqrt{3}\]W
done
clear
C)
50 W
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 9) A block of mass 2 kg rests on a horizontal surface. If a horizontal force of 5 N is applied on the block the frictional force on, it is: (\[{{\mu }_{k}}=0.4,{{\mu }_{s}}=0.5\])
A)
5N
done
clear
B)
10 N
done
clear
C)
8 N
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 10) Three identical spheres of mass M each are placed at the corners of an equilateral triangle of side 2m. Taking one of the comer as the origin, the position vector of the centre of mass is :
A)
\[\sqrt{3}(\hat{i}-\hat{j})\]
done
clear
B)
\[\frac{{\hat{i}}}{\sqrt{3}}+\hat{j}\]
done
clear
C)
\[\frac{\hat{i}+\hat{j}}{3}\]
done
clear
D)
\[\hat{i}+\frac{{\hat{j}}}{\sqrt{3}}\]
done
clear
View Answer play_arrow
question_answer 11) A shell initially at rest explodes into two pieces of equal mass, the two pieces will:
A)
move with different velocities in different directions
done
clear
B)
move with the same velocity in opposite directions
done
clear
C)
move with the same velocity in the same direction
done
clear
D)
be at rest
done
clear
View Answer play_arrow
question_answer 12) Impulse is :
A)
a scalar
done
clear
B)
equal to change in the momentum of a body
done
clear
C)
equal to rate of change of momentum of a body
done
clear
D)
a force
done
clear
View Answer play_arrow
question_answer 13) In a head on elastic collision of a very heavy body moving at V with a light body at rest, velocity of heavy body after collision is:
A)
V
done
clear
B)
2 V
done
clear
C)
zero
done
clear
D)
\[\frac{v}{2}\]
done
clear
View Answer play_arrow
question_answer 14)
A T joint is formed by two identical rods A and B each of mass m and length L in the XY plane as shown. Its moment of inertia about axis coinciding with A is :
A)
\[\frac{2m{{L}^{2}}}{3}\]
done
clear
B)
\[\frac{m{{L}^{2}}}{12}\]
done
clear
C)
\[\frac{m{{L}^{2}}}{6}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 15) A disc of moment of inertia 5 kg-m is acted upon by a constant torque of 40 Nm. Starting from rest the time taken by it to acquire an angular velocity of 24 rad/s is:
A)
3 s
done
clear
B)
4 s
done
clear
C)
2.5 s
done
clear
D)
120 s
done
clear
View Answer play_arrow
question_answer 16) If the angular momentum of a rotating body about a fixed axis is increased by 10%. Its kinetic energy will be increased by :
A)
10%
done
clear
B)
20%
done
clear
C)
21%
done
clear
D)
5%
done
clear
View Answer play_arrow
question_answer 17) If the earth were to spin faster, acceleration due to gravity at the poles :
A)
increases
done
clear
B)
decreases
done
clear
C)
remains the same
done
clear
D)
depends on how fast it spins
done
clear
View Answer play_arrow
question_answer 18) The time period of an artificial satellite in a circular orbit is independent of:
A)
the mass of the satellite
done
clear
B)
radius of the orbit
done
clear
C)
mass of the earth and radius of the earth
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 19) Angle of contact of a liquid with a solid depends on:
A)
solid only
done
clear
B)
liquid only
done
clear
C)
both on solid and liquid
done
clear
D)
orientation of the solid surface in liquid
done
clear
View Answer play_arrow
question_answer 20) If longitudinal strain for a wire is 0.03 and its Poisson?s ratio is 0.5, then its lateral strain is :
A)
0.003
done
clear
B)
0.0075
done
clear
C)
0.015
done
clear
D)
0.4
done
clear
View Answer play_arrow
question_answer 21) When the temperature increases, the viscosity of:
A)
gase decreases and liquid increases
done
clear
B)
gase increases and liquid decreases
done
clear
C)
gase and liquid increases
done
clear
D)
gase and liquid decreases
done
clear
View Answer play_arrow
question_answer 22) Water flows steadily through a horizontal pipe of variable cross-section. If the pressure of water is P at a point where flow speed is v, the pressure at another point where the flow of speed is 2 v, is (take density of water as \[\rho \]) :
A)
\[p-\frac{3\rho {{v}^{2}}}{2}\]
done
clear
B)
\[p-\frac{\rho {{v}^{2}}}{2}\]
done
clear
C)
\[p-\frac{3\rho {{v}^{2}}}{4}\]
done
clear
D)
\[p-\rho {{v}^{2}}\]
done
clear
View Answer play_arrow
question_answer 23) Which of the following statements is true?
A)
Internal energy of a gas depends only on the state of the gas
done
clear
B)
In an isothermal process change in internal energy is maximum
done
clear
C)
Area under pressure, volume graph equals heat supplied in any process
done
clear
D)
Work done is state dependent but not path dependent
done
clear
View Answer play_arrow
question_answer 24) A gas\[\left( \gamma =\frac{5}{3} \right)\]expands isobarically. The percentage of heat supplied that increases thermal energy and that involved in doing work for expansion is :
A)
40 : 60
done
clear
B)
60 : 40
done
clear
C)
50 : 50
done
clear
D)
25 : 30
done
clear
View Answer play_arrow
question_answer 25) A perfect black body is one whose emissive power is :
A)
maximum
done
clear
B)
zero
done
clear
C)
unity
done
clear
D)
minimum
done
clear
View Answer play_arrow
question_answer 26) A black body at a temperature T radiates energy at E. It the temperature falls to\[\frac{T}{2},\] the radiated energy will be :
A)
\[\frac{E}{4}\]
done
clear
B)
\[\frac{E}{2}\]
done
clear
C)
\[2E\]
done
clear
D)
\[\frac{E}{16}\]
done
clear
View Answer play_arrow
question_answer 27) If the units of mass, length and time, are doubled unit of angular momentum will be :
A)
doubled
done
clear
B)
tripled
done
clear
C)
quadrupled
done
clear
D)
8 timer the original value
done
clear
View Answer play_arrow
question_answer 28) Which of the following graphs shows variation of potential energy (U) with position x?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 29) Choose the correct statement:
A)
Time period of a simple pendulum depends on amplitude
done
clear
B)
Time shown by a spring watch varies with acceleration due to gravity
done
clear
C)
In a simple pendulum time period varies linearly with the length of the pendulum
done
clear
D)
The graph between length of the pendulum and time period is a parabola
done
clear
View Answer play_arrow
question_answer 30) Time period of a spring mass system is T. If this spring is cut into two parts whose lengths are in the ratio 1: 3 and the same mass is attached to the longer pan, the new time period will be :
A)
\[\sqrt{\frac{3}{2}}\,T\]
done
clear
B)
\[\frac{T}{\sqrt{3}}\]
done
clear
C)
\[\frac{\sqrt{3}T}{2}\]
done
clear
D)
\[\sqrt{3}T\]
done
clear
View Answer play_arrow
question_answer 31) Equation of progressive wave is \[y=A\sin \left( 10\pi x+11\pi t+\frac{\pi }{3} \right):\]
A)
its wavelength is 2 units
done
clear
B)
it is travelling in the positive x-direction
done
clear
C)
wave velocity is 1.5 units
done
clear
D)
time period of SHM is 1 s
done
clear
View Answer play_arrow
question_answer 32) The phenomenon of sound propagation in air is :
A)
isothermal process
done
clear
B)
isobaric process
done
clear
C)
adiabatic process
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 33) The second overtone of an open pipe is in resonance with the first overtone of a dosed pipe of length 2 m. Length of the open pipe is:
A)
4 m
done
clear
B)
2 m
done
clear
C)
8 m
done
clear
D)
1 m
done
clear
View Answer play_arrow
question_answer 34) Choose the correct statement:
A)
Beats are due to destructive interference
done
clear
B)
Maximum beat frequency audible to a human being is 20
done
clear
C)
Beats are as a result of Doppler's effect
done
clear
D)
Beats are due to superposition of two waves of nearly equal frequencies
done
clear
View Answer play_arrow
question_answer 35) A motor car is approaching towards a crossing with a velocity of 72 km/h. The frequency of sound of its horn as heard by a policeman standing on the crossing is 260 Hz. The frequency of horn is:
A)
200 Hz
done
clear
B)
244 Hz
done
clear
C)
150 Hz
done
clear
D)
80 Hz
done
clear
View Answer play_arrow
question_answer 36) Forces exerted by a uniform electric field on an electron having mass me and proton of mass mp are represented as Fe and Fp respectively are related as:
A)
\[{{F}_{p}}={{F}_{e}}\]
done
clear
B)
\[\frac{{{F}_{e}}}{{{F}_{p}}}=\frac{{{m}_{e}}}{{{m}_{p}}}\]
done
clear
C)
\[\frac{{{F}_{e}}}{{{F}_{p}}}=\frac{{{m}_{e}}}{{{m}_{p}}}\]
done
clear
D)
\[\frac{{{F}_{e}}}{{{F}_{p}}}=\frac{m_{e}^{2}}{m_{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 37) Electric field strength due to a dipole at a point on the axial line of dipole is :
A)
from positive charge to negative charge
done
clear
B)
from negative charge to positive charge
done
clear
C)
along the equatorial line
done
clear
D)
at an angle to axial line
done
clear
View Answer play_arrow
question_answer 38) Potential and field strength at a certain distance from a point charge are 600 V and 200 N/C. Distance of the point from the charge is:
A)
2 m
done
clear
B)
4 m
done
clear
C)
8 m
done
clear
D)
3 m
done
clear
View Answer play_arrow
question_answer 39) Two identical spheres with charges 4q, - 2q kept some distance apart exert a force F on each other. If they are made to touch each other and replaced at their old positions, the force between them will be :
A)
\[\frac{1}{9}F\]
done
clear
B)
\[\frac{1}{8}F\]
done
clear
C)
\[\frac{9}{8}F\]
done
clear
D)
\[\frac{8}{9}F\]
done
clear
View Answer play_arrow
question_answer 40) Two capacitors each of capacity 2 \[\mu \]F are connected in parallel. If they are connected to 100 V battery, then energy stored in them is :
A)
0.02 J
done
clear
B)
0.04 J
done
clear
C)
0.01 J
done
clear
D)
200 J
done
clear
View Answer play_arrow
question_answer 41) Which factor is immaterial for the wire used in electric fuse?
A)
Length
done
clear
B)
Radius
done
clear
C)
Material
done
clear
D)
Current
done
clear
View Answer play_arrow
question_answer 42) A battery of emf 2 V and internal resistance 0.1 \[\Omega \] is being charged by a current of 5 A. The potential difference between the terminals of the battery is :
A)
2.5 V
done
clear
B)
1.5 V
done
clear
C)
0.5 V
done
clear
D)
IV
done
clear
View Answer play_arrow
question_answer 43) An electric bulb is rated at 220 V, 200 W. Power consumed by it when operated at 110 V is:
A)
25 W
done
clear
B)
50 W
done
clear
C)
75 W
done
clear
D)
90 W
done
clear
View Answer play_arrow
question_answer 44)
Two cells having emf 4 V, 2 V and internal resistances 1 \[\Omega \], 1\[\Omega \] are connected as shown in figure below. Current through 6\[\Omega \] resistance is :
A)
\[\frac{1}{3}A\]
done
clear
B)
\[\frac{2}{3}A\]
done
clear
C)
\[1A\]
done
clear
D)
\[\frac{2}{9}A\]
done
clear
View Answer play_arrow
question_answer 45) For a given thermocouple neutral temperature:
A)
is a constant
done
clear
B)
depends on cold junction temperature
done
clear
C)
depends on inversion temperature
done
clear
D)
double that of cold junction temperature
done
clear
View Answer play_arrow
question_answer 46) A charged particle enters a uniform magnetic field with a certain speed at right angles to it. In the magnetic field a change could occur in its:
A)
kinetic energy
done
clear
B)
angular momentum
done
clear
C)
linear momentum
done
clear
D)
speed
done
clear
View Answer play_arrow
question_answer 47) A coil having 500 turns of square shape each of side 10 cm is placed normal to a magnetic field which is increasing at 1 T/s. The induced emf is :
A)
0.1 V
done
clear
B)
0.5 V
done
clear
C)
IV
done
clear
D)
5 V
done
clear
View Answer play_arrow
question_answer 48) A galvanometer has a resistance 50 Q. A resistance of 5\[\Omega \] is connected parallel to it. Fraction of the total current flowing through galvanometer is :
A)
\[\frac{1}{10}\]
done
clear
B)
\[\frac{1}{11}\]
done
clear
C)
\[\frac{1}{50}\]
done
clear
D)
\[\frac{2}{15}\]
done
clear
View Answer play_arrow
question_answer 49) A wire oriented in the east-west direction carries a current eastward. Direction of the magnetic field at a point to the south of the wire is:
A)
vertically down
done
clear
B)
vertically up
done
clear
C)
north-east
done
clear
D)
south-east
done
clear
View Answer play_arrow
question_answer 50) An inductor is connected to an AC source. When compared to voltage, the current in the lead wires :
A)
is ahead in phase by\[\pi \]
done
clear
B)
lags in phase by \[\pi \]
done
clear
C)
is ahead in phase by\[\frac{\pi }{2}\]
done
clear
D)
lags in phase by \[\frac{\pi }{2}\]
done
clear
View Answer play_arrow
question_answer 51) Curie temperature is the one above which :
A)
paramagnetic substance changes to ferromagnetic
done
clear
B)
paramagnetic changes to diamagnetic
done
clear
C)
diamagnetic changes to paramagnetic
done
clear
D)
ferromagnetic changes to paramagnetic
done
clear
View Answer play_arrow
question_answer 52) Energy in a current carrying coil is stored in the form of:
A)
electric field
done
clear
B)
magnetic field
done
clear
C)
heat
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 53) Graph of force per unit length between two long parallel currents carrying conductor and the distance between them is :
A)
straight line
done
clear
B)
parabola
done
clear
C)
ellipse
done
clear
D)
rectangular hyperbola
done
clear
View Answer play_arrow
question_answer 54) A bar magnet is held at right angles to a uniform magnetic field. The couple acting on the magnet is to be halved by rotating it from this position. The angle of rotation is :
A)
\[60{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[30{}^\circ \]
done
clear
D)
\[75{}^\circ \]
done
clear
View Answer play_arrow
question_answer 55) A point source of light is kept below the surface of water in a pond :
A)
light emerges from every point of the surface of the pond
done
clear
B)
no light is transmitted from the surface of the pond
done
clear
C)
all the light emitted by the source emerges from a circular region of the pond
done
clear
D)
some of the light emitted by the source emerges from a circular region of the pond
done
clear
View Answer play_arrow
question_answer 56) If white light is used in Young?s double slit experiment:
A)
no interference pattern is formed
done
clear
B)
white fringes are formed
done
clear
C)
central bright fringe is white
done
clear
D)
central bright fringe is coloured
done
clear
View Answer play_arrow
question_answer 57) Electromagnetic waves can be deflected by :
A)
electric fields only
done
clear
B)
magnetic fields only
done
clear
C)
both and
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 58) Maximum lateral displacement of a ray of light incident on a slab of thickness t is :
A)
\[\frac{t}{2}\]
done
clear
B)
\[\frac{t}{3}\]
done
clear
C)
\[\frac{t}{4}\]
done
clear
D)
t
done
clear
View Answer play_arrow
question_answer 59) Mercury vapour lamp gives :
A)
continuous spectrum
done
clear
B)
line spectrum
done
clear
C)
band spectrum
done
clear
D)
absorption spectrum
done
clear
View Answer play_arrow
question_answer 60) Pick the correct statement from tne following:
A)
Primary rainbow is a virtual image and secondary rainbow is a real image
done
clear
B)
Primary rainbow is a real image and secondary rainbow is a virtual image
done
clear
C)
Both primary and secondary rainbows are virtual images
done
clear
D)
Both primary and secondary rainbows are real images
done
clear
View Answer play_arrow
question_answer 61) The refractive index of water, glass and diamond are 1.33, 1.50, 2.40 respectively. The refractive index of diamond relative to water and of glass relative to diamond, respectively are nearly:
A)
1.80, 0.625
done
clear
B)
0.554, 0.625
done
clear
C)
1.80, 1.6
done
clear
D)
0.554, 1.6
done
clear
View Answer play_arrow
question_answer 62) In the diffraction pattern of a single slit:
A)
all bands are uniformly bright
done
clear
B)
all bands are uniformly wide
done
clear
C)
central band is narrower
done
clear
D)
central band is wider
done
clear
View Answer play_arrow
question_answer 63) A radioactive substancc has a half-life of four months. Three-fourth of the substance will decay in :
A)
3 months
done
clear
B)
4 months
done
clear
C)
8 months
done
clear
D)
12 months
done
clear
View Answer play_arrow
question_answer 64) Electrons in the atom are held to the nucleus by:
A)
Coulomb's forces
done
clear
B)
nuclear forces
done
clear
C)
van der Waal's forces
done
clear
D)
gravitational forces
done
clear
View Answer play_arrow
question_answer 65) Mass of the nucleons together in a heavy nucleus is :
A)
greater than mass of nucleus
done
clear
B)
equal to mass of nucleus
done
clear
C)
same as mass of nucleus
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 66) Ratio of the radii of the nuclei with mass numbers 8 and 27 would be :
A)
27/8
done
clear
B)
8/27
done
clear
C)
2/3
done
clear
D)
3/2
done
clear
View Answer play_arrow
question_answer 67) Stopping potential required to reduce the photoelectric current to zero :
A)
is directly proportional to the wavelength of the incident radiation
done
clear
B)
increases uniformly with wavelength of the incident radiation
done
clear
C)
is directly proportional to the frequency of the incident radiation
done
clear
D)
decreases uniformly with the frequency of the incident radiation
done
clear
View Answer play_arrow
question_answer 68) The first member of the Balmer?s series of the hydrogen has a wavelength a, the wavelength of the second member of its series is :
A)
\[\frac{27}{20}\lambda \]
done
clear
B)
\[\frac{20}{27}\lambda \]
done
clear
C)
\[\frac{27}{20}\lambda \]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 69) The photoelectric threshold frequency of a metal is v. When light of frequency 4v is incident on the metal. The maximum kinetic energy of the emitted photoelectrons is :
A)
4 hv
done
clear
B)
3 hv
done
clear
C)
5 hv
done
clear
D)
\[\frac{5}{2}hv\]
done
clear
View Answer play_arrow
question_answer 70) The shortest wavelength in Lyman series is 91.2 nm. The longest wavelength of the series is:
A)
121.6 nm
done
clear
B)
182.4 nm
done
clear
C)
243.4 nm
done
clear
D)
364.8 nm
done
clear
View Answer play_arrow
question_answer 71) Energy gap of a semiconductor is of the order of:
A)
1 eV
done
clear
B)
10 eV
done
clear
C)
0.1 eV
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 72) Majority charge carriers in p-type material are:
A)
holes
done
clear
B)
electrons
done
clear
C)
both and
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 73) Resistance of a semiconductor:
A)
increases with increase in temperature
done
clear
B)
decreases with increase in temperature
done
clear
C)
is not affected by change in temperature
done
clear
D)
increases for germanium and decreases for silicon
done
clear
View Answer play_arrow
question_answer 74) Semiconductor material having fewer free electrons than pure germanium or silicon is:
A)
p-type
done
clear
B)
n-type
done
clear
C)
both and
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 75) The concentrations of impurities in a transistor are :
A)
equal for the emitter, base and collector regions
done
clear
B)
least for the emitter region
done
clear
C)
largest for the emitter region
done
clear
D)
least for the base region
done
clear
View Answer play_arrow
question_answer 76) At\[{{25}^{o}}C,\]the total pressure of an ideal solution obtained by mixing 3 moles of "A" and 2 moles of "B", is 184 torr. What is the vapour pressure (in torr) of pure "B" at the same temperature? (vapour pressure of pure "A" at \[{{25}^{o}}C,\] is 200 torr):
A)
180
done
clear
B)
160
done
clear
C)
16
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 77) Relative lowering of vapour pressure of a dilute solution is 0.2. What is the mole fraction of the non-volatile solute?
A)
0.8
done
clear
B)
0.5
done
clear
C)
0.3
done
clear
D)
0.2
done
clear
View Answer play_arrow
question_answer 78)
Match the following: List A List B (1) \[PhC{{O}_{2}}C{{H}_{3}}\] (A) 2, 4-DNP (2) \[{{C}_{6}}{{H}_{5}}C{{H}_{2}}C{{O}_{2}}H\] (B) Amdt-Eister synthesis (3) \[{{C}_{6}}{{H}_{5}}CHO\] (C) Hydrolysis
Correct answer is:
A)
1-A, 2-B, 3-C
done
clear
B)
1-B, 2-C, 3-A
done
clear
C)
1-C, 2-B, 3-A
done
clear
D)
1-B, 2-A, 3-C
done
clear
View Answer play_arrow
question_answer 79) 2-pentanone and 3-methyl-2-butanone are a pair of ....... isomers:
A)
functional
done
clear
B)
chain
done
clear
C)
positional
done
clear
D)
stereo
done
clear
View Answer play_arrow
question_answer 80) What are the units of entropy?
A)
cal K
done
clear
B)
\[cal\,{{K}^{-1}}\]
done
clear
C)
\[cm\text{ }{{K}^{-1}}\]
done
clear
D)
cm K
done
clear
View Answer play_arrow
question_answer 81) Calculate\[\Delta H\](in Joules) for, C (graphite)\[\to \]C (diamond), from the following data \[C(graphite)+{{O}_{2}}(g)\to C{{O}_{2}}(g);\] \[\Delta H=-393.5kJ\] \[C(diamond)+{{O}_{2}}\to C{{O}_{2}}(g);\] \[\Delta H=-395.4\text{ }kJ\]
A)
1,900
done
clear
B)
\[-788.9\times {{10}^{3}}\]
done
clear
C)
1,90,000
done
clear
D)
\[+788.9\times {{10}^{3}}\]
done
clear
View Answer play_arrow
question_answer 82) \[C{{H}_{3}}Br+\overline{O}H\xrightarrow{{}}C{{H}_{3}}OH+B{{r}^{-}}\]reaction proceeds by \[{{S}_{N}}2-\]mechanism. Its rate is dependent on the concentration of:
A)
\[C{{H}_{3}}Br,\overline{O}H\]
done
clear
B)
\[C{{H}_{3}}Br\]only
done
clear
C)
OH only
done
clear
D)
\[C{{H}_{3}}Br,C{{H}_{3}}OH\]
done
clear
View Answer play_arrow
question_answer 83) The end products in the Cannizaro reaction of benzaldehyde is:
A)
\[PhC{{O}_{2}}H,\text{ }PhC{{H}_{2}}OH\]
done
clear
B)
\[PhC{{O}_{2}}H,\text{ }PhC{{H}_{2}}C{{O}_{2}}H\]
done
clear
C)
\[PhC{{H}_{2}}OH,\text{ }PhCOC{{H}_{3}}\]
done
clear
D)
\[PhC{{O}_{2}}H,\text{ }PhCOC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 84) For a chemical reaction, the free energy change\[(\Delta G)\]is negative. The reaction is:
A)
a spontaneous reaction
done
clear
B)
an equilibrium reaction
done
clear
C)
a non-spontaneous reaction
done
clear
D)
characterised by\[{{r}_{f}}={{r}_{b}}\](where\[{{r}_{f}}\]and\[{{r}_{b}}\]are rates of forward and backward reactions respectively)
done
clear
View Answer play_arrow
question_answer 85) In which of the following reactions, the heat liberated is known as "heat of combustion"?
A)
\[{{H}^{+}}(aq)+O{{H}^{-}}(aq)\to {{H}_{2}}O(l)+heat\]
done
clear
B)
\[C(graphite)+\frac{1}{2}{{O}_{2}}(g)\to CO(g)+heat\]
done
clear
C)
\[C{{H}_{4}}(g)+2{{O}_{2}}(g)\to C{{O}_{2}}(g)+2{{H}_{2}}O(l)\]\[+heat\]
done
clear
D)
\[{{H}_{2}}S{{O}_{4}}(aq)+{{H}_{2}}O(l)\to {{H}_{2}}S{{O}_{4}}(aq)+heat\]
done
clear
View Answer play_arrow
question_answer 86) The number of chiral centres in (+) -glucose:
A)
4
done
clear
B)
3
done
clear
C)
2
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 87) Aniline on oxidation with\[N{{a}_{2}}C{{r}_{2}}{{O}_{7}}\]and\[{{H}_{2}}S{{O}_{4}}\] gives:
A)
benzoic acid
done
clear
B)
m-amino benzoic acid
done
clear
C)
Schiff?s base
done
clear
D)
p-benzoquinone
done
clear
View Answer play_arrow
question_answer 88) What are the units of equivalent conductivity of a solution?
A)
\[mho\text{ }c{{m}^{-1}}\]
done
clear
B)
\[ohm\text{ }c{{m}^{-1}}g\text{ }equi{{v}^{-1}}\]
done
clear
C)
\[mho\text{ }c{{m}^{-2}}g\text{ }equi{{v}^{-1}}\]
done
clear
D)
\[mho\text{ }c{{m}^{2}}g\text{ }equi{{v}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 89) What is the cell reaction occurring in Daniel cell (Galvanic cell)?
A)
\[Cu(s)+ZnS{{O}_{4}}(aq)\to CuS{{O}_{4}}(aq)+Zn(s)\]
done
clear
B)
\[Zn(s)+CuS{{O}_{4}}(aq)\to Cu(s)+ZS{{O}_{4}}(aq)\]
done
clear
C)
\[Ni(s)+ZnS{{O}_{4}}(aq)\to NiS{{O}_{4}}(aq)+Zn(s)\]
done
clear
D)
\[2Na(s)+CdS{{O}_{4}}(aq)\to N{{a}_{2}}S{{O}_{4}}(aq)\] \[+Cd(s)\]
done
clear
View Answer play_arrow
question_answer 90) n-propylamine yields a volatile compound\[\underline{X}\] on warming with ale. alkali and chloroform. \[\underline{X}\]has an offensive odour. The structure of\[\underline{X}\]is:
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CN\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CHCN\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}NC\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}CHNC\]
done
clear
View Answer play_arrow
question_answer 91) The molecular formula of benzonitrile:
A)
\[{{C}_{6}}{{H}_{5}}CN\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}NC\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}CNO\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}NCO\]
done
clear
View Answer play_arrow
question_answer 92) Which of the following statements (or equation) is correct?
A)
The units of cell emf are\[V.\text{ }c{{m}^{-1}}\]
done
clear
B)
\[\Delta G=-\frac{nF}{{{E}_{ccell}}}\]
done
clear
C)
In Galvanic cell, chemical energy is transformed into electrical energy
done
clear
D)
Oxidation state of Mn in potassium permanganate is + 6
done
clear
View Answer play_arrow
question_answer 93) Which of the following metal can be obtained by the electrolysis of the aqueous solution of their salts?
A)
Cu
done
clear
B)
Na
done
clear
C)
Mg
done
clear
D)
K
done
clear
View Answer play_arrow
question_answer 94) The magnetic moment (in BM) of\[Z{{n}^{2+}}\]ion according to spin-only formula is:
A)
zero
done
clear
B)
1.73
done
clear
C)
2.84
done
clear
D)
3.87
done
clear
View Answer play_arrow
question_answer 95) The 3d-block element that exhibits maximum number of oxidation states in:
A)
Sc
done
clear
B)
Ti
done
clear
C)
Mn
done
clear
D)
Zn
done
clear
View Answer play_arrow
question_answer 96) Which of the following molecule in its valence shell has three bond pairs of electrons and one lone pair of electrons?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[B{{F}_{3}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 97) Which of the following set of properties belong to\[PC{{l}_{5}}\]?
A)
\[s{{p}^{3}},\]tetrahedral, 4 valence shell pairs of electrons
done
clear
B)
\[s{{p}^{3}}d,\]trigonal bipyramidal, 5 valence shell pairs of electrons
done
clear
C)
\[s{{p}^{3}}{{d}^{2}},\]octahedral, 6 valence shell pairs of electrons
done
clear
D)
\[s{{p}^{3}}d,\]square planar, 4 valence shell pairs of electrons
done
clear
View Answer play_arrow
question_answer 98) The metal ion in complex A has EAN identical to the atomic number of krypton. A is: (At. no. of\[Cr=24,\text{ }Fe=\text{ }26,\text{ }Pd=46\]):
A)
\[[Pd{{(N{{H}_{3}})}_{6}}]C{{l}_{4}}\]
done
clear
B)
\[[Co{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]
done
clear
C)
\[N{{a}_{4}}[Fe{{(CN)}_{6}}]\]
done
clear
D)
\[[Cr{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl\]
done
clear
View Answer play_arrow
question_answer 99) The complex that does not given a precipitate with \[AgN{{O}_{3}}\]solution:
A)
\[[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]\]
done
clear
B)
\[[Co{{(N{{H}_{3}})}_{5}}Cl]S{{O}_{4}}\]
done
clear
C)
\[[Ag{{(N{{H}_{3}})}_{2}}]Cl\]
done
clear
D)
\[[Cr{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl\]
done
clear
View Answer play_arrow
question_answer 100) According to bond order concept, the correct order of stability of\[{{O}_{2}},O_{2}^{+}\]and\[O_{2}^{-}\]is:
A)
\[{{O}_{2}}>O_{2}^{+}>O_{2}^{-}\]
done
clear
B)
\[O_{2}^{-}>{{O}_{2}}>O_{2}^{+}\]
done
clear
C)
\[{{O}_{2}}>O_{2}^{-}>O_{2}^{+}\]
done
clear
D)
\[O_{2}^{+}>{{O}_{2}}>O_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 101) Zero dipole moment is possessed by:
A)
\[PC{{l}_{3}}\]
done
clear
B)
\[B{{F}_{3}}\]
done
clear
C)
\[Cl{{F}_{3}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 102) The number of moles of ions given on complete ionization of one mole of:
A)
4
done
clear
B)
3
done
clear
C)
2
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 103) The co-ordination number in an ........ complex may increase to 8:
A)
cobalt
done
clear
B)
osmium
done
clear
C)
nickel
done
clear
D)
iron
done
clear
View Answer play_arrow
question_answer 104) The radius ratio\[\left( \frac{{{r}^{+}}}{{{r}^{-}}} \right)\]of an ionic solid\[({{A}^{+}}{{B}^{-}})\]is 0.69. What is the coordination number of\[{{B}^{-}}\]?
A)
6
done
clear
B)
8
done
clear
C)
2
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 105) A, B and C are ideal gases. Their molecular weights are 2, 4 and 28 respectively. The rate of diffusion of these gases follow the order:
A)
\[C>A>B\]
done
clear
B)
\[C>B>A\]
done
clear
C)
\[A=B=C\]
done
clear
D)
\[A>B>C\]
done
clear
View Answer play_arrow
question_answer 106) Among the following ions (hydrated), the colorless metal ion:
A)
\[C{{u}^{+}}\]
done
clear
B)
\[C{{u}^{2+}}\]
done
clear
C)
\[F{{e}^{2+}}\]
done
clear
D)
\[M{{n}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 107) German silver is an alloy of:
A)
\[Cu,Zn\]
done
clear
B)
\[Cu,\text{ }Ni,\text{ }Zn\]
done
clear
C)
\[Cu,\text{ }Sn,\text{ }Zn\]
done
clear
D)
\[Cu,\text{ }Zn\]
done
clear
View Answer play_arrow
question_answer 108) Which of the following statements is not correct?
A)
The units of surface tension are dynes\[c{{m}^{-1}}\]
done
clear
B)
The units of viscosity coefficient of a liquid are "poise"
done
clear
C)
\[CsCl\]crystallizes in body centred cubic type of lattice
done
clear
D)
The co-ordination number of\[{{S}^{2-}}\]in\[ZnS\]is 6
done
clear
View Answer play_arrow
question_answer 109) Which of the following is not a method of preparation of colloidal solution?
A)
Electrical dispersion
done
clear
B)
Peptization
done
clear
C)
Coagulation
done
clear
D)
Mechanical dispersion
done
clear
View Answer play_arrow
question_answer 110) Pauling's equation for determining the electronegativity of an element:
A)
\[{{X}_{A}}-{{X}_{B}}=0.208\sqrt{\Delta }\]
done
clear
B)
\[{{X}_{A}}+{{X}_{B}}=0.208\sqrt{\Delta }\]
done
clear
C)
\[{{X}_{A}}-{{X}_{B}}={{\Delta }^{2}}\]
done
clear
D)
\[{{X}_{A}}-{{X}_{B}}=\sqrt{\Delta }\]
done
clear
View Answer play_arrow
question_answer 111) The ionic radii \[(\overset{\text{o}}{\mathop{\text{A}}}\,)\] of \[{{C}^{4-}}\] and \[{{O}^{2-}}\] respectively are 2.60 and 1.40. The ionic radius of the isoelectronic ion \[{{N}^{3-}}\] would be:
A)
2.6
done
clear
B)
1.71
done
clear
C)
1.4
done
clear
D)
0.95
done
clear
View Answer play_arrow
question_answer 112)
The gold numbers of some colloidal solutions are given below: Colloidal solution Gold number A 0.01 B 2.5 C 20
The protective nature of these colloidal solutions follow the order:
A)
\[C>B>A\]
done
clear
B)
\[A>B>C\]
done
clear
C)
\[A=B=C\]
done
clear
D)
\[B>A>C\]
done
clear
View Answer play_arrow
question_answer 113) Which of the following leaction is an example for homogeneous catalysis?
A)
\[2{{H}_{2}}{{O}_{2}}(l)\xrightarrow{Mn{{O}_{2}}(s)}2{{H}_{2}}O(l)+{{O}_{2}}(g)\]
done
clear
B)
\[2S{{O}_{2}}(g)+{{O}_{2}}(g)2S{{O}_{3}}(g)\]
done
clear
C)
\[2CO(g)+{{O}_{2}}(g)\xrightarrow{NO(g)}2C{{O}_{2}}(g)\]
done
clear
D)
\[{{H}_{2}}(g)+{{C}_{2}}{{H}_{4}}(g)\xrightarrow{Ni(s)}{{C}_{2}}{{H}_{6}}(g)\]
done
clear
View Answer play_arrow
question_answer 114) Consider the following abbreviations for hydrated alkali ions: \[X={{[Li{{({{H}_{2}}O)}_{n}}]}^{+}}\] \[Y={{[K{{({{H}_{2}}O)}_{n}}]}^{+}}\] \[Z={{[Cs{{({{H}_{2}}O)}_{n}}]}^{+}}\] What is the correct order of size of these hydrated alkali ions?
A)
\[X>Y>Z\]
done
clear
B)
\[Z>Y>X\]
done
clear
C)
\[X=Y=Z\]
done
clear
D)
\[Z>X>Y\]
done
clear
View Answer play_arrow
question_answer 115) What is the product formed when phosphorus trioxide is dissolved in water?
A)
\[HP{{O}_{3}}\]
done
clear
B)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
C)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
D)
\[HP{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 116) The molecrlar formula of dithionic acid is:
A)
\[{{H}_{2}}{{S}_{2}}{{O}_{4}}\]
done
clear
B)
\[{{H}_{2}}{{S}_{2}}{{O}_{6}}\]
done
clear
C)
\[{{H}_{2}}{{S}_{2}}{{O}_{5}}\]
done
clear
D)
\[{{H}_{2}}{{S}_{2}}{{O}_{7}}\]
done
clear
View Answer play_arrow
question_answer 117) The bond dissociation energy of\[C{{l}_{2}},B{{r}_{2}}\]and \[{{I}_{2}}\]follow the order:
A)
\[C{{l}_{2}}>{{I}_{2}}>B{{r}_{2}}\]
done
clear
B)
\[{{I}_{2}}>B{{r}_{2}}>C{{l}_{2}}\]
done
clear
C)
\[{{I}_{2}}=C{{l}_{2}}=B{{r}_{2}}\]
done
clear
D)
\[C{{l}_{2}}>B{{r}_{2}}>{{I}_{2}}\]
done
clear
View Answer play_arrow
question_answer 118) During the extraction of copper, the impurity \[(FeS)\]is removed as slag by mixing the contaminated copper ore with silica and coke. The molecular formula of slag is:
A)
\[FeSi{{O}_{3}}\]
done
clear
B)
\[F{{e}_{2}}{{O}_{3}}\]
done
clear
C)
\[FeSi\](solid)
done
clear
D)
\[FeSi\](vapour)
done
clear
View Answer play_arrow
question_answer 119) Which of the following is used as indelible ink?
A)
Aqueous\[CuS{{O}_{4}}\]solution
done
clear
B)
Aqueous\[AgN{{O}_{3}}\]solution
done
clear
C)
Aqueous\[NaCl\]solution
done
clear
D)
Aqueous\[NaOH\]solution
done
clear
View Answer play_arrow
question_answer 120) Which of the following ores is an ore of copper?
A)
Argentite
done
clear
B)
Haematite
done
clear
C)
Malachite
done
clear
D)
Calamine
done
clear
View Answer play_arrow
question_answer 121) \[KMn{{O}_{4}}\](mol. wt. = 158) oxidizes oxalic acid in acid medium to\[C{{O}_{2}}\]and water as follows: \[5{{C}_{2}}O_{4}^{2-}+2MnO_{4}^{-}+16{{H}^{+}}\to 10C{{O}_{2}}+2M{{n}^{2+}}\]\[+\text{ }8{{H}_{2}}O\] What is the equivalent weight of\[KMn{{O}_{4}}\]?
A)
158
done
clear
B)
31.6
done
clear
C)
39.5
done
clear
D)
79
done
clear
View Answer play_arrow
question_answer 122) Sodium bicarbonate on heating decomposes to form sodium carbonate,\[C{{O}_{2}}\]and water. If 0.2 moles of sodium bicarbonate is completely decomposed, how many moles of sodium carbonate is formed?
A)
0.1
done
clear
B)
0.2
done
clear
C)
0.05
done
clear
D)
0.025
done
clear
View Answer play_arrow
question_answer 123) What is the energy (in eV) required to excite the electron from\[n=1\]to\[n=2\]state in hydrogen atom? (\[n=\]principal quantum number)
A)
13.6
done
clear
B)
3.4
done
clear
C)
17.0
done
clear
D)
10.2
done
clear
View Answer play_arrow
question_answer 124) According to aufbau principle, the correctorder of energy of 3d, 45 and 4p orbitals is:
A)
\[4p<3d<4s\]
done
clear
B)
\[4s<4p<3d\]
done
clear
C)
\[4s<3d<4p\]
done
clear
D)
\[3d<4s<4p\]
done
clear
View Answer play_arrow
question_answer 125) n-pentane and 2-methylbutane are a pair of:
A)
enantiomers
done
clear
B)
stereoisomers
done
clear
C)
diastereomers
done
clear
D)
constitutional isomers
done
clear
View Answer play_arrow
question_answer 126)
A)
geometrical isomerism
done
clear
B)
tautomerism
done
clear
C)
optical isomerism
done
clear
D)
geometrical and optical isomerism
done
clear
View Answer play_arrow
question_answer 127) The activity of a radioactive nuclide is\[2\times {{10}^{7}}\]disintegrations per minute (dpm). After 23.03 min, its activity is reduced to\[2\times {{10}^{6}}\] dpm. What is the average life (in min) of this nuclide?
A)
100
done
clear
B)
10
done
clear
C)
1
done
clear
D)
0.1
done
clear
View Answer play_arrow
question_answer 128) What is the correct order of velocity of alpha , beta (P) and gamma (y) rays?
A)
\[\alpha >\beta >\gamma \]
done
clear
B)
\[\alpha >\gamma >\beta \]
done
clear
C)
\[\gamma >\alpha >\beta \]
done
clear
D)
\[\gamma >\beta >\alpha \]
done
clear
View Answer play_arrow
question_answer 129) The isomers which are interconverted through rotation around a single bond are:
A)
conformers
done
clear
B)
diastereomers
done
clear
C)
enantiomers
done
clear
D)
position isomers
done
clear
View Answer play_arrow
question_answer 130) The major product in the reaction of 2-butyne with Li/liq.\[N{{H}_{3}}\]is:
A)
done
clear
B)
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
D)
\[{{H}_{2}}C=CH-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 131) What is\[\underline{X}\]in the following nuclear reaction? \[_{11}N{{a}^{23}}{{+}_{0}}{{n}^{1}}{{\to }_{11}}N{{a}^{24}}+\underline{X}\]
A)
\[_{1}{{H}^{1}}\]
done
clear
B)
\[_{2}H{{e}^{4}}\]
done
clear
C)
\[_{1}{{H}^{2}}\]
done
clear
D)
\[\gamma -\]ray (gamma ray)
done
clear
View Answer play_arrow
question_answer 132) HA is a weak acid. The pH of 0.1 M HA solution is 2. What is the degree of dissociation\[(\alpha )\]of HA?
A)
0.5
done
clear
B)
0.2
done
clear
C)
0.1
done
clear
D)
0.301
done
clear
View Answer play_arrow
question_answer 133) In the following reaction, A and B, respectively are: \[\underline{A}\xrightarrow[{}]{HBr}{{C}_{2}}{{H}_{5}}Br,\xrightarrow[{}]{B}\underline{A}\]
A)
\[{{C}_{2}}{{H}_{4}},alc\,KOH/\Delta \]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}Cl,aq\,KOH/\Delta \]
done
clear
C)
\[C{{H}_{3}}OH,aq\,KOH/\Delta \]
done
clear
D)
\[{{C}_{2}}{{H}_{2}},\,PB{{r}_{3}}\]
done
clear
View Answer play_arrow
question_answer 134) The reagent(s) used in the preparation of aspirin from salicylic acid:
A)
\[SOC{{l}_{2}},\]pyridine
done
clear
B)
\[{{(C{{H}_{3}}CO)}_{2}}O,{{H}^{+}}\]
done
clear
C)
\[C{{H}_{3}}C{{O}_{2}}H,HCl\]
done
clear
D)
\[C{{H}_{3}}Cl,AlC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 135) The equilibrium reaction that is not influenced by volume change at constant temperature is:
A)
\[{{H}_{2}}(g)+{{I}_{2}}(g)2HI(g)\]
done
clear
B)
\[{{N}_{2}}(g)+3{{H}_{2}}(g)2N{{H}_{3}}(g)\]
done
clear
C)
\[{{N}_{2}}{{O}_{4}}(g)2N{{O}_{2}}(g)\]
done
clear
D)
\[2NO(g)+{{O}_{2}}2N{{O}_{2}}(g)\]
done
clear
View Answer play_arrow
question_answer 136) Consider the following solutions of equal concentrations: \[A=N{{H}_{4}}Cl\] \[B=C{{H}_{3}}COONa\] \[C=N{{H}_{4}}OH\] \[D=C{{H}_{3}}COOH\] A buffer solution can be obtained by mixing equal volumes of:
A)
C and D
done
clear
B)
A and B
done
clear
C)
A and C
done
clear
D)
C and D
done
clear
View Answer play_arrow
question_answer 137) In the following reaction, X and Y respectively are: \[{{C}_{2}}{{H}_{5}}OH\xrightarrow[{}]{KMn{{O}_{4}}/{{H}^{+}}}\underline{X}\xrightarrow[{{H}_{2}}S{{O}_{4}}/\Delta ]{Y}C{{H}_{2}}C{{O}_{2}}{{C}_{2}}{{H}_{5}}\]
A)
\[C{{H}_{3}}OH,{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[C{{H}_{3}}CHO,C{{H}_{3}}OH\]
done
clear
C)
\[C{{H}_{3}}C{{O}_{2}}H,{{C}_{2}}{{H}_{5}}OH\]
done
clear
D)
\[{{C}_{2}}{{H}_{4}},C{{H}_{3}}C{{O}_{2}}H\]
done
clear
View Answer play_arrow
question_answer 138) The reaction conditions used for converting 1, 2-dibromopropane to propylene are:
A)
KOH, alcohol\[/\Delta \]
done
clear
B)
KOH, water\[/\Delta \]
done
clear
C)
Zn, alcohol \[/\Delta \]
done
clear
D)
Na, alcohol\[/\Delta \]
done
clear
View Answer play_arrow
question_answer 139) Which of the following is a Lewis acid?
A)
\[AlC{{l}_{3}}\]
done
clear
B)
\[C{{l}^{-}}\]
done
clear
C)
\[CO\]
done
clear
D)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 140) If "a" and\[''{{t}_{1/2}}''\]are initial concentration of reactant and half-life of a zero order reaction respectively, which of the following is correct?
A)
\[{{t}_{1/2}}\propto \frac{1}{a}\]
done
clear
B)
\[{{t}_{1/2}}\propto a\]
done
clear
C)
\[{{t}_{1/2}}\propto \frac{1}{{{a}^{2}}}\]
done
clear
D)
\[{{t}_{1/2}}\propto {{a}^{2}}\]
done
clear
View Answer play_arrow
question_answer 141) All monosaccharides ....... Tollen's reagent:
A)
oxidises
done
clear
B)
condense with
done
clear
C)
reduces
done
clear
D)
add to
done
clear
View Answer play_arrow
question_answer 142) The product formed in the reaction of glycine with benzoyl chloride 4- aq\[NaOH\]:
A)
\[PhCOC{{H}_{2}}N{{H}_{2}}\]
done
clear
B)
\[PhC{{H}_{2}}N{{H}_{2}}\]
done
clear
C)
\[PhCONHC{{H}_{3}}\]
done
clear
D)
\[PhCONHC{{H}_{2}}C{{O}_{2}}H\]
done
clear
View Answer play_arrow
question_answer 143) The rate constant of a reaction is found to be\[3\times {{10}^{-3}}mol.\text{ }{{L}^{-1}}mi{{n}^{-1}}\]. The order of the reaction is:
A)
zero
done
clear
B)
1
done
clear
C)
2
done
clear
D)
1.5
done
clear
View Answer play_arrow
question_answer 144) What is the two third life of a first order reaction having\[k=5.48\times {{10}^{-14}}{{s}^{-1}}\]:
A)
\[2.01\times {{10}^{11}}s\]
done
clear
B)
\[2.01\times {{10}^{13}}s\]
done
clear
C)
\[8.08\times {{10}^{13}}s\]
done
clear
D)
\[16.04\times {{10}^{11}}s\]
done
clear
View Answer play_arrow
question_answer 145) Which of the following solvents are aprotic? \[N{{H}_{3}}\] \[S{{O}_{2}}\] \[C{{H}_{3}}CN\] \[C{{H}_{3}}C{{O}_{2}}H\]
A)
A, B, C
done
clear
B)
A, C, D
done
clear
C)
B, C
done
clear
D)
A, C
done
clear
View Answer play_arrow
question_answer 146) Chlorobenzene is o, p-directing in electrophilic substitution reaction. The directing influence is explained by:
A)
+ M of\[Ph\]
done
clear
B)
\[+I\] of\[Cl\]
done
clear
C)
+M of\[Cl\]
done
clear
D)
\[-I\] of\[Ph\]
done
clear
View Answer play_arrow
question_answer 147) 5 L of a solution contains 25 mg of\[CaC{{O}_{3}}\]. What is its concentration in ppm?(mol. wt. of \[CaC{{O}_{3}}\]is 100):
A)
25
done
clear
B)
1
done
clear
C)
5
done
clear
D)
2500
done
clear
View Answer play_arrow
question_answer 148) Observe the following abbrevations: \[{{\pi }_{obs}}=\]observed colligative property \[{{\pi }_{cal}}=\]theoretical colligative property assuming normal behaviour of solute. van't Hoff factor (i) is given by:
A)
\[i={{\pi }_{obs}}\times {{\pi }_{cal}}\]
done
clear
B)
\[i={{\pi }_{obs}}+{{\pi }_{cal}}\]
done
clear
C)
\[i={{\pi }_{obs}}-{{\pi }_{cal}}\]
done
clear
D)
\[i=\frac{{{\pi }_{obs}}}{{{\pi }_{cal}}}\]
done
clear
View Answer play_arrow
question_answer 149) The correct order for homolytic bond dissociation energies (\[\Delta H\]in kcal/Mol) for \[C{{H}_{4}}(A),{{C}_{2}}{{H}_{6}}(B)\]and\[C{{H}_{3}}Br(C),\]under identical experimental conditions:
A)
\[C>B>A\]
done
clear
B)
\[B>C>A\]
done
clear
C)
\[C>A>B\]
done
clear
D)
\[A>B>C\]
done
clear
View Answer play_arrow
question_answer 150) \[RX+{{I}^{-}}\xrightarrow{{}}R-I+{{X}^{-}}\]is an example of ....... reaction:
A)
nucleophilic addition
done
clear
B)
nucleophilic substitution
done
clear
C)
electrophilic addition
done
clear
D)
elimination
done
clear
View Answer play_arrow
question_answer 151) Protista includes:
A)
dinoflagellates, Amoeba, Paramecium
done
clear
B)
mushroom, Paramecium, Euglena
done
clear
C)
Hydra, Amoeba, Paramecium
done
clear
D)
yeast, Euglena, dinoflagellates
done
clear
View Answer play_arrow
question_answer 152) Ranthambore National Park is situated in :
A)
Assam
done
clear
B)
Jharkhand
done
clear
C)
Uttaranchal
done
clear
D)
Rajasthan
done
clear
View Answer play_arrow
question_answer 153) Scientific name of king cobra is :
A)
Naja naja
done
clear
B)
Bungarus coerulus
done
clear
C)
Naja hunnah
done
clear
D)
Viper a russelli
done
clear
View Answer play_arrow
question_answer 154) Select the correct statement:
A)
Birds are poikilothermic
done
clear
B)
Flatworms are coelomic animals
done
clear
C)
Earthworm is metamerically segmented
done
clear
D)
Fishes are radially symmetrical
done
clear
View Answer play_arrow
question_answer 155) Select the correct statement:
A)
cleavage follows gastrulation
done
clear
B)
yolk content in egg has no role in cleavage
done
clear
C)
cleavage is repeated mitotic division of zygote
done
clear
D)
gastrulation and blastulation are followed by each other
done
clear
View Answer play_arrow
question_answer 156) Select the correct set of animals of class mammalia :
A)
lion, hippopotamus, penguin, bat
done
clear
B)
lion, bat, whale, ostrich
done
clear
C)
hippopotamus, penguin, whale, kangaroo
done
clear
D)
whale, bat, kangaroo, hippopotamus
done
clear
View Answer play_arrow
question_answer 157) Select the statement which is not correct:
A)
Polygenic character is controlled by multiple alleles
done
clear
B)
In case of polygenic inheritance thousands of intermediate phenotypes are found between 2 extreme ones
done
clear
C)
Height, weight, skin colour are polygenic
done
clear
D)
Polygenic character is controlled by multiple genes
done
clear
View Answer play_arrow
question_answer 158) Serum is :
A)
blood without corpuscles
done
clear
B)
blood without fibrinogen
done
clear
C)
blood without fibrinogen and corpuscles
done
clear
D)
otherwise called as plasma
done
clear
View Answer play_arrow
question_answer 159) The blood cell involved in production of humoral immunity is :
A)
B-lymphocyte
done
clear
B)
T-lymphocyte
done
clear
C)
eosinophil
done
clear
D)
monocytes
done
clear
View Answer play_arrow
question_answer 160) The difference between blood and lymph is:
A)
blood has RBC and WBC while lymph has no cells
done
clear
B)
blood has RBC and WBC while lymph only WBC
done
clear
C)
blood has WBC while lymph has RBC
done
clear
D)
blood has dissolve salt while lymph has no cells
done
clear
View Answer play_arrow
question_answer 161) The hormone insulin is secreted by :
A)
pancreas
done
clear
B)
liver
done
clear
C)
kidney
done
clear
D)
adrenal gland
done
clear
View Answer play_arrow
question_answer 162) The outermost covering of brain is:
A)
duramater
done
clear
B)
arachnoid
done
clear
C)
piamater
done
clear
D)
choroid layer
done
clear
View Answer play_arrow
question_answer 163) The primary host of Plasmodium is :
A)
man
done
clear
B)
male Culex
done
clear
C)
sheep
done
clear
D)
female Anopheles
done
clear
View Answer play_arrow
question_answer 164) Characteristic features such as four-chambered heart, feather and pneumatic bone are applicable to which class of vertebrate?
A)
Cyclostomata
done
clear
B)
Aves
done
clear
C)
Reptilia
done
clear
D)
Mammals
done
clear
View Answer play_arrow
question_answer 165) The statement, ?Tiger is in the apex of food chain?, indicates:
A)
tiger has many enemies
done
clear
B)
tiger has maximum biomass
done
clear
C)
tiger is omnivorous
done
clear
D)
tiger is dependent upon large number of herbivores and even more number of trees in forest
done
clear
View Answer play_arrow
question_answer 166) The term test cross refers to a cross between :
A)
\[{{F}_{1}}\] hybird with another \[{{F}_{1}}\]hybrid
done
clear
B)
\[{{F}_{1}}\]hybrid and a double recessive individual
done
clear
C)
\[{{F}_{1}}\]hybrid and either of the parents
done
clear
D)
\[{{F}_{1}}\]hybrid and \[{{F}_{2}}\]individual
done
clear
View Answer play_arrow
question_answer 167) Tusk of elephant is modified :
A)
incisor
done
clear
B)
canine
done
clear
C)
premolar
done
clear
D)
molar
done
clear
View Answer play_arrow
question_answer 168) Water vascular system is characteristic feature of phylum:
A)
Porifera
done
clear
B)
Colenterata
done
clear
C)
Mollusca
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow
question_answer 169) What is cholecystokinin?
A)
Enzyme
done
clear
B)
Bile-pigement
done
clear
C)
Gastro-intestinal hormone
done
clear
D)
Lipid
done
clear
View Answer play_arrow
question_answer 170) Which of the following is correct about mammalian testes?
A)
Graafian follicles, Sertoli cells, Leydig cells
done
clear
B)
Graafian follicles, Sertoli cells, seminiferous tubules
done
clear
C)
Sertoli cells, seminiferous tubules, cells
done
clear
D)
Graafian follicle, Leydig cells seminiferous tubules
done
clear
View Answer play_arrow
question_answer 171) Which is not correct with respect to human kidney?
A)
The peripheral region is called cortex and central medulla
done
clear
B)
Malpighian capsule are present in the cortex region
done
clear
C)
Blood enters glomerulus trough efferent arterioles
done
clear
D)
The concave part of kidney is called hilus
done
clear
View Answer play_arrow
question_answer 172) Which of the following is correct?
A)
Leukemia - skin cancer
done
clear
B)
Diabetes - sugar free
done
clear
C)
Rheumatic fever - defective pacemaker
done
clear
D)
Heart attack - radiation therapy
done
clear
View Answer play_arrow
question_answer 173) Which of the following is exsitu conservation'
A)
Banning of Akhard Sikar in Similipal
done
clear
B)
Breeding of animals in Nandan Kanaha
done
clear
C)
Protecting migration of birds in Chilka lake
done
clear
D)
Protecting fishing in Bhitar Kanika
done
clear
View Answer play_arrow
question_answer 174) Which of the following is not a correct match?
A)
Sex determination - a chromosomal phenomenon
done
clear
B)
Y chromosome - autosomal
done
clear
C)
Red green colour blindness in human - a sex linked character
done
clear
D)
An abnormal chromosome number in each cell - a case of polyploidy
done
clear
View Answer play_arrow
question_answer 175) Which of the following is not a correct pair?
A)
Mesozoic era-age of mammals
done
clear
B)
Origin of species-Charles Darwin
done
clear
C)
Study of fossil-Palaeontology
done
clear
D)
Mutation theory-Hugo de Vries
done
clear
View Answer play_arrow
question_answer 176) Which of the following pyramid of numbers in ecology is rot upright?
A)
Pond ecosystem
done
clear
B)
Desert ecosystem
done
clear
C)
Tree ecosystem
done
clear
D)
Forest ecosystem
done
clear
View Answer play_arrow
question_answer 177) Which of the following sets of characters are applicable in metamorphosis of tadpole larva of frog and toads?
A)
Reabsorption of gills and resorption of tail
done
clear
B)
Reabsorption of gills and lengthening of tail
done
clear
C)
Complete development of gills and reabsorption of tail
done
clear
D)
Complex development of gills and lengthening of tail
done
clear
View Answer play_arrow
question_answer 178) Which of the following is wrongly matched?
A)
Arthropoda-cockroach
done
clear
B)
Annelida-Hydra
done
clear
C)
Echinodermata-star fish
done
clear
D)
Nemathelminthes-Ascaris
done
clear
View Answer play_arrow
question_answer 179) Wuchereria bancrofti 'S :
A)
a Platyhelminthes
done
clear
B)
only host in man
done
clear
C)
causing blockage of lymphatic vessel
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 180) Middle piece of sperm contains:
A)
mitochondria, Golgi bodies, centriole
done
clear
B)
axial filament, centriole, Golgi bodies
done
clear
C)
mitochondria, centriole, axial filament
done
clear
D)
Golgi bodies, axial filament, centriole
done
clear
View Answer play_arrow
question_answer 181) Statement 1: Cephalochordata bears noto-chord all along the body throughout life. Statement 2 : Urochordate bears vertebral column only in tail throughout the life. Which statement is correct?
A)
Both 1st and 2nd are correct
done
clear
B)
1st correct, 2nd wrong
done
clear
C)
1st wrong, 2nd correct
done
clear
D)
Both 1st and 2nd are wrong
done
clear
View Answer play_arrow
question_answer 182) Secretin hormone is secreted from :
A)
stomach and stimulates gastric gland
done
clear
B)
duodenum and stimulates liver
done
clear
C)
thyroid and stimulates thyroid gland
done
clear
D)
duodenum and stimulates pancreas
done
clear
View Answer play_arrow
question_answer 183) The main point of Darwin?s theory is:
A)
variation
done
clear
B)
natural selection
done
clear
C)
enormous fertility
done
clear
D)
mutation
done
clear
View Answer play_arrow
question_answer 184) Which of the following is discovered by Kendall?
A)
FSH and LH
done
clear
B)
Corticotrophin
done
clear
C)
Thyroxine
done
clear
D)
Insulin
done
clear
View Answer play_arrow
question_answer 185) Reserpine is obtained from :
A)
Asafoetida
done
clear
B)
Rauwolfia serpentine
done
clear
C)
Curcuma longa
done
clear
D)
Papaver somniferum
done
clear
View Answer play_arrow
question_answer 186) The coiling of tendril around some base in response to touch is called :
A)
hydrotaxis
done
clear
B)
chemotaxis
done
clear
C)
thigmotropism
done
clear
D)
geotaxis
done
clear
View Answer play_arrow
question_answer 187) Sperm enters from which part of egg :
A)
any where in unfertilized egg from animal pole
done
clear
B)
from animal pole in unfertilized egg
done
clear
C)
in unfertilized egg from vegetai pole
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 188) Seed coat is derived from :
A)
pericarp
done
clear
B)
epicarp
done
clear
C)
integuments of ovule
done
clear
D)
nucellus
done
clear
View Answer play_arrow
question_answer 189) 6 molecules glucose + 6 molecules of \[{{O}_{2}}\] and 38 ADP combined to form \[6{{H}_{2}}O,\text{ }6C{{O}_{2}}\] and :
A)
38 molecules of ATP
done
clear
B)
28 ATP
done
clear
C)
38 ADP
done
clear
D)
28 ADP
done
clear
View Answer play_arrow
question_answer 190) A characteristic feature of ovary of Brassica campestris is:
A)
presence of feplum
done
clear
B)
axile placentation
done
clear
C)
epignous
done
clear
D)
multilocular nature
done
clear
View Answer play_arrow
question_answer 191) One of the characteristic of Hydra is :
A)
predation
done
clear
B)
metamerism
done
clear
C)
hibernation
done
clear
D)
mimicry
done
clear
View Answer play_arrow
question_answer 192) Where does exoerythrocytic cycle take place in life cycle of Plasmodium?
A)
RLC of human
done
clear
B)
Human liver
done
clear
C)
Stomach of Anopheles mosquito
done
clear
D)
Salivary gland of Anopheles mosquito
done
clear
View Answer play_arrow
question_answer 193) Primary acceptor of \[C{{O}_{2}}\] in photosynthesis is:
A)
phosphoric acid
done
clear
B)
ribulose phosphate
done
clear
C)
glucose
done
clear
D)
ribulose 1, 5 biphosphate
done
clear
View Answer play_arrow
question_answer 194) If Hydra is cut transversely in three equal parts then :
A)
all three parts will die
done
clear
B)
regeneration will occur in all the three parts
done
clear
C)
regeneration will occur only in anterior
done
clear
D)
regeneration occurs only in middle part
done
clear
View Answer play_arrow
question_answer 195) Which of the following pair lack the unit membrane?
A)
Nucleus and ER
done
clear
B)
Mitochondria and chloroplast
done
clear
C)
Ribosome and nucleolus
done
clear
D)
Golgi body and lysosome
done
clear
View Answer play_arrow
question_answer 196) Which of the following statement is correct ?
A)
In Cycas, megasporophyll produce pollen grains
done
clear
B)
In Agaricus, gills produce basidiospores
done
clear
C)
In Aspergillus, fruiting body is perithecium
done
clear
D)
In Funaria, capsule represents gametophytic generation
done
clear
View Answer play_arrow
question_answer 197) The number of DNA is chromosome at \[{{G}_{2}}\]stage:
A)
one
done
clear
B)
two
done
clear
C)
four
done
clear
D)
eight
done
clear
View Answer play_arrow
question_answer 198) How many egg are found in egg chamber of female cockroach ?
A)
2
done
clear
B)
4
done
clear
C)
8
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 199) Which of the following is a disaccharide?
A)
Glucose
done
clear
B)
Fructose
done
clear
C)
Sucrose
done
clear
D)
Galactose
done
clear
View Answer play_arrow
question_answer 200) If all the peptide bonds of protein are broken, then the remaining part is :
A)
amide
done
clear
B)
oligosaccharide
done
clear
C)
polypeptide
done
clear
D)
amino acid
done
clear
View Answer play_arrow
question_answer 201) Periplaneta has no respiratory pigment in its blood because:
A)
air is conducted directly to the body tissues
done
clear
B)
it has haemocoelom
done
clear
C)
it has anaerobic respiration
done
clear
D)
it lacks blood cell in blood
done
clear
View Answer play_arrow
question_answer 202) Which is correct for meiotic metaphase-I?
A)
Bivalents are arranged at equator
done
clear
B)
Univalents are arranged at equator
done
clear
C)
Non-homologous chromosomes forms pair
done
clear
D)
Spindle fibres are attached at chromomere
done
clear
View Answer play_arrow
question_answer 203) Hydrolysis of lipid yields :
A)
fats
done
clear
B)
fatty acids and glycerol
done
clear
C)
mannose and glycerol
done
clear
D)
maltose and fatty acid
done
clear
View Answer play_arrow
question_answer 204) In the following how the sap wood is converted into heart wood?
A)
By degeneration of protoplast of living cells
done
clear
B)
Tylosis formation
done
clear
C)
By deposition of resins, oil, gums etc
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 205) In which of the following animal cleavage divisions are restricted to a small part of cytoplasm and nucleus in animal pole of egg?
A)
Cockroach
done
clear
B)
Frog
done
clear
C)
Chick
done
clear
D)
Rabbit
done
clear
View Answer play_arrow
question_answer 206) Exception of Mendel?s law is:
A)
independent assortment
done
clear
B)
linkage
done
clear
C)
purity of gametes
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 207) What type of gametes will form by genotype RrYy ?
A)
RY, Ry, rY, ry
done
clear
B)
RY, Ry, ry, ry
done
clear
C)
Ry, Ry, Yy, ry
done
clear
D)
Rr, RR, Yy, YY
done
clear
View Answer play_arrow
question_answer 208) The position of protoxylem in leaf is :
A)
adaxial
done
clear
B)
abaxial
done
clear
C)
surrounded by metaxylem
done
clear
D)
lateral
done
clear
View Answer play_arrow
question_answer 209) In Pheretima, locomotion occurs with the help of:
A)
circular muscles
done
clear
B)
longitudinal muscles and setae
done
clear
C)
circular, longitudinal muscles and setae
done
clear
D)
parapodia
done
clear
View Answer play_arrow
question_answer 210) The difference in phloem of gymnosperms and angiosperms is due to :
A)
parenchyma
done
clear
B)
sieve cell
done
clear
C)
companion cell
done
clear
D)
fibres
done
clear
View Answer play_arrow
question_answer 211) A type of life cycle in which plasmogamy, karyogamy, haplodization takes place but not at specific place in life cycle of an organism is called as:
A)
parasexuality
done
clear
B)
heterozygosity
done
clear
C)
homozygosity
done
clear
D)
asexuality
done
clear
View Answer play_arrow
question_answer 212) In Amoeba which controls the cytoplasmic osmality:
A)
nucleus
done
clear
B)
ectoplasm
done
clear
C)
biurets
done
clear
D)
contractile vacuole
done
clear
View Answer play_arrow
question_answer 213) Branched, aseptate. coenocytic mycelium present in :
A)
Aspergillus
done
clear
B)
Albugo
done
clear
C)
Penicillium
done
clear
D)
Erysiphe
done
clear
View Answer play_arrow
question_answer 214) In which of the following aestivation sepal?s/ petal's one margin covers the other and its margin is covered by previous one?
A)
Valvate
done
clear
B)
Twisted
done
clear
C)
Imbricate
done
clear
D)
Quincuncial
done
clear
View Answer play_arrow
question_answer 215) In Cockroach vision is due to :
A)
one compound eye
done
clear
B)
only two compound eyes
done
clear
C)
two simple eyes
done
clear
D)
two compound and two simple eyes
done
clear
View Answer play_arrow
question_answer 216) The economically important plant of Malvaceae :
A)
Gossypium kirsutum
done
clear
B)
Hibiscus cannabis
done
clear
C)
Abelmoschus esculentum
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 217) The cells without nuclei are present in :
A)
vascular cambium
done
clear
B)
root hair
done
clear
C)
companion cell
done
clear
D)
members of sieve tube
done
clear
View Answer play_arrow
question_answer 218) Which of the following is not correctly matched ?
A)
Rhinoncephalon-olfactory
done
clear
B)
Hypothalamus-pituitary
done
clear
C)
Cerebellum-balance
done
clear
D)
Medulla oblongata-temperature regulation
done
clear
View Answer play_arrow
question_answer 219) Pyruvic acid is the end product of which process?
A)
Krebs cycle
done
clear
B)
Calvin cycle
done
clear
C)
Pentose phosphate pathway
done
clear
D)
Glycolysis
done
clear
View Answer play_arrow
question_answer 220) The nutritive layer of microsporangia of cypsella:
A)
endothecium
done
clear
B)
exothecium
done
clear
C)
sporogenous tissue
done
clear
D)
tapetum
done
clear
View Answer play_arrow
question_answer 221) The scientist who was awarded Nobel prize in 1959 for in vitro synthesis of polyribonucleotide :
A)
Kornberg
done
clear
B)
Calvin
done
clear
C)
Khorana
done
clear
D)
Ochoa
done
clear
View Answer play_arrow
question_answer 222) Which of the following have sunken stomata?
A)
Nerium
done
clear
B)
Mangifera
done
clear
C)
Hydrilla
done
clear
D)
Zea mays
done
clear
View Answer play_arrow
question_answer 223) Heterocercal tail is found in :
A)
cartilaginous fishes
done
clear
B)
bony fishes
done
clear
C)
whale
done
clear
D)
amphibians
done
clear
View Answer play_arrow
question_answer 224) When a plasmolysed cells is placed in a hypotonic solution then water will move inside the cell. Which force causes this?
A)
DPD
done
clear
B)
OP
done
clear
C)
WP
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 225) Dormancy of seed is broken by :
A)
auxin
done
clear
B)
gibberellins
done
clear
C)
ethylene
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
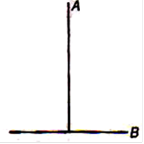
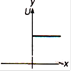
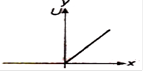
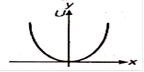
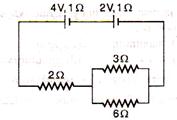
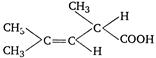 Compound can exhibit:
Compound can exhibit:
