question_answer 1) A solid cylinder is rolling down on an inclined plane of angle\[\theta \]. The coefficient of static friction between the plane and cylinder \[{{\mu}_{s}}\]. Then condition for the cylinder not to slip is
A)
\[\tan \theta \ge 3{{\mu }_{s}}\]
done
clear
B)
\[\tan \theta >3{{\mu }_{s}}\]
done
clear
C)
\[\tan \theta \le 3{{\mu }_{s}}\]
done
clear
D)
\[\tan \theta <3{{\mu }_{s}}\]
done
clear
View Answer play_arrow
question_answer 2) The moment of inertia of a circular ring of mass kg about an axis passing through its centre and perpendicular to its plane is \[4\text{ }kg-{{m}^{2}}.\] The diameter of the ring is
A)
2 m
done
clear
B)
4 m
done
clear
C)
5 m
done
clear
D)
6 m
done
clear
View Answer play_arrow
question_answer 3) If g is the acceleration due to gravity on the surface of earth, its value at a height equal to double the radius of earth is
A)
g
done
clear
B)
\[\frac{g}{2}\]
done
clear
C)
\[\frac{g}{3}\]
done
clear
D)
\[\frac{g}{9}\]
done
clear
View Answer play_arrow
question_answer 4) On bombardment of \[{{U}^{235}}\]by slow neutrons, 200 MeV energy is released. If the power output of atomic reactor is 1.6 MW, then the rate of fission will be
A)
\[5\times {{10}^{16}}{{/}_{s}}\]
done
clear
B)
\[10\times {{10}^{16}}{{/}_{s}}\]
done
clear
C)
\[15\times {{10}^{16}}{{/}_{s}}\]
done
clear
D)
\[20\times {{10}^{-16}}{{/}_{s}}\]
done
clear
View Answer play_arrow
question_answer 5) A stress of \[3.18\times {{10}^{8}}N-{{m}^{-2}}\] is applied to a steel rod of length 1 m along its length. Its Youngs modulus is \[2\times {{10}^{11}}\text{ }N-{{m}^{-2}}.\] Then, the elongation produced in the rod (in mm) is
A)
3.18
done
clear
B)
6.36
done
clear
C)
5.18
done
clear
D)
1.59
done
clear
View Answer play_arrow
question_answer 6) A liquid is allowed into a tube of truncated cone shape. Identify the correct statement from the following.
A)
The speed is high at the wider end and low at the narrow end
done
clear
B)
The speed is low at the wider end and high at the narrow end
done
clear
C)
The speed is same at both ends in a stream line flow
done
clear
D)
The liquid flows with uniform velocity in the tube
done
clear
View Answer play_arrow
question_answer 7) A sphere of radius R is gently dropped into liquid of viscosity \[\eta \] in a vertical uniform tube. It attains a terminal velocity v. Another sphere of radius 2 R when dropped into the same liquid, will attain its terminal velocity
A)
v
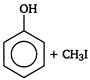
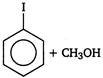
done
clear
B)
2v
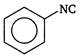
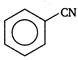
done
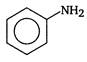
clear
C)
4v
done
clear
D)
9v
done
clear
View Answer play_arrow
question_answer 8) The excess pressure in a bubble of radius R of a gas in a liquid of surface tension S is
A)
\[\frac{2S}{R}\]
done
clear
B)
\[\frac{2R}{S}\]
done
clear
C)
\[\frac{2S}{{{R}^{2}}}\]
done
clear
D)
\[\frac{2{{R}^{2}}}{S}\]
done
clear
View Answer play_arrow
question_answer 9) The average kinetic energy of a gas molecule is
A)
Proportional to pressure of gas
done
clear
B)
inversely proportional to volume of gas
done
clear
C)
inversely proportional to absolute temperature of gas
done
clear
D)
directly proportional to absolute temperature of gas
done
clear
View Answer play_arrow
question_answer 10) Ideal gas undergoes an adiabatic change in its state from \[({{p}_{1}},{{V}_{1}},{{T}_{1}})\]to \[({{p}_{2}},{{V}_{2}},{{T}_{2}}).\] The work done (W) in the process is (\[\mu \] = number of molecules, \[{{C}_{p}}\]and \[{{C}_{v}}\]are molar specific heats of gas)
A)
\[w=\mu {{C}_{p}}({{T}_{1}}-{{T}_{2}})\]
done
clear
B)
\[w=\mu {{C}_{v}}({{T}_{1}}-{{T}_{2}})\]
done
clear
C)
\[w=\mu {{C}_{p}}({{T}_{1}}+{{T}_{2}})\]
done
clear
D)
\[w=\mu {{C}_{v}}({{T}_{1}}+{{T}_{2}})\]
done
clear
View Answer play_arrow
question_answer 11) For an ideal gas
A)
\[{{C}_{p}}\]is less than\[{{C}_{v}}\]
done
clear
B)
\[{{C}_{p}}\]is equal to \[{{C}_{v}}\]
done
clear
C)
\[{{C}_{p}}\]is greater than\[{{C}_{v}}\]
done
clear
D)
\[{{C}_{p}}={{C}_{v}}=0\]
done
clear
View Answer play_arrow
question_answer 12) For a gas molecule with 6 degrees of freedom the law of equipartition of energy gives the following relation between the molecular specific heat \[({{C}_{v}})\] and gas constant (R)
A)
\[{{C}_{v}}=\frac{R}{2}\]
done
clear
B)
\[{{C}_{v}}=R\]
done
clear
C)
\[{{C}_{v}}=2R\]
done
clear
D)
\[{{C}_{v}}=3R\]
done
clear
View Answer play_arrow
question_answer 13) Wiens displacement law for emission of radiation can be written as
A)
\[{{\lambda }_{\max }}\] is proportional to absolute temperature (T)
done
clear
B)
\[{{\lambda }_{\max }}\] is proportional to square of absolute temperature \[({{T}^{2}})\]
done
clear
C)
\[{{\lambda }_{\max }}\] is inversely proportional to absolute temperature (T)
done
clear
D)
\[{{\lambda }_{\max }}\] is inversely proportional to square of absolute temperature \[({{T}^{2}})\] (\[{{\lambda }_{\max }}\] = wavelength whose energy density is greatest)
done
clear
View Answer play_arrow
question_answer 14) The frequency of the fundamental note in a wire stretched under tension T is f. If the tension is increased to 25 T, then the frequency of the fundamental note will be
A)
25f
done
clear
B)
5f
done
clear
C)
10f
done
clear
D)
f
done
clear
View Answer play_arrow
question_answer 15) The frequency of fundamental note in an organ pipe is 240 Hz. On blowing air, frequencies 720 Hz and 1200 Hz are heard. This indicates that organ pipe is
A)
a pipe closed at one end
done
clear
B)
a pipe open at both ends
done
clear
C)
closed at both ends
done
clear
D)
having holes like flute
done
clear
View Answer play_arrow
question_answer 16) If \[{{L}_{1}}\]and \[{{L}_{2}}\]are the lengths of the first and second resonating air columns in a resonance tube, then the wavelength of the note produced is
A)
\[2({{L}_{2}}+{{L}_{1}})\]
done
clear
B)
\[2({{L}_{2}}-{{L}_{1}})\]
done
clear
C)
\[2\left( {{L}_{2}}-\frac{{{L}_{1}}}{2} \right)\]
done
clear
D)
\[2\left( {{L}_{2}}+\frac{{{L}_{1}}}{2} \right)\]
done
clear
View Answer play_arrow
question_answer 17) Beats are produced by frequencies \[{{f}_{1}}\]and \[{{f}_{2}}\left( {{f}_{1}}>{{f}_{2}} \right).\]The duration of time between two successive maxima or minima is equal to
A)
\[\frac{1}{{{f}_{1}}+{{f}_{2}}}\]
done
clear
B)
\[\frac{2}{{{f}_{1}}-{{f}_{2}}}\]
done
clear
C)
\[\frac{2}{{{f}_{1}}+{{f}_{2}}}\]
done
clear
D)
\[\frac{2}{{{f}_{1}}-{{f}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 18) If a body is executing simple harmonic motion, then
A)
at extreme positions, the total energy is zero
done
clear
B)
at equilibrium position, the total energy is in the form of potential energy
done
clear
C)
at equilibrium position, the total energy is in the form of kinetic energy
done
clear
D)
at extreme position, the total energy is infinite
done
clear
View Answer play_arrow
question_answer 19) An electric dipole has a pair of equal and opposite point charges q and - q separated by a distance 2x. The axis of the dipole is defined as
A)
direction from positive charge to negative charge
done
clear
B)
direction from negative charge to positive charge
done
clear
C)
perpendicular to the line joining the two charges drawn at the centre and pointing upward direction
done
clear
D)
perpendicular to the line joining the two charges drawn at the centre and pointing downward direction
done
clear
View Answer play_arrow
question_answer 20) The dipole moment of a dipole in an uniform external field E is P. Then, the torque (x) acting on the dipole is
A)
\[\vec{t}=\vec{p}\times \vec{E}\]
done
clear
B)
\[\vec{t}=\vec{p}.\vec{E}\]
done
clear
C)
\[\vec{t}=2(\vec{p}+\vec{E})\]
done
clear
D)
\[\vec{t}=(\vec{p}+\vec{E})\]
done
clear
View Answer play_arrow
question_answer 21) The electric flux through a closed surface area S enclosing charge Q is \[\phi .\] If the surface area is doubled, then the flux is
A)
\[2\phi \]
done
clear
B)
\[\phi /2\]
done
clear
C)
\[\phi /4\]
done
clear
D)
\[\phi \]
done
clear
View Answer play_arrow
question_answer 22) Consider a thin spherical shell of radius R consisting of uniform surface charge density 0. The electric field at a point of distance x from its centre and outside the shell is
A)
inversely proportional to\[\sigma \]
done
clear
B)
directly proportional to \[{{x}^{2}}\]
done
clear
C)
directly proportional to \[\sigma \]
done
clear
D)
inversely proportional to \[{{x}^{2}}\]
done
clear
View Answer play_arrow
question_answer 23) The work done in bringing at a unit positive charge from infinity distance to a point at distance X from a positive charge Q is W. Then, the potential \[^{\phi }\] at that point is
A)
\[\frac{wQ}{x}\]
done
clear
B)
\[w\]
done
clear
C)
\[\frac{w}{Q}\]
done
clear
D)
\[wQ\]
done
clear
View Answer play_arrow
question_answer 24) The capacitance C of a capacitor is
A)
independent of the charge and potential of the capacitor
done
clear
B)
dependent on the charge and independent of potential
done
clear
C)
independent of the geometrical configuration of the capacitor
done
clear
D)
independent of the dielectric medium between the two conducting surfaces of the capacitor
done
clear
View Answer play_arrow
question_answer 25)
Four capacitors are connected in a circuit as shown in the following figure. Calculate the effective capacitance between the points A and B.
A)
\[\frac{4}{3}\mu F\]
done
clear
B)
\[\frac{24}{5}\mu F\]
done
clear
C)
\[9\mu F\]
done
clear
D)
\[5\mu F\]
done
clear
View Answer play_arrow
question_answer 26) Metals have
A)
zero resistivity
done
clear
B)
high resistivity
done
clear
C)
low resistivity
done
clear
D)
infinite resistivity
done
clear
View Answer play_arrow
question_answer 27) The electric potential inside a conducting sphere
A)
increases from centre to surface
done
clear
B)
decreases from centre to surface
done
clear
C)
remains constant from centre to surface
done
clear
D)
is zero at every point inside
done
clear
View Answer play_arrow
question_answer 28) Electron of mass m and charge e in external field E experiences acceleration
A)
\[\frac{e}{mE}\] in the opposite direction to the field
done
clear
B)
\[\frac{eE}{m}\] in the direction of the field m
done
clear
C)
\[\frac{em}{E}\], in the direction of the field
done
clear
D)
\[\frac{eE}{m}\], in the opposite direction of the field m
done
clear
View Answer play_arrow
question_answer 29) Kirchhof Ps second law for the analysis of circuit is based on
A)
conservation of charge
done
clear
B)
conservation of energy
done
clear
C)
conservation of both charge and energy
done
clear
D)
conservation of momentum of electron
done
clear
View Answer play_arrow
question_answer 30)
In circuit shown below, the resistances are given in ohm and the battery is assumed ideal with emf equal to 3 V. The voltage across the resistance \[{{R}_{4}}\] is
A)
0.4 V
done
clear
B)
0.6V
done
clear
C)
1.2 V
done
clear
D)
1.5 V
done
clear
View Answer play_arrow
question_answer 31) The direction of induced magnetic field d \[\mathbf{\vec{B}}\] due to current element id \[\vec{L}\] at a point of distance r from it, when a current i passes through a long conductor is in the direction
A)
of position vector \[\vec{r}\] of the point
done
clear
B)
of current element d \[\vec{L}\]
done
clear
C)
perpendicular to both d \[\vec{L}\]and \[\vec{r}\]
done
clear
D)
perpendicular to d \[\vec{L}\] only
done
clear
View Answer play_arrow
question_answer 32) The magnetic force on a charged particle moving in the field does not work, because
A)
kinetic energy of the charged particle does not change
done
clear
B)
the charge of the particle remains same
done
clear
C)
the magnetic force is parallel to velocity of the particle
done
clear
D)
the magnetic force is parallel to magnetic field
done
clear
View Answer play_arrow
question_answer 33) To convert a moving coil galvanometer (MCG) into a voltmeter
A)
a high resistance R is connected in parallel with MCG
done
clear
B)
a low resistance r is connected in parallel with MCG
done
clear
C)
a low resistance r is connected in series with MCG
done
clear
D)
a high resistance R is connected in series with MCG
done
clear
View Answer play_arrow
question_answer 34) Identify the correct statement from the following.
A)
Cyclotron frequency is dependent on speed of the charged particle
done
clear
B)
Kinetic energy of charged particle in cyclotron does not dependent on its mass
done
clear
C)
Cyclotron frequency does not depend on speed of charged particle
done
clear
D)
Kinetic energy of charged particle in cyclotron is independent of its charge
done
clear
View Answer play_arrow
question_answer 35) Consider two straight parallel conductors A and B separated by a distance x and carrying individual currents and iB respectively. If the two conductors attract each other, it indicates that
A)
the two currents are parallel in direction
done
clear
B)
the two currents are anti-parallel in direction
done
clear
C)
the magnetic lines of induction are parallel
done
clear
D)
the magnetic lines of induction are parallel to length of conductors
done
clear
View Answer play_arrow
question_answer 36) The magnetic susceptibility of paramagnetic materials is
A)
positive, but very high
done
clear
B)
negative, but small
done
clear
C)
negative but very high
done
clear
D)
positive, but small
done
clear
View Answer play_arrow
question_answer 37) According to Lenzs law of electromagnetic induction
A)
the induced emf is not in the direction opposing the change in magnetic flux
done
clear
B)
the relative motion between the coil and magnet produces change in magnetic flux
done
clear
C)
only the magnet should be moved towards coil
done
clear
D)
only the coil should be moved towards magnet
done
clear
View Answer play_arrow
question_answer 38) According to phenomenon of mutual inductance
A)
the mutual inductance does not dependent on geometry of the two coils involved
done
clear
B)
the mutual inductance depends on the intrinsic magnetic property, like relative permeability of the material
done
clear
C)
the mutual inductance is independent of the magnetic property of the material
done
clear
D)
ratio of magnetic flux produced by the coil 1 at the place of the coil 2 and the current in the coil 2 will be different from that of the ratio defined by interchanging the coils
done
clear
View Answer play_arrow
question_answer 39) The natural frequency (\[{{\omega }_{0}}\]) of oscillations in L-C circuit is given by
A)
\[\frac{1}{2\pi }\frac{1}{\sqrt{LC}}\]
done
clear
B)
\[\frac{1}{2\pi }\sqrt{LC}\]
done
clear
C)
\[\frac{1}{\sqrt{LC}}\]
done
clear
D)
\[\sqrt{LC}\]
done
clear
View Answer play_arrow
question_answer 40) In L-C-R series circuit the resonance condition in terms of capacitive reactance \[({{X}_{c}})\] and inductive reactance \[({{X}_{L}})\] is
A)
\[{{X}_{c}}+{{X}_{L}}=0\]
done
clear
B)
\[{{X}_{c}}=0\]
done
clear
C)
\[{{X}_{L}}=0\]
done
clear
D)
\[{{X}_{c}}-{{X}_{L}}=0\]
done
clear
View Answer play_arrow
question_answer 41) In step-up transformer, relation between number of turns in primary \[({{N}_{p}})\] and number of turns in secondary \[({{N}_{s}})\] coils is
A)
\[{{N}_{s}}\]is greater than \[{{N}_{p}}\]
done
clear
B)
\[{{N}_{p}}\]is greater than \[{{N}_{s}}\]
done
clear
C)
\[{{N}_{s}}\]is equal to \[{{N}_{p}}\]
done
clear
D)
\[{{N}_{p}}=\text{ }2{{N}_{s}}\]
done
clear
View Answer play_arrow
question_answer 42) Two light sources are said to be of coherent nature
A)
when they have same frequency and a varying phase difference
done
clear
B)
when they have same frequency and a constant phase difference
done
clear
C)
when they have constant phase difference and different frequencies
done
clear
D)
when they have varying phase difference and different frequencies
done
clear
View Answer play_arrow
question_answer 43) In Youngs double slit interference pattern the fringe width
A)
can be changed only by changing the wavelength of incident light
done
clear
B)
can be changed only by changing the separation between the two slits
done
clear
C)
can be changed either by changing the wavelength or by changing the separation between two sources
done
clear
D)
is a universal constant and hence cannot be changed
done
clear
View Answer play_arrow
question_answer 44) Colours in thin films are due to
A)
diffraction phenomenon
done
clear
B)
scattering phenomenon
done
clear
C)
interference phenomenon
done
clear
D)
polarization phenomenon
done
clear
View Answer play_arrow
question_answer 45) Brewsters angle in terms of refractive index (n) of the medium
A)
\[{{\tan }^{-1}}\sqrt{n}\]
done
clear
B)
\[{{\sin }^{-1}}n\]
done
clear
C)
\[{{\sin }^{-1}}\sqrt{n}\]
done
clear
D)
\[{{\tan }^{-1}}n\]
done
clear
View Answer play_arrow
question_answer 46) The angle of incidence of light is equal to Brewsters angle, then A reflected ray is perpendicular to refracted ray B refracted ray is parallel to reflected ray C reflected light is polarized having its electric vector in the plane of incidence D refracted light is polarized
A)
and are true
done
clear
B)
and are true
done
clear
C)
and are true
done
clear
D)
and are true
done
clear
View Answer play_arrow
question_answer 47) The working of optical fibres is based on
A)
dispersion of light
done
clear
B)
total internal reflection
done
clear
C)
polarization of light
done
clear
D)
interference of light
done
clear
View Answer play_arrow
question_answer 48) First Bohr radius of an atom with Z = 82 is R. Radius of its third orbit is
A)
9 R
done
clear
B)
6 R
done
clear
C)
3 R
done
clear
D)
R
done
clear
View Answer play_arrow
question_answer 49) The de-Broglie wavelength associated with a particle moving with momentum (p) and mass 56.
A)
\[\frac{h}{p}\]
done
clear
B)
\[\frac{h}{mp}\]
done
clear
C)
\[\frac{h}{{{p}^{2}}}\]
done
clear
D)
\[\frac{{{h}^{2}}}{{{p}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 50) The angular momentum (L) of an electron moving in a stable orbit around mucleus is
A)
half integral multiple of\[\frac{h}{2\pi }\]
done
clear
B)
integral multiple of h
done
clear
C)
integral multiple of\[\frac{h}{2\pi }\]
done
clear
D)
half integral multiple of h
done
clear
View Answer play_arrow
question_answer 51) According to Moseleys law of X-rays the frequency (v) of a particular characteristic X-ray and the atomic number (Z) of the element depend on each other as
A)
\[\sqrt{v}=k{{Z}^{2}}\]
done
clear
B)
\[\sqrt{v}=\frac{h}{{{Z}^{2}}}\]
done
clear
C)
\[v=kZ\]
done
clear
D)
\[\sqrt{v}=kZ\]
done
clear
View Answer play_arrow
question_answer 52) If \[\lambda \] is decay constant and N the number of radioactive nuclei of an element, then the decay rate (R) of that element is
A)
\[\lambda {{N}^{2}}\]
done
clear
B)
\[\lambda N\]
done
clear
C)
\[\frac{\lambda }{N}\]
done
clear
D)
\[{{\lambda }^{2}}N\]
done
clear
View Answer play_arrow
question_answer 53) The ratio of half-life times of two elements A and B is \[\frac{{{T}_{A}}}{{{B}_{B}}}\] The ratio of respective decay constants \[\frac{{{\lambda }_{A}}}{{{\lambda }_{B}}}\]is
A)
\[\frac{{{T}_{B}}}{{{T}_{A}}}\]
done
clear
B)
\[\frac{{{T}_{A}}}{{{T}_{B}}}\]
done
clear
C)
\[\frac{{{T}_{A}}+{{T}_{B}}}{{{T}_{A}}}\]
done
clear
D)
\[\frac{{{T}_{A}}-{{T}_{B}}}{{{T}_{A}}}\]
done
clear
View Answer play_arrow
question_answer 54) For a nuclear to be in critical condition, the value of neutron multiplication factor (k) must be
A)
\[K>1\]
done
clear
B)
\[K<1\]
done
clear
C)
\[K=1\]
done
clear
D)
\[K=0\]
done
clear
View Answer play_arrow
question_answer 55) If nE and nH represent the number of free electrons and holes respectively in a semiconducting material, then for n-type semiconducting material
A)
\[{{n}_{g}}<<{{n}_{H}}\]
done
clear
B)
\[{{n}_{g}}>>{{n}_{H}}\]
done
clear
C)
\[{{n}_{E}}={{n}_{H}}\]
done
clear
D)
\[{{n}_{E}}={{n}_{H}}=0\]
done
clear
View Answer play_arrow
question_answer 56) An intrinsic semiconductor at 0 K temperature behaves like
A)
conductor
done
clear
B)
p-type semiconductor
done
clear
C)
n-type semiconductor
done
clear
D)
insulator
done
clear
View Answer play_arrow
question_answer 57) When a p-n junction diode is connected in forward bias its barrier potential
A)
decreases and less current flows in the circuit
done
clear
B)
decreases and more current flows in the circuit
done
clear
C)
increases and more current flows in the circuit
done
clear
D)
decreases and no current flows in the circuit
done
clear
View Answer play_arrow
question_answer 58) The main cause of zener breakdown is
A)
the base semiconductor being germanium
done
clear
B)
production of electron-hole pairs due to thermal excitation
done
clear
C)
low doping
done
clear
D)
high doping
done
clear
View Answer play_arrow
question_answer 59) The depletion layer in a silicon diode is 1 \[\mu m\] wide and its knee potential is 0.6 V, then the electric field in the depletion layer will be
A)
\[0.6\text{ }V/m\]
done
clear
B)
\[6\times 104\text{ }V/m\]
done
clear
C)
\[6\times 105\text{ }V/m\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 60) Identify the true statement for OR gate
A)
Output Y will be 1 when input A or B or both are 1
done
clear
B)
Output Y will be 0 when the either of the inputs A or B is 1
done
clear
C)
Output Y will be 1 only when both the inputs A and B are 1
done
clear
D)
Output Y will be 1 only when either of the inputs A and B are 1
done
clear
View Answer play_arrow
question_answer 61) Dimensional formula for force is
A)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
B)
\[[ML{{T}^{-2}}]\]
done
clear
C)
\[[M{{L}^{-1}}{{T}^{-2}}]\]
done
clear
D)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 62) \[\vec{A}\] is a vector with magnitude A, then the unit vector \[\vec{a}\]in the direction of vector \[\vec{A}\]is
A)
\[A\,\vec{A}\]
done
clear
B)
\[\vec{A}\,\,\,\vec{A}\]
done
clear
C)
\[\vec{A}\times \vec{A}\]
done
clear
D)
\[\frac{\left| {\vec{A}} \right|}{A}\]
done
clear
View Answer play_arrow
question_answer 63) A body is under the action of two mutually perpendicular forces of 3 N and 4 N. The resultant force acting on the body is
A)
7 N
done
clear
B)
1 N
done
clear
C)
5 N
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 64) If the scalar and vector products of two vectors \[\vec{A}\] and \[\vec{B}\]are equal in magnitude, then the angle between the two vectors is
A)
\[45{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
\[180{}^\circ \]
done
clear
D)
\[360{}^\circ \]
done
clear
View Answer play_arrow
question_answer 65) A body is moving along a straight line path with constant velocity. At an instant of time the distance travelled by it is s and its displacement is D, then
A)
D < s
done
clear
B)
D > s
done
clear
C)
D = s
done
clear
D)
D < s
done
clear
View Answer play_arrow
question_answer 66) A body is projected at an angle 0 with respect to horizontal direction with velocity u. The maximum range of the body is
A)
\[R=\frac{{{u}^{2}}\sin 2\theta }{g}\]
done
clear
B)
\[R=\frac{{{u}^{2}}{{\sin }^{2}}\theta }{2g}\]
done
clear
C)
\[R=\frac{{{u}^{2}}}{g}\]
done
clear
D)
\[R={{u}^{2}}\sin \theta \]
done
clear
View Answer play_arrow
question_answer 67) A body moving along a circular path of radius R with velocity v, has centripetal acceleration a. If its velocity is made equal to 2v, then its centripetal acceleration is
A)
4a
done
clear
B)
2 a
done
clear
C)
\[\frac{a}{4}\]
done
clear
D)
\[\frac{a}{2}\]
done
clear
View Answer play_arrow
question_answer 68) A cyclist is travelling with velocity v on a banked curved road of radius R. The angle 0 through which the cyclist leans inwards is given by
A)
\[\tan \theta =\frac{Rg}{{{v}^{2}}}\]
done
clear
B)
\[\tan \theta ={{v}^{2}}Rg\]
done
clear
C)
\[\tan \theta =\frac{{{v}^{2}}g}{R}\]
done
clear
D)
\[\tan \theta =\frac{{{v}^{2}}}{Rg}\]
done
clear
View Answer play_arrow
question_answer 69) A body starts from rest and moves with uniform acceleration. Which of the following graphs represent its motion ?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 70) A gun fires N bullets per second, each of mass m with velocity v. The force exerted by the bullets on the gun is
A)
\[vNm\]
done
clear
B)
\[\frac{mv}{N}\]
done
clear
C)
\[mv{{N}^{2}}\]
done
clear
D)
\[\frac{m{{v}^{2}}}{N}\]
done
clear
View Answer play_arrow
question_answer 71) The rate of mass of the gas emitted from rear of a rocket is initially 0.1 kg/s. If the speed of the gas relative to the rocket is 50 m/s and mass of the rocket is 2 kg, then the acceleration of the rocket (in\[m/{{s}^{2}}\]) is
A)
5
done
clear
B)
5.2
done
clear
C)
2.5
done
clear
D)
25
done
clear
View Answer play_arrow
question_answer 72) The area under the displacement-force curve gives
A)
distance travelled
done
clear
B)
total force
done
clear
C)
momentum
done
clear
D)
work done
done
clear
View Answer play_arrow
question_answer 73) Identify the correct statement for the rotational motion of a rigid body.
A)
Individual particles of the body do not undergo accelerated motion
done
clear
B)
The centre of mass of the body remains unchanged
done
clear
C)
The centre of mass of the body moves uniformly in a circular path.
done
clear
D)
Individual particles and centre of mass the body undergo an accelerated motion
done
clear
View Answer play_arrow
question_answer 74) The moment of inertia about an axis of a body which is rotating with angular velocity 1 rad/s is numerically equal to
A)
one-fourth of its rotational kinetic energy
done
clear
B)
half of the rotational kinetic energy
done
clear
C)
rotational kinetic energy
done
clear
D)
twice the rotational kinetic energy
done
clear
View Answer play_arrow
question_answer 75) The moment of inertia of a circular disc of radius 2 m and mass 2 kg, about an axis passing through its centre of mass is \[2\text{ }kg-{{m}^{2}}.\] Its moment of inertia about an axis parallel to this axis and passing through its edge (in \[2\text{ }kg-{{m}^{2}}.\]) is
A)
10
done
clear
B)
8
done
clear
C)
6
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 76) The phenomenon observed when a beam of light is passed through a colloidal solution, is
A)
cataphoresis
done
clear
B)
delectrophoresis
done
clear
C)
coagulation
done
clear
D)
Tyndall effect
done
clear
View Answer play_arrow
question_answer 77) In case of condensation of polymers
A)
high molecular weight polymers are formed all at once
done
clear
B)
lower molecular weight polymers are formed all at once
done
clear
C)
molecular weight of polymer rises throughout the reaction
done
clear
D)
have no specific relation to their molecular weight
done
clear
View Answer play_arrow
question_answer 78) The element with the lowest ionization potential is
A)
Na
done
clear
B)
K
done
clear
C)
Rb
done
clear
D)
Cs
done
clear
View Answer play_arrow
question_answer 79) Differentiating electron in inner transition elements enters the ......... orbital.
A)
\[s\]
done
clear
B)
\[p\]
done
clear
C)
\[d\]
done
clear
D)
\[f\]
done
clear
View Answer play_arrow
question_answer 80) Which one of the following is a non-polar molecule?
A)
\[CC{{l}_{4}}\]
done
clear
B)
\[CHC{{l}_{3}}\]
done
clear
C)
\[C{{H}_{2}}C{{l}_{2}}\]
done
clear
D)
\[C{{H}_{3}}Cl\]
done
clear
View Answer play_arrow
question_answer 81) The nature of the bond in diamond is
A)
ionic
done
clear
B)
covalent
done
clear
C)
metallic
done
clear
D)
coordinate covalent
done
clear
View Answer play_arrow
question_answer 82) According to VSEPR theory the repulsion between different pair (lone or bond) of electrons obey the order
A)
\[lp-bp>lp-lp>bp-bp\]
done
clear
B)
\[lp-bp>bp-bp>lp-lp\]
done
clear
C)
\[lp-lp>lp-bp>bp-bp\]
done
clear
D)
\[bp-bp>lp-lp>lp-bp\]
done
clear
View Answer play_arrow
question_answer 83) From the molecular orbital theory, one can show that the bond order in molecule as
A)
2
done
clear
B)
1
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 84) Which of the following metal oxides is most basic?
A)
\[ZnO\]
done
clear
B)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
C)
\[As{{p}^{3}}\]
done
clear
D)
\[{{K}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 85) In the laboratory\[{{H}_{2}}S\]gas is prepared by using black lumps and dil.\[{{H}_{2}}S{{O}_{4}}\]The black lumps are
A)
\[FeS{{O}_{4}}\]
done
clear
B)
\[Mn{{O}_{2}}\]
done
clear
C)
\[FeS\]
done
clear
D)
\[FeS{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 86) The order of electron affinity of halogens is
A)
\[F>Cl>Br>I\]
done
clear
B)
\[Cl>F>Br>I\]
done
clear
C)
\[Cl>F>I>Br\]
done
clear
D)
\[Br>Cl>F>I\]
done
clear
View Answer play_arrow
question_answer 87) When chlorine reacts with dil.\[NaOH\]under cold conditions, the oxidation state of chlorine changes from zero to
A)
- 1 and + 5
done
clear
B)
+ 1 and + 4
done
clear
C)
+ 5 and + 3
done
clear
D)
- 1 and + 1
done
clear
View Answer play_arrow
question_answer 88) The highest oxidation state exhibited by transition metals is
A)
+7
done
clear
B)
+ 8
done
clear
C)
+6
done
clear
D)
+5
done
clear
View Answer play_arrow
question_answer 89) Which one of the following statements is not true with regard to transition elements?
A)
They readily form complex compounds
done
clear
B)
They show variable oxidation states
done
clear
C)
All their ions are colorless
done
clear
D)
Their ions contain partially filled d-electrons
done
clear
View Answer play_arrow
question_answer 90) The catalyst used in the manufacture of ammonia is
A)
\[{{V}_{2}}{{O}_{5}}\]
done
clear
B)
\[Pt\]
done
clear
C)
\[Fe\]
done
clear
D)
\[Ni{{(CO)}_{4}}\]
done
clear
View Answer play_arrow
question_answer 91) The most stable oxidation state of lanthanides is
A)
+2
done
clear
B)
+4
done
clear
C)
0
done
clear
D)
+ 3
done
clear
View Answer play_arrow
question_answer 92) The number of ions formed when hexamine copper (II) sulphate is dissolved in water is
A)
1
done
clear
B)
2
done
clear
C)
4
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 93) The number of unpaired electrons in the square planar\[{{[pt{{(CN)}_{4}}]}^{2-}}\]ion is
A)
2
done
clear
B)
1
done
clear
C)
0
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 94) In metal carbonyl (organometallic) complexes, the M-C bond is
A)
ionic
done
clear
B)
covalent with ionic character
done
clear
C)
covalent
done
clear
D)
coordinate covalent
done
clear
View Answer play_arrow
question_answer 95) The complexes\[[ptC{{l}_{2}}{{(N{{H}_{3}})}_{4}}]B{{r}_{2}}\]and \[[ptB{{r}_{2}}{{(N{{H}_{3}})}_{4}}]C{{l}_{2}}\]are examples for isomerism
A)
geometrical
done
clear
B)
optical
done
clear
C)
ionisation
done
clear
D)
linkage
done
clear
View Answer play_arrow
question_answer 96) The metallurgical process in which a metal is obtained in a fused state is called
A)
smelting
done
clear
B)
roasting
done
clear
C)
calcination
done
clear
D)
froth floatation
done
clear
View Answer play_arrow
question_answer 97) Metallic silver may be obtained from\[AgCl\]by
A)
heating it in the current of Ha
done
clear
B)
fusing it with sand
done
clear
C)
treating with carbon monoxide
done
clear
D)
fusing it with\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 98) Which one of the following metals is extracted by a carbon reduction process?
A)
Copper
done
clear
B)
Iron
done
clear
C)
Aluminium
done
clear
D)
Magnesium
done
clear
View Answer play_arrow
question_answer 99) IUPAC name of\[C{{H}_{3}}\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{CH}}\,C{{H}_{2}}CHO\]is
A)
3-chlorobutanol
done
clear
B)
3-chlorobutanaldehyde
done
clear
C)
3-chlorobutanal
done
clear
D)
2-chlorobutanol
done
clear
View Answer play_arrow
question_answer 100) Di-chloroacetic acid is a stronger acid than acetic acid. This is due to occurrence of
A)
mesomeric effect
done
clear
B)
hyperconjugation
done
clear
C)
inductive effect
done
clear
D)
steric effect
done
clear
View Answer play_arrow
question_answer 101) Dehydration of alcohol is an example of which type of reaction?
A)
Substitution
done
clear
B)
Elimination
done
clear
C)
Addition
done
clear
D)
Rearrangement
done
clear
View Answer play_arrow
question_answer 102) Number of monochloro derivatives obtained when neo-pentane is chlorinated, is
A)
one
done
clear
B)
two
done
clear
C)
three
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 103) Which of the following alkenes gives only acetaldehyde on ozonolysis?
A)
Ethene
done
clear
B)
Propene
done
clear
C)
1-butene
done
clear
D)
2-butene
done
clear
View Answer play_arrow
question_answer 104) 1-butyne on hydration gives
A)
butan-1, 2-diol
done
clear
B)
butan-1-ol
done
clear
C)
butan-2-ol
done
clear
D)
butan-2-one
done
clear
View Answer play_arrow
question_answer 105) Least stable conformer of cyclohexane is
A)
chair
done
clear
B)
boat
done
clear
C)
twist boat
done
clear
D)
planar hexagon
done
clear
View Answer play_arrow
question_answer 106) Which one of the following monoenes does not exhibit geometric isomerism?
A)
\[{{C}_{4}}{{H}_{8}}\]
done
clear
B)
\[{{C}_{3}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{5}}{{H}_{10}}\]
done
clear
D)
\[{{C}_{8}}{{H}_{16}}\]
done
clear
View Answer play_arrow
question_answer 107) Which one of the following chlorohydrocarbons readily undergoes solvolysis?
A)
\[C{{H}_{2}}=CHCl\]
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 108) Conversion of chlorobenzene to phenol involves
A)
electrophilic substitution
done
clear
B)
nucleophilic substitution
done
clear
C)
free radical substitution
done
clear
D)
electrophilic addition
done
clear
View Answer play_arrow
question_answer 109)
A)
an ester
done
clear
B)
an anhydride
done
clear
C)
acetal
done
clear
D)
hemiacetal
done
clear
View Answer play_arrow
question_answer 110) Which one of the following does not give iodoform?
A)
done
clear
B)
\[C{{H}_{3}}OH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
D)
\[C{{H}_{3}}\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 111) The products obtained when anisole is heated in a sealed tube with HI are
A)
done
clear
B)
done
clear
C)
done
clear
D)
\[C{{H}_{3}}OH+C{{H}_{3}}I\]
done
clear
View Answer play_arrow
question_answer 112) Which of the following diacid readily gives anhydride on heating?
A)
Fumaric
done
clear
B)
Maleic acid
done
clear
C)
Malic acid
done
clear
D)
Terephthalic acid
done
clear
View Answer play_arrow
question_answer 113) Hydroxamic acid test is employed to detect
A)
ketones
done
clear
B)
aldehydes
done
clear
C)
esters
done
clear
D)
amides
done
clear
View Answer play_arrow
question_answer 114) Picric acid is a stronger acid than acetic acid and benzoic acid. It contains
A)
\[\text{ }S{{O}_{3}}H\]group
done
clear
B)
two -COOH groups
done
clear
C)
phenolic group
done
clear
D)
three - COOH groups
done
clear
View Answer play_arrow
question_answer 115) Benzamide can be converted into benzonitrile with
A)
\[{{H}_{3}}{{O}^{+}}\]
done
clear
B)
\[O{{H}^{-}}/{{H}_{2}}O\]
done
clear
C)
\[KCN\]
done
clear
D)
\[{{P}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 116) The most basic compound in the following is
A)
\[N{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[HN{{(C{{H}_{3}})}_{2}}\]
done
clear
D)
\[N{{(C{{H}_{3}})}_{3}}\]
done
clear
View Answer play_arrow
question_answer 117) The compound with foul odour among the following is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 118) Nitration of nitrobenzene at\[125{}^\circ C\]with mixed acids gives
A)
meta-dinitrobenzene
done
clear
B)
ortho-dinitrobenzene
done
clear
C)
para-dinitrobenzene
done
clear
D)
1, 3, 5-trinitro benzene
done
clear
View Answer play_arrow
question_answer 119) The a-amino acid which does not give purple colour in the ninhydrin test is
A)
proline
done
clear
B)
glycine
done
clear
C)
lysine
done
clear
D)
aspartic acid
done
clear
View Answer play_arrow
question_answer 120) The anomeric carbon in D(+) glucose is
A)
C-1 carbon
done
clear
B)
C-2 carbon
done
clear
C)
C-5 carbon
done
clear
D)
C-6 carbon
done
clear
View Answer play_arrow
question_answer 121) The stoichiometry of the following reaction is\[{{K}_{2}}{{S}_{2}}{{O}_{8}}(aq)+2KI(aq)\to 2{{K}_{2}}S{{O}_{4}}(aq)+{{I}_{2}}(aq)\]
A)
2 : 2
done
clear
B)
1 : 1
done
clear
C)
1 : 2
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 122) Of two oxides of iron, the first contained 22% and the second contained 30% of oxygen by weight. The ratio of weights of iron in the two oxides that combine with the same weight of oxygen, is
A)
3 : 2
done
clear
B)
2 : 1
done
clear
C)
1 : 2
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 123) The scientist who proposed the atomic model based on the quantization of energy for the first time is
A)
Max Planck
done
clear
B)
Niels Bohr
done
clear
C)
de-Broglie
done
clear
D)
Heisenberg
done
clear
View Answer play_arrow
question_answer 124) Which one of the following is the set of correct quantum numbers of an electron in 3d orbital?
A)
\[n=3,l=0,m=0,s=-1/2\]
done
clear
B)
\[n=2,l=3,m=0,s=+1/2\]
done
clear
C)
\[n=3,l=1,m=0,s=-1/2\]
done
clear
D)
\[n=3,Z=2,m=1,s=+\text{ }1/2\]
done
clear
View Answer play_arrow
question_answer 125) Electron density in the YZ plane of\[3{{d}_{{{x}^{2}}-{{y}^{2}}}}\] orbital is
A)
zero
done
clear
B)
0.50
done
clear
C)
0.75
done
clear
D)
0.90
done
clear
View Answer play_arrow
question_answer 126) The half-life period of a radioactive isotope is 4.8 min. Starting with 1 mg of the isotope, how much of it would remain after 10 min?
A)
0.5 mg
done
clear
B)
0.726 mg
done
clear
C)
0.126 mg
done
clear
D)
0.236 mg
done
clear
View Answer play_arrow
question_answer 127) The number of beta particles emitted in the radioactive decay series from\[^{238}{{U}_{92}}\]to\[^{206}P{{b}_{82}}\]is
A)
10
done
clear
B)
8
done
clear
C)
6
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 128) What happens to the yield on application of high pressure in the Habers synthesis of ammonia?
A)
Increases
done
clear
B)
Decreases
done
clear
C)
Unaffected
done
clear
D)
Reaction stops
done
clear
View Answer play_arrow
question_answer 129) In the reaction\[{{H}_{2}}(g)+C{{l}_{2}}(g)2HCl(g)\]
A)
\[{{K}_{p}}\ne {{K}_{c}}\]
done
clear
B)
\[{{K}_{p}}={{K}_{c}}\]
done
clear
C)
\[{{K}_{p}}>{{K}_{c}}\]
done
clear
D)
\[{{K}_{p}}<{{K}_{c}}\]
done
clear
View Answer play_arrow
question_answer 130) pH of an aqueous solution containing\[{{10}^{8}}\,mol/L\]of\[HCl\]is
A)
8
done
clear
B)
10
done
clear
C)
6.96
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 131) An aqueous solution contains\[N{{i}^{2+}},C{{o}^{-28}}\]and\[P{{b}^{2+}}\]ions at equal concentrations. The solubility product of\[NiS,\text{ }PbS\]and\[CoS\]in water at\[25{}^\circ C\]are\[1.4\times {{10}^{-24}},\text{ }3.4\times {{10}^{-28}}\]and \[3\times {{10}^{-26}},\]respectively. Indicate which of these ions will be precipitated first and last when sulphide concentration is progressively increased from zero?
A)
\[NiS\]and \[PbS\]
done
clear
B)
\[NiS\]and \[CoS\]
done
clear
C)
\[CoS\]and\[NiS\]
done
clear
D)
\[PbS\]and\[NiS\]
done
clear
View Answer play_arrow
question_answer 132) A reaction involving A, B and C as reactants is found to obey the rate law, rate = \[=k{{[A]}^{x}}{{[B]}^{y}}{{[C]}^{z}}\]. When the concentrations of A, B and C are doubled separately, the rate is also found to increase two, zero and four times respectively. The overall order of the reaction is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 133) The units of the rate of a second order reaction are
A)
\[tim{{e}^{-1}}\]
done
clear
B)
\[mol\text{ }{{L}^{-1}}tim{{e}^{-1}}\]
done
clear
C)
\[L\text{ }mo{{l}^{-1}}tim{{e}^{-1}}\]
done
clear
D)
\[{{L}^{2}}\text{ }mo{{l}^{-2}}tim{{e}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 134) Activation energy of a reaction
A)
is independent of temperature
done
clear
B)
increases with temperature
done
clear
C)
gets doubled for every 10 degree rise in temperature
done
clear
D)
decreases with temperature
done
clear
View Answer play_arrow
question_answer 135) Which one of the following concentration units is independent of temperature?
A)
Normality
done
clear
B)
Molarity
done
clear
C)
Molality
done
clear
D)
ppm
done
clear
View Answer play_arrow
question_answer 136) Maximum lowering of vapour pressure is observed in the case of
A)
0.1 M glucose
done
clear
B)
\[0:1\,M\,BaC{{l}_{2}}\]
done
clear
C)
\[0.1\,M\,MgS{{O}_{4}}\]
done
clear
D)
\[0.1\text{ }NaCl\]
done
clear
View Answer play_arrow
question_answer 137) A solution containing 4 g of polyvinyl chloride polymer in one litre of dioxane was found to have an osmotic pressure of\[4.1\times {{10}^{-4}}\]atm at\[{{27}^{o}}C\]. The approximate molecular weight of the polymer is
A)
1500
done
clear
B)
10,000
done
clear
C)
\[2.4\times {{10}^{5}}\]
done
clear
D)
\[2\times {{10}^{12}}\]
done
clear
View Answer play_arrow
question_answer 138) Abnormal colligative properties are observed only when the dissolved non-volatile solute in a given dilute solution
A)
is a non-electrolyte
done
clear
B)
offers an intense colour
done
clear
C)
associates or dissociates
done
clear
D)
offers no colour
done
clear
View Answer play_arrow
question_answer 139) We believe in the laws of thermodynamics because they are
A)
theoretical
done
clear
B)
derived based on mathematical analysis
done
clear
C)
empirical and nobody disproved
done
clear
D)
mere statements
done
clear
View Answer play_arrow
question_answer 140) The latent heat of fusion of ice at\[{{0}^{o}}C\]is 80 cal/g. Entropy change\[(\Delta S)\]accompanying the melting of 1 g of ice at\[{{0}^{o}}C\]would be (units : cal/g/K)
A)
273
done
clear
B)
8.0
done
clear
C)
0.0
done
clear
D)
0.293
done
clear
View Answer play_arrow
question_answer 141) \[\Delta H\]for the reaction, \[C(graphite)+2{{H}_{2}}(g)\xrightarrow{{}}C{{H}_{4}}(g)\,at\,298K\] and 1 atm is - 17900 cal. The\[\Delta E\]for the above conversion would be
A)
\[-17900\text{ }cal\]
done
clear
B)
\[17900\text{ }cal\]
done
clear
C)
\[17308\text{ }cal\]
done
clear
D)
\[-17308\text{ }cal\]
done
clear
View Answer play_arrow
question_answer 142) Which one of the following is spontaneous at all temperatures?
A)
\[{{H}_{2}}(g)\xrightarrow{{}}2{{H}_{atom}}\]\[\Delta {{H}^{o}}=436\text{ }kJ,\text{ }\Delta {{S}^{o}}=90.7\text{ }eu\]
done
clear
B)
\[\frac{1}{2}{{N}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}NO(g);\]\[\Delta {{H}^{o}}=90.3\text{ }kJ,\text{ }\Delta {{S}^{o}}=3.0\text{ }eu\]
done
clear
C)
\[2N{{O}_{2}}(g)\xrightarrow[{}]{{}}{{N}_{2}}{{O}_{4}}(g)\]\[\Delta {{H}^{o}}=-\text{ }56.0\text{ }kJ\text{ }\Delta {{S}^{o}}=-17.7\text{ }eu\]
done
clear
D)
\[{{H}_{2}}{{O}_{2}}(g)\xrightarrow{{}}{{H}_{2}}O(l)+\frac{1}{2}{{O}_{2}}(g)\]\[\Delta {{H}^{o}}=-98.3\text{ }kJ\text{ }\Delta {{S}^{o}}=80.0\text{ }eu\]
done
clear
View Answer play_arrow
question_answer 143) During a redox titration involving a solution containing\[F{{e}^{2+}}\]ions against\[MnO_{4}^{-}\]in the presence of excess of\[{{H}^{+}}\]ions, the number of electrons that gets transferred is
A)
6
done
clear
B)
5
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 144) The oxidation state of sulphur in sodium tetrathionate\[(N{{a}_{2}}{{S}_{4}}{{O}_{6}})\]is
A)
2
done
clear
B)
0
done
clear
C)
2.5
done
clear
D)
3.5
done
clear
View Answer play_arrow
question_answer 145) Galvanic cell is a device in which
A)
chemical energy is converted into electrical energy
done
clear
B)
electrical energy is converted into chemical energy
done
clear
C)
chemical energy is seen in the form of heat
done
clear
D)
thermal energy from an outside source is used to drive the cell reaction
done
clear
View Answer play_arrow
question_answer 146) The relationship between Gibbs free energy change\[(\Delta G)\]and emf\[(\Delta S)\]of a reversible electrochemical cell is given by
A)
\[\Delta G=nFE\]
done
clear
B)
\[\Delta G=nF/E\]
done
clear
C)
\[\Delta G=-nFE\]
done
clear
D)
\[\Delta G=E/nF\]
done
clear
View Answer play_arrow
question_answer 147) The units of van der Waals constants a, b respectively, are
A)
\[L\text{ }at{{m}^{2}}\text{ }mo{{l}^{-1}}and\text{ }mol\text{ }{{L}^{-1}}\]
done
clear
B)
\[L\text{ }atm\text{ }mo{{l}^{2}}\text{ }and\text{ }mol\text{ }L\]
done
clear
C)
\[{{L}^{2}}\text{ }atm\text{ }mo{{l}^{-2}}\text{ }and\text{ }mo{{l}^{-1}}\text{ }L\]
done
clear
D)
\[{{L}^{-2}}at{{m}^{-1}}mo{{l}^{-1}}and\text{ }L\text{ }mo{{l}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 148) Identify the pair of gases that have equal rates of diffusion
A)
\[CO,\text{ }NO\]
done
clear
B)
\[{{N}_{3}}O,\text{ }CO\]
done
clear
C)
\[{{N}_{2}}O,C{{O}_{2}}\]
done
clear
D)
\[C{{O}_{2}},N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 149) For AX ionic crystal to exist in bcc structure, the ratio of radii\[\left( \frac{{{r}_{cation}}}{{{r}_{anion}}} \right)\]should be
A)
between 0.41 and 0.73
done
clear
B)
greater than 0.73
done
clear
C)
less than 0.41
done
clear
D)
equal to 1.0
done
clear
View Answer play_arrow
question_answer 150) Identify the correct statement for the adsorption of a real gas on charcoal at 1 atm and\[{{15}^{o}}C\].
A)
gases which are small in molecular size are adsorbed more
done
clear
B)
decrease in pressure increases the extent of adsorption
done
clear
C)
gases which are easily liquefiable are adsorbed more in quantity
done
clear
D)
gas which has a behaviour similar to an inert gas is adsorbed more
done
clear
View Answer play_arrow
question_answer 151) The entry of pollen tube into the ovule through micropyle is called
A)
porogamy
done
clear
B)
misogamy
done
clear
C)
anisogamy
done
clear
D)
chalazogamy
done
clear
View Answer play_arrow
question_answer 152) Type of pollination of Commelina is
A)
chasmogamy
done
clear
B)
geitonogamy
done
clear
C)
xenogamy
done
clear
D)
cleistogamy
done
clear
View Answer play_arrow
question_answer 153) The process of embryo formation without fertilization is known as
A)
apospory
done
clear
B)
apogamy
done
clear
C)
parthenocarpy
done
clear
D)
polyembryony
done
clear
View Answer play_arrow
question_answer 154) Which of the process Clolodny-Went theory is concerned with?
A)
Photomorphogenesis
done
clear
B)
Photoperiodism
done
clear
C)
Phototropism
done
clear
D)
Photorespiration
done
clear
View Answer play_arrow
question_answer 155) The hormone present in the liquid endosperm of coconut is
A)
cytokinin
done
clear
B)
gibberellins
done
clear
C)
ethylene
done
clear
D)
auxin
done
clear
View Answer play_arrow
question_answer 156) The phytohormone, which induces triple response growth is
A)
\[IAA\]
done
clear
B)
\[ABA\]
done
clear
C)
\[G{{A}_{3}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 157) An example of short day plant is
A)
wheat
done
clear
B)
maize
done
clear
C)
Chrysanthemum
done
clear
D)
radish
done
clear
View Answer play_arrow
question_answer 158) The ovary after fertilization is converted into
A)
embryo
done
clear
B)
endosperm
done
clear
C)
fruit
done
clear
D)
seed
done
clear
View Answer play_arrow
question_answer 159) The molecular formula of chlorophyll-a is
A)
\[{{C}_{55}}{{H}_{72}}{{O}_{5}}{{N}_{4}}Mg\]
done
clear
B)
\[~{{C}_{55}}{{H}_{70}}{{O}_{6}}{{N}_{4}}Mg\]
done
clear
C)
\[{{C}_{55}}{{H}_{72}}{{O}_{4}}{{N}_{4}}Mg\]
done
clear
D)
\[{{C}_{50}}{{H}_{72}}{{O}_{5}}{{N}_{4}}Mg\]
done
clear
View Answer play_arrow
question_answer 160) The first compound that accepts \[C{{O}_{2}}\] during dark phase is
A)
NADP
done
clear
B)
ferredoxin
done
clear
C)
RuBP
done
clear
D)
cytochrome
done
clear
View Answer play_arrow
question_answer 161) Initiation codon for methionine is
A)
AAA
done
clear
B)
UUU
done
clear
C)
UAA
done
clear
D)
AUG
done
clear
View Answer play_arrow
question_answer 162) The deficiency of this micro-nutrient results in little leaf disease
A)
copper
done
clear
B)
zinc
done
clear
C)
boron
done
clear
D)
iron
done
clear
View Answer play_arrow
question_answer 163) Kranz anatomy is a morphological diversity in the leaves of
A)
\[{{C}_{3}}-\]plants
done
clear
B)
\[{{C}_{4}}-\]plants
done
clear
C)
Both (a) and (b)
done
clear
D)
CAM plants
done
clear
View Answer play_arrow
question_answer 164) Prechilling treatment to break seed dormancy is
A)
scarification
done
clear
B)
stratification
done
clear
C)
impaction
done
clear
D)
vernalization
done
clear
View Answer play_arrow
question_answer 165) The pyruvic acid formed during glycolysis is oxidized to \[C{{O}_{2}}\]and \[{{H}_{2}}O\] in a cycle called
A)
Calvin cycle
done
clear
B)
Nitrogen cycle
done
clear
C)
Hill reaction
done
clear
D)
Krebs cycle
done
clear
View Answer play_arrow
question_answer 166) Respiratory Quotient (RQ) is one in case of
A)
fatty acids
done
clear
B)
nucleic acids
done
clear
C)
carbohydrates
done
clear
D)
organic acids
done
clear
View Answer play_arrow
question_answer 167) An example of free living nitrogen fixing aerobic bacteria is
A)
Clostridium
done
clear
B)
Rhizobium
done
clear
C)
Azotobacter
done
clear
D)
Rhodospirillum
done
clear
View Answer play_arrow
question_answer 168) Identify the plant belonging to the Reed-Swamp stage in hydrarch succession
A)
Juncus
done
clear
B)
Sagittaria
done
clear
C)
Salix
done
clear
D)
Trapa
done
clear
View Answer play_arrow
question_answer 169) A gas produced by paddy fields and connected with global warming is
A)
\[C{{O}_{2}}\]
done
clear
B)
chlorine
done
clear
C)
\[{{H}_{2}}S\]
done
clear
D)
methane
done
clear
View Answer play_arrow
question_answer 170) If the strong partner is benefitted and the weak partner is damaged, it is known as
A)
predation
done
clear
B)
allelopathy
done
clear
C)
symbiosis
done
clear
D)
commensalism
done
clear
View Answer play_arrow
question_answer 171) Acid rain is mainly caused due to increase in the levels of the gas(es)
A)
\[S{{O}_{2}}\] only
done
clear
B)
\[C{{O}_{2}}\] only
done
clear
C)
\[S{{O}_{2}},\text{ }C{{O}_{2}}\]
done
clear
D)
\[N{{O}_{2}}\] and \[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 172) The flow of energy among various trophic levels of an ecosystem is
A)
unidirectional
done
clear
B)
bidirectional
done
clear
C)
multidirectional
done
clear
D)
circular
done
clear
View Answer play_arrow
question_answer 173) Increase in atmospheric temperature due to \[C{{O}_{2}}\]is called
A)
Pasteur effect
done
clear
B)
Green-house effect
done
clear
C)
Blackman effect
done
clear
D)
Emerson effect
done
clear
View Answer play_arrow
question_answer 174) The protective ozone layer is present in
A)
ionosphere
done
clear
B)
stratosphere
done
clear
C)
troposphere
done
clear
D)
lithosphere
done
clear
View Answer play_arrow
question_answer 175) Decomposition of organic matter is brought about by
A)
Protozoa
done
clear
B)
plants
done
clear
C)
micro-organisms
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 176) The smallest taxon is called
A)
class
done
clear
B)
order
done
clear
C)
genus
done
clear
D)
species
done
clear
View Answer play_arrow
question_answer 177) Which one of the following is the first National Park in India?
A)
Kanha National Park
done
clear
B)
Periyar National Park
done
clear
C)
Corbett National Park
done
clear
D)
Bandipur National Park
done
clear
View Answer play_arrow
question_answer 178) Which one of the following is having ssRNA?
A)
TMV
done
clear
B)
\[{{T}_{2}}-\]bacteriophage
done
clear
C)
Reovirus
done
clear
D)
CMV
done
clear
View Answer play_arrow
question_answer 179) In Whittakers system of classification, prokaryotes are placed in the kingdom
A)
Protista
done
clear
B)
Monera
done
clear
C)
Plantae
done
clear
D)
Animalia
done
clear
View Answer play_arrow
question_answer 180) Virus consists of
A)
Nucleic acid
done
clear
B)
Protein
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 181) This substance is present in the cell walls of Gram-positive bacteria only
A)
peptidoglycan
done
clear
B)
lipopolysaccharides
done
clear
C)
teichoic acids
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 182) Stem is modified into cladode
A)
Casuarina
done
clear
B)
Asparagus
done
clear
C)
Opuntia
done
clear
D)
Euphorbia
done
clear
View Answer play_arrow
question_answer 183) Verticillaster type of inflorescence is found in
A)
Cotton
done
clear
B)
Datura
done
clear
C)
Leucas
done
clear
D)
Ocimum
done
clear
View Answer play_arrow
question_answer 184) A simple one seeded fruit, in which pericarp is fused with seed coat is
A)
achene
done
clear
B)
caryopsis
done
clear
C)
cypsela
done
clear
D)
nut
done
clear
View Answer play_arrow
question_answer 185) The portion of DNA which contains information for an entire polypeptide is called as
A)
cistron
done
clear
B)
muton
done
clear
C)
recon
done
clear
D)
operon
done
clear
View Answer play_arrow
question_answer 186) Bicarpellary, syncarpous ovary with axile placentation is seen in
A)
Solanaceae
done
clear
B)
Caesalpinaceae
done
clear
C)
Asteraceae
done
clear
D)
Malvaceae
done
clear
View Answer play_arrow
question_answer 187) Alburnum is also called as
A)
autumn wood
done
clear
B)
heart wood
done
clear
C)
sap wood
done
clear
D)
spring wood
done
clear
View Answer play_arrow
question_answer 188) Endothelium of blood vessels is made up of
A)
simple cuboidal epithelium
done
clear
B)
simple squamous epithelium
done
clear
C)
simple columnar epithelium
done
clear
D)
simple non-ciliated columnar
done
clear
View Answer play_arrow
question_answer 189) In humans, sphincter of Oddi is associated with the opening of
A)
common heap to pancreatic duct
done
clear
B)
pyloric stomach
done
clear
C)
oesophagus
done
clear
D)
colon
done
clear
View Answer play_arrow
question_answer 190) In human beings, the duration of cardiac cycle is
A)
0.08 sec
done
clear
B)
0.5 sec
done
clear
C)
0.8 sec
done
clear
D)
8.0 sec
done
clear
View Answer play_arrow
question_answer 191) In which part of nephron, reabsorption is minimum from filtrate?
A)
Henles loop
done
clear
B)
Proximal convoluted tubule
done
clear
C)
Distal convoluted tubule
done
clear
D)
Collecting duct
done
clear
View Answer play_arrow
question_answer 192) Which hormone level reaches peak during luteal phase of menstrual cycle?
A)
Luteinizing hormone
done
clear
B)
Progesterone
done
clear
C)
Follicle stimulating hormone
done
clear
D)
Estrogen
done
clear
View Answer play_arrow
question_answer 193) The largest subunit of prokaryotic ribosomes is
A)
30S
done
clear
B)
40S
done
clear
C)
50S
done
clear
D)
60S
done
clear
View Answer play_arrow
question_answer 194) Which of the following is a part of endomembrane system of eukaryotic cell ?
A)
Peroxisomes
done
clear
B)
Chloroplasts
done
clear
C)
Mitochondria
done
clear
D)
Golgi complexes
done
clear
View Answer play_arrow
question_answer 195) Inflammation of joints due to accumulation of uric acid crystals is called as
A)
gout
done
clear
B)
myasthenia gravis
done
clear
C)
osteoporosis
done
clear
D)
osteomalacia
done
clear
View Answer play_arrow
question_answer 196) Chromosomes are visible with chromatids at this phase of mitosis
A)
interphase
done
clear
B)
prophase
done
clear
C)
metaphase
done
clear
D)
anaphase
done
clear
View Answer play_arrow
question_answer 197) Inheritance of ABO blood grouping is an example of
A)
dominance
done
clear
B)
codominance
done
clear
C)
incomplete dominance
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 198) In a dihybrid cross between RRYY and rryy parents, the number of RrYy genotypes in \[{{F}_{2}}\] generation will be
A)
4
done
clear
B)
3
done
clear
C)
2
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 199) Identify a Mendelian disorder from the following
A)
Downs syndrome
done
clear
B)
Turners syndrome
done
clear
C)
Phenylketonuria
done
clear
D)
Klinefelters syndrome
done
clear
View Answer play_arrow
question_answer 200) Uracil is present in RNA at the place of
A)
adenine
done
clear
B)
guanine
done
clear
C)
cytosine
done
clear
D)
thymine
done
clear
View Answer play_arrow
question_answer 201) Copying genetic information from one strand of DNA into RNA is
A)
translation
done
clear
B)
transcription
done
clear
C)
transformation
done
clear
D)
transduction
done
clear
View Answer play_arrow
question_answer 202) S L Millers closed flask contained
A)
\[C{{H}_{4}}\]
done
clear
B)
\[{{H}_{2}}\]
done
clear
C)
\[N{{H}_{3}}\]and water vapour
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 203) Change of frequency of alleles in a population results in evolution is proposed in
A)
Darwins theory
done
clear
B)
Lamarcks theory
done
clear
C)
Hardy-Weinberg principle
done
clear
D)
de Vries theory
done
clear
View Answer play_arrow
question_answer 204) One of the following is the vestigial organ in human beings
A)
nictitating membrane
done
clear
B)
spleen
done
clear
C)
femur
done
clear
D)
tibia
done
clear
View Answer play_arrow
question_answer 205) The golden age of reptiles is
A)
Coenozoic era
done
clear
B)
Palaeozoic era
done
clear
C)
Mesozoic era
done
clear
D)
Silurian period
done
clear
View Answer play_arrow
question_answer 206) The theory of use and disuse of organ was proposed by
A)
Darwin
done
clear
B)
Lamarck
done
clear
C)
de Vries
done
clear
D)
Hooker
done
clear
View Answer play_arrow
question_answer 207) One of the following theories were proposed by Weismann
A)
law of inheritance
done
clear
B)
theory of inheritance of acquired characters
done
clear
C)
theory of natural selection
done
clear
D)
theory of germplasm
done
clear
View Answer play_arrow
question_answer 208) Ontogeny recapitulates phylogeny-this theory is called as
A)
biogenetic law
done
clear
B)
law of embryology
done
clear
C)
law of acquired characters
done
clear
D)
law of bridges
done
clear
View Answer play_arrow
question_answer 209) The brain capacity of Homo erectus is
A)
800 cc
done
clear
B)
900 cc
done
clear
C)
1200 cc
done
clear
D)
1400 cc
done
clear
View Answer play_arrow
question_answer 210) An example of innate immunity is
A)
PMNL-neutrophils
done
clear
B)
T-lymphocytes
done
clear
C)
B-lymphocytes
done
clear
D)
TH cells
done
clear
View Answer play_arrow
question_answer 211) Heroin is extracted from
A)
Erythroxylum coca
done
clear
B)
Cannabis sativa
done
clear
C)
Papaver somniferum
done
clear
D)
Atropa belladonna
done
clear
View Answer play_arrow
question_answer 212) The enzyme that cuts DNA is
A)
DNA-polymerase
done
clear
B)
DNA-ligase
done
clear
C)
DNA-lyase
done
clear
D)
Restriction endonuclease
done
clear
View Answer play_arrow
question_answer 213) In the association between two organisms, if one organism is benefitted and the other is not benefitted, this relationship is known as
A)
symbiotism
done
clear
B)
mutualism
done
clear
C)
commensalism
done
clear
D)
parasitism
done
clear
View Answer play_arrow
question_answer 214) Opiate narcotics drugs are
A)
antianxiety
done
clear
B)
analgesic
done
clear
C)
hypnotic
done
clear
D)
antihistamine
done
clear
View Answer play_arrow
question_answer 215) The drug useful to increase cardiovascular effects in human beings is
A)
cocaine
done
clear
B)
barbiturate
done
clear
C)
benzodiazetine
done
clear
D)
insulin
done
clear
View Answer play_arrow
question_answer 216) In echolocation, the animal that produces high frequency sounds is
A)
monkey
done
clear
B)
butterfly
done
clear
C)
squirrel
done
clear
D)
bat
done
clear
View Answer play_arrow
question_answer 217) Two kingdoms constantly figured in all biological classifications are
A)
PIantae and Animalia
done
clear
B)
Monera and Animalia
done
clear
C)
Protista and Animalia
done
clear
D)
Protista and Plantae
done
clear
View Answer play_arrow
question_answer 218) The dioecious animal is
A)
liver fluke
done
clear
B)
hookworm
done
clear
C)
tapeworm
done
clear
D)
earthworm
done
clear
View Answer play_arrow
question_answer 219) Comb plates are found in
A)
Adamsia
done
clear
B)
Aurelia
done
clear
C)
Nereis
done
clear
D)
Pleurobrachia
done
clear
View Answer play_arrow
question_answer 220) Highest degree of polymorphism is found in
A)
Protozoa
done
clear
B)
Cnidaria
done
clear
C)
Platyhelminthes
done
clear
D)
Arthropoda
done
clear
View Answer play_arrow
question_answer 221) Sea mouse belongs to phylum
A)
Mollusca
done
clear
B)
Cnidaria
done
clear
C)
Arthropoda
done
clear
D)
Annelida
done
clear
View Answer play_arrow
question_answer 222) One of the following animal belongs to Cyclostomata
A)
Channa
done
clear
B)
Loris
done
clear
C)
Dodo
done
clear
D)
Petromyzon
done
clear
View Answer play_arrow
question_answer 223) An egg laying mammal is
A)
Delphinus
done
clear
B)
Macaca
done
clear
C)
Ornithorhynchus
done
clear
D)
Macropus
done
clear
View Answer play_arrow
question_answer 224) In sharks, one of the following is absent
A)
claspers
done
clear
B)
placoid scales
done
clear
C)
cartilaginous endoskeleton
done
clear
D)
air bladder
done
clear
View Answer play_arrow
question_answer 225) The devil fish and sea hare are
A)
mollusks
done
clear
B)
crustaceans
done
clear
C)
coelenterates
done
clear
D)
marine fish and mammal
done
clear
View Answer play_arrow
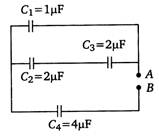
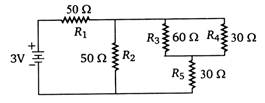

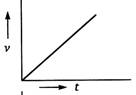

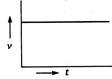
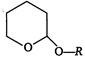 is
is