A) \[NaCl,MgC{{l}_{2}},MgO\]
B) \[PC{{l}_{3}},N{{H}_{3}},{{H}_{2}}O\]
C) \[{{N}_{2}}{{O}_{4}},CC{{l}_{4}},{{N}_{2}}{{O}_{5}}\]
D) \[B{{F}_{3}},BeC{{l}_{2}}\]and \[N{{O}_{2}}\]
Correct Answer: D
Solution :
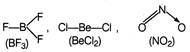 In all these species, central atoms-B, Be and N have less than eight electrons. Thus, octet role is not followed here.
In all these species, central atoms-B, Be and N have less than eight electrons. Thus, octet role is not followed here.
You need to login to perform this action.
You will be redirected in
3 sec
