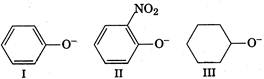
A) III > I > II
B) I > III > II
C) lI > III > I
D) III > II > I
Correct Answer: A
Solution :
In phenoxide ion (I), the negative charge is delcalised in benzene ring, therefore less available for donation to a Lewis acid, hence weaker base. In cyclohexanoide ion, negative charge is not delocalised anywhere, stronger base.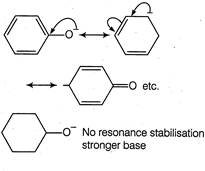 Both phenoxide and nitrophenoxide ion have resonance delocalisation but in case of nitrophenoxide ion the negative charge of oxygen is absorbed by nitro group through resonance. Hence, less available on oxygen for donation to a Lewis acid. Due to this reason, nitrophenoxide is weaker base than the unsubstituted phenoxide ion. Therefore, correct order is III > I > II.
Both phenoxide and nitrophenoxide ion have resonance delocalisation but in case of nitrophenoxide ion the negative charge of oxygen is absorbed by nitro group through resonance. Hence, less available on oxygen for donation to a Lewis acid. Due to this reason, nitrophenoxide is weaker base than the unsubstituted phenoxide ion. Therefore, correct order is III > I > II.
You need to login to perform this action.
You will be redirected in
3 sec
