-
question_answer1) A diamagnetic material in a magnetic field moves
A)
from stronger to weaker parts of the field
done
clear
B)
perpendicular to the field
done
clear
C)
from weaker to stronger parts of the field
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer2) A source and a listener are both moving towards each other with speed \[1.11f\], where v is the speed of sound. If the frequency of the note emitted by the source is \[1.22f\], the frequency heard by the listener would be nearly
A)
f
done
clear
B)
\[1.27f\]
done
clear
C)
\[\text{1}.0\text{1}\times \text{1}{{0}^{\text{5}}}\text{ N}/{{\text{m}}^{\text{2}}}\]
done
clear
D)
\[\text{9}.\text{13}\times \text{1}{{0}^{\text{4}}}\text{ N}/{{\text{m}}^{\text{2}}}\]
done
clear
View Answer play_arrow
-
question_answer3) A 5g droplet of liquid nitrogen is enclosed in a 50mL tube which is sealed at very low ressure. When the tube is warmed to 35°C, the nitrogen pressure in the tube is (molecular weight of nitrogen = 28, R = 8.3 J/mol K)
A)
\[\text{9}.\text{13}\times \text{1}{{0}^{\text{3}}}\text{N}/{{\text{m}}^{\text{2}}}\]
done
clear
B)
\[\text{18}.\text{26 N}/{{\text{m}}^{\text{2}}}\]
done
clear
C)
\[\text{2}.\text{25}\times \text{1}{{0}^{\text{3}}}\text{min}\]
done
clear
D)
\[\text{3}.\text{97}\times \text{1}{{0}^{\text{3}}}\text{min}\]
done
clear
View Answer play_arrow
-
question_answer4) A chef, on finding his stove out of order, decides to boil the water for his wife's coeffe by shaking it in a thermos flask. Suppose, that he uses tap water at 15°C and chef making 30 shakes each minute. Neglecting any loss of thermal energy by the flask, how long must he shake the flask until the water reaches 100°C?
A)
\[9.13\times {{10}^{3}}N/{{m}^{2}}\]
done
clear
B)
\[\text{5}.\text{25}\times \text{1}{{0}^{\text{3}}}\text{min}\]
done
clear
C)
\[\left[ \text{FL}{{\text{T}}^{-\text{2}}} \right]\]
done
clear
D)
\[\left[ \text{F}{{\text{L}}^{\text{2}}}{{T}^{-\text{2}}} \right]\]
done
clear
View Answer play_arrow
-
question_answer5) If force (F), length (L) and time (T) be considered fundamental units, then units of mass will be
A)
\[\left[ \text{F}{{\text{L}}^{-\text{1}}}{{\text{T}}^{\text{2}}} \right]\]
done
clear
B)
\[\left[ {{\text{F}}^{2}}\text{L}{{\text{T}}^{\text{-2}}} \right]\]
done
clear
C)
\[-\text{273}.\text{15}{}^\circ \text{F}\]
done
clear
D)
\[-\text{453}.\text{15}{}^\circ \text{F}\]
done
clear
View Answer play_arrow
-
question_answer6) If absolute zero is -273.15°C on Celsius temperature scale, then the absolute zero on the fahrenheit scale is
A)
\[-\text{459}.\text{67}{}^\circ \text{F}\]
done
clear
B)
\[-\text{491}.\text{67}{}^\circ \text{F}\]
done
clear
C)
\[\text{52}00\text{{ }\!\!\mathrm{\AA}\!\!\text{ }}\]
done
clear
D)
\[\text{Vc}=\text{1}.\text{5V}\]
done
clear
View Answer play_arrow
-
question_answer7) What should be the velocity of an electron so that its momentum, becomes equal to that of a photon of wavelength\[\text{1}00\text{ }\mu \text{A}\]?
A)
700 m/s
done
clear
B)
1000m/s
done
clear
C)
1400 m/s
done
clear
D)
2800 m/s
done
clear
View Answer play_arrow
-
question_answer8) A transistor is operated in common emitter configuration at constant collector voltage \[\text{15}0\text{ }\mu \text{A}\]such that a change in the base current from \[\text{5 mA}\]to\[\text{10 mA}\]. Produces a change in the collector current from \[\text{ }\!\!\beta\!\!\text{ }\]to \[\left( \frac{1}{V(volume)} \right)\]. The current gain (\[\frac{3}{4}\text{m}/\text{s}\]) is
A)
67
done
clear
B)
75
done
clear
C)
100
done
clear
D)
50
done
clear
View Answer play_arrow
-
question_answer9) The graph of pressure p and \[\frac{1}{3}\text{m}/\text{s}\] of1 mole of an ideal at constant temperature is
A)
done
clear
B)
.
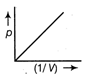
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer10) Gas exerts pressure on the walls of the container, because
A)
gas has weight
done
clear
B)
gas molecules have momentum
done
clear
C)
gas molecules collide with each other
done
clear
D)
gas molecules collide with the wails of the container
done
clear
View Answer play_arrow
-
question_answer11) An astronaut is approching the moon. He sends out a radio signal of frequency 5000 MHz and the frequency of echo is different from that of the original frequency by 100 kHz. His velocity of approach with respect to the moon is
A)
2km/s
done
clear
B)
3 km/s
done
clear
C)
4 km/s
done
clear
D)
5 km/s
done
clear
View Answer play_arrow
-
question_answer12) A 10 kg ball moving with velocity 2 m/s collides with a 20 kg mass initially at rest. If both of them coalesce, the final velocity of combined mass is
A)
\[\frac{3}{2}\text{m}/\text{s}\]
done
clear
B)
\[\frac{2}{3}\text{m}/\text{s}\]
done
clear
C)
\[{{\lambda }_{0}},\]
done
clear
D)
\[\frac{25}{16}{{\lambda }_{0}}\]
done
clear
View Answer play_arrow
-
question_answer13) The wavelength of radiation emitted is \[\frac{27}{20}{{\lambda }_{0}}\] when an electron in hydrogen atom jumps from 3rd to 2nd orbit. If in the hydrogen atom itself, the electron jumps from fourth orbit to second orbit, then wavelength of emitted radiation will be
A)
\[\frac{20}{27}{{\lambda }_{0}}\]
done
clear
B)
\[\frac{16}{25}{{\lambda }_{0}}\]
done
clear
C)
\[3\Omega \]
done
clear
D)
\[4\Omega \]
done
clear
View Answer play_arrow
-
question_answer14)
Seven resistances are connected as shown in the figure. The equivalent resistance between A and B is 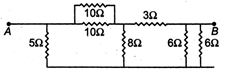
A)
\[4.5\Omega \]
done
clear
B)
\[5\Omega \]
done
clear
C)
\[\frac{\sqrt{3}}{1}\]
done
clear
D)
\[\frac{(\sqrt{3}+1)}{(\sqrt{3}-1)}\]
done
clear
View Answer play_arrow
-
question_answer15) Two tangent galvanometer having coils of same radius are connected in series. A current flowing in them produces deflections of 60° and 45° respectively. Ratio of number of turns in the coils is
A)
\[\frac{(\sqrt{3}+1)}{1}\]
done
clear
B)
\[\frac{4}{3}\]
done
clear
C)
\[4\mu F\]
done
clear
D)
\[10\mu F\]
done
clear
View Answer play_arrow
-
question_answer16)
Equivalent capacitor of the given combination of five capacitors is 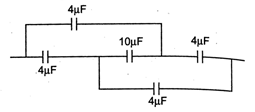
A)
\[8\mu F\]
done
clear
B)
\[120\mu F\]
done
clear
C)
\[\omega \]
done
clear
D)
\[R/2\]
done
clear
View Answer play_arrow
-
question_answer17) A disc having mass M and radius R is rotating with angular velocity \[\frac{4\omega }{5}\], another disc of mass 2M and radius \[\frac{2\omega }{5}\] is placed coaxially on the ' first disc gently. The angular velocity of the system will be
A)
\[\frac{3\omega }{5}\]
done
clear
B)
\[\frac{2\omega }{3}\]
done
clear
C)
\[\mu =\frac{3}{2}\]
done
clear
D)
\[\mu =\frac{4}{3}\]
done
clear
View Answer play_arrow
-
question_answer18) Light is refracted \[{{\sin }^{-1}}\left( \frac{9}{8} \right)\]to water \[{{45}^{o}}\] for total internal reflection sin i will be equal to
A)
\[{{60}^{o}}\]
done
clear
B)
\[{{\sin }^{-1}}\left( \frac{8}{9} \right)\]
done
clear
C)
\[\beta =0.\text{1}\]
done
clear
D)
\[{{P}_{1}}\]
done
clear
View Answer play_arrow
-
question_answer19) The voltage of an amplifier without feedback is 100 V. If a negative feedback is introduced, with a feedback fraction\[{{P}_{2}}\], then the gain of the feedback amplifier is
A)
90
done
clear
B)
100.1
done
clear
C)
10
done
clear
D)
9.0
done
clear
View Answer play_arrow
-
question_answer20) An organ pipe \[{{P}_{1}}\] closed at one end vibrating in its first overtone and other pipe \[{{P}_{2}}\] open at both ends, vibrating in its third overtone are in resonance with a given tuning forks, then ratio of length of \[\text{2}\times \text{1}{{0}^{\text{7}}}\text{m}/\text{s}\] and \[\text{2}\times \text{1}{{0}^{-2}}T\] will be
A)
3: 8
done
clear
B)
3:4
done
clear
C)
3:2
done
clear
D)
3: 1
done
clear
View Answer play_arrow
-
question_answer21) An electron moving with velocity \[\left( \frac{e}{m} \right)\] describes a circle in a magnetic field of strength\[\text{1}.\text{76}\times \text{1}{{0}^{\text{11}}}\text{C}/\text{kg}\]. If \[2B\]of electron is\[\frac{B}{4}\], then the diameter of the circle is nearly
A)
1.1 cm
done
clear
B)
1.1 mm
done
clear
C)
1.1 m
done
clear
D)
11 cm
done
clear
View Answer play_arrow
-
question_answer22) A solenoid of 30 cm long is made by winding 2000 loops of wire on an iron rod whose 1 cross-section is 1.5 \[c{{m}^{2}}.\]. If the relative permeability of the iron is 6000, what is the self-inductance of the solenoid?
A)
1.5H
done
clear
B)
2.5H
done
clear
C)
3.5H
done
clear
D)
0.5H
done
clear
View Answer play_arrow
-
question_answer23) An electric current passes through a long straight wire. At a distance 5 cm from the wire, the magnetic field is B. The field at 20 cm from the wire would be
A)
\[\frac{B}{2}\]
done
clear
B)
\[y=A\sin (Bx+Ct+D)\]
done
clear
C)
\[[{{m}^{0}}{{L}^{-1}}{{T}^{0}}]\]
done
clear
D)
B
done
clear
View Answer play_arrow
-
question_answer24) Suitable impurities are added to a semiconductor depending on its use. This is done to
A)
increase its life
done
clear
B)
enable it to withstand high voltage
done
clear
C)
increases its electrical conductivity
done
clear
D)
increases its electrical resistivity
done
clear
View Answer play_arrow
-
question_answer25) Given that the displacement of an oscillating particle is given by\[[{{m}^{0}}{{L}^{0}}{{T}^{-1}}]\]. The dimensional formula for (ABCD) is
A)
\[[{{m}^{0}}{{L}^{-1}}{{T}^{-2}}]\]
done
clear
B)
\[[{{m}^{0}}{{L}^{0}}{{T}^{0}}]\]
done
clear
C)
\[1.5\mu \]
done
clear
D)
\[\mu \]
done
clear
View Answer play_arrow
-
question_answer26) A vessel is half-filled with a liquid of refractive index \[\mu \]. The other half of the vessel is filled with an immiscible liquid of refractive index. \[W\] The apparent depth of the vessel is 50% of the actual depth, then \[\frac{4W}{3}\] is
A)
1.4
done
clear
B)
1.5
done
clear
C)
1.6
done
clear
D)
1.67
done
clear
View Answer play_arrow
-
question_answer27) The work done in increasing the voltage across the plates of a capacitor from 5V to 10V is W. The work done in increasing the voltage from 10V to 15 V will be
A)
\[\frac{5W}{2}\]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\sigma =\text{5}.\text{67}\times \text{1}{{0}^{-\text{8}}}\text{W}-{{\text{m}}^{\text{2}}}{{\text{K}}^{\text{-4}}}\]
done
clear
D)
2W
done
clear
View Answer play_arrow
-
question_answer28) A copper ring is held horizontally and a bar magnet is dropped through the ring with its length along the axis of the ring. The acceleration of the falling magnet is
A)
equal to that due to gravity
done
clear
B)
less than that due to gravity
done
clear
C)
more than that due to gravity
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer29) A particle is executing two different simple harmonic motions, mutually perpendicular of different amplitudes and having phase difference of \[y=5\sin \frac{\pi x}{3}\cos 40\pi t\]. The path of the particle will be 4U
A)
circular
done
clear
B)
straight line
done
clear
C)
parabolic
done
clear
D)
elliptical
done
clear
View Answer play_arrow
-
question_answer30) A source is moving towards an observer with a speed of 20 m/s and having frequency of 240 Hz. The observer now moving towards the source with a speed of 20 m/s. Apparent frequency heard by the observer is (velocity of sound = 340 m/s)
A)
270 Hz
done
clear
B)
540 Hz
done
clear
C)
135 Hz
done
clear
D)
370 Hz
done
clear
View Answer play_arrow
-
question_answer31) A thin square plate with each side equal to 10 cm, is heated by a blacksmith. The rate of radiated energy by the heated plate is 1134 W. The temperature of hot square plate is (Stefan's constant\[t\], emissivity of plate =1)
A)
1000 K
done
clear
B)
1989 K
done
clear
C)
2000 K
done
clear
D)
2378 K
done
clear
View Answer play_arrow
-
question_answer32) The equation of stationary wave along a stretched string is given by \[{{(Kg)}^{1/2}}\], where x and y are in cm and \[{{(Kg)}^{-1/2}}\]in second. The separation between two adjacent nodes is
A)
1.5 cm
done
clear
B)
3 cm
done
clear
C)
6 cm
done
clear
D)
4 cm
done
clear
View Answer play_arrow
-
question_answer33) There are two planets and the ratio of radius of the two planets is k but ratio of acceleration due to gravity of both planets is g. What will be the ratio of their escape velocity?
A)
\[{{(Kg)}^{2}}\]
done
clear
B)
\[{{(Kg)}^{-2}}\]
done
clear
C)
\[\frac{pV}{nT}\]
done
clear
D)
\[\frac{pV}{nT}\frac{pV}{nT}\upsilon ersus\]
done
clear
View Answer play_arrow
-
question_answer34)
The figure below shows the plot of \[{{T}_{1}}>{{T}_{2}}\]\[\frac{pV}{nT}\]p for oxygen gas at two different temperatures. 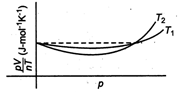 Read the following statements concerning the above curves. (i) The dotted line corresponds to the ideal gas behavior . (ii) \[4\times {{10}^{3}}A{{m}^{-1}}\] (iii)The value of \[\text{1}{{0}^{-\text{2}}}\] at the point, where the curves meet on the y-axis is the same for all edges. Which of the above statement is true?
Read the following statements concerning the above curves. (i) The dotted line corresponds to the ideal gas behavior . (ii) \[4\times {{10}^{3}}A{{m}^{-1}}\] (iii)The value of \[\text{1}{{0}^{-\text{2}}}\] at the point, where the curves meet on the y-axis is the same for all edges. Which of the above statement is true?
A)
Only (i)
done
clear
B)
(i) and (ii)
done
clear
C)
(i), (ii) and (iii)
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer35) A bar magnet has a coercivity \[\text{1}{{0}^{-3}}\]. It is desired to demagnetise it by inserting it inside a solenoid 12 cm long and having 60 turns. The current carried by solenoid should be
A)
8 A
done
clear
B)
6 A
done
clear
C)
4.5A
done
clear
D)
2 A
done
clear
View Answer play_arrow
-
question_answer36) A Carnot engine has efficiency 1/5. Efficiency becomes 1/3 when temperature of sink is decreased by 50 K. What is the temperature of sink?
A)
325 K
done
clear
B)
375 K
done
clear
C)
300 K
done
clear
D)
380 K
done
clear
View Answer play_arrow
-
question_answer37)
Following diagram performs the logic function 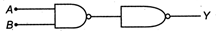
A)
OR gate
done
clear
B)
AND gate
done
clear
C)
XOR gate
done
clear
D)
NAND gate
done
clear
View Answer play_arrow
-
question_answer38) Parsec is the unit of
A)
time
done
clear
B)
distance
done
clear
C)
frequency
done
clear
D)
angular acceleration
done
clear
View Answer play_arrow
-
question_answer39) A bread gives a boy of mass 40 kg an energy of 21 kJ. If the efficiency is 28%, then the height can be climbed by him using this energy
A)
2.25 m
done
clear
B)
15 m
done
clear
C)
10 m
done
clear
D)
5m
done
clear
View Answer play_arrow
-
question_answer40) If the radius of a coil is changing at the rate of \[1\mu V\]units in a normal magnetic field \[\text{1}.\text{96}\times \text{1}{{0}^{-\text{8}}}\text{ m}/\text{s}\] units, the induced emf is \[\text{2}.\text{12}\times \text{1}{{0}^{\text{8}}}\text{ m}/\text{s}\]. What is the final radius of the coil?
A)
1.6 cm
done
clear
B)
16 cm
done
clear
C)
12 cm
done
clear
D)
1.2 cm
done
clear
View Answer play_arrow
-
question_answer41) A ray of light is incident on the surface of 47 separation of a medium with the velocity of light at an angle 45° and is refracted in the medium at an angle 30°. What will be the velocity of light in the medium?
A)
\[\text{3}.\text{18}\times \text{1}{{0}^{8}}m/s\]
done
clear
B)
\[\text{3}.\text{33}\times {{10}^{\text{8}}}\text{ m}/\text{s}\]
done
clear
C)
\[\theta =\text{45}{}^\circ \]
done
clear
D)
\[\frac{1}{3}M{{L}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer42) A spot of lights rotates in a horizontal plane with a constant angular velocity of 0.1rad/s. The spot of light P moves along the wall at a distance of 3 m from S. The velocity of spot P, where, \[\frac{3}{2}M{{L}^{2}}\]is
A)
0.5 m/s
done
clear
B)
0.6 m/s
done
clear
C)
0.7 m/s
done
clear
D)
0.8 m/s
done
clear
View Answer play_arrow
-
question_answer43) Three point masses, each of mass M are placed at the corners of an equilateral triangle of side L. The moment of inertia of the system about an axis along one side of the triangle is
A)
\[\frac{3}{4}M{{L}^{2}}\]
done
clear
B)
\[M{{L}^{2}}\]
done
clear
C)
\[{{R}_{1}}\]
done
clear
D)
\[{{R}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer44) Two conducting spheres of radii \[{{Q}_{1}}\] and \[{{Q}_{2}}\]. are charged with charges \[{{Q}_{1}}{{R}_{2}}\ne {{Q}_{2}}{{R}_{1}}\] and\[{{Q}_{1}}{{R}_{2}}={{Q}_{2}}{{R}_{1}}\] respectively. On bringing them in contact, there is
A)
no change in the energy of the system
done
clear
B)
an increase in the energy of the system, if \[s=\frac{{{t}^{2}}}{4}\]
done
clear
C)
always a decrease in the energy of the system
done
clear
D)
a decrease in the energy of the system, if \[T\propto V\]
done
clear
View Answer play_arrow
-
question_answer45) A body of mass 6 kg is under a force which causes displacement in it given by\[T\propto {{V}^{2}}\]where t is time. The work done by the force in 2s is
A)
12 J
done
clear
B)
9 J
done
clear
C)
6 J
done
clear
D)
3 J
done
clear
View Answer play_arrow
-
question_answer46) Which of the following statements is/are true?
A)
A clock when taken on a mountain can be made to give correct time, if we change the length of pendulum suitably
done
clear
B)
An increase in value of g makes a clock to slow
done
clear
C)
If the length of a pendulum is increased the clock becomes fast
done
clear
D)
A clock when taken to a top mine or carried to the top of mountain become slow
done
clear
View Answer play_arrow
-
question_answer47) In case of a forced vibration, the resonance wave becomes very sharp, when the
A)
applied periodic force is small
done
clear
B)
quality factor is small
done
clear
C)
damping force is small
done
clear
D)
restoring force is small
done
clear
View Answer play_arrow
-
question_answer48) The difference in length of a mean solar day and a sideral day is about
A)
1 min
done
clear
B)
4 min
done
clear
C)
15 min
done
clear
D)
56 min
done
clear
View Answer play_arrow
-
question_answer49) If the size of aperture is decreased
A)
intensity of image is decreased
done
clear
B)
no effect in formation of image
done
clear
C)
any of the above
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer50) If T is the reverberation time of an auditorium of volume V then
A)
\[T\propto \frac{1}{{{V}^{2}}}\]
done
clear
B)
\[T\propto \frac{1}{V}\]
done
clear
C)
\[\text{6}\times \text{1}{{0}^{-\text{7}}}\text{A}-{{\text{m}}^{\text{2}}}\]
done
clear
D)
\[\text{5 g}/\text{c}{{\text{m}}^{\text{3}}}\]
done
clear
View Answer play_arrow
-
question_answer51) The magnetic moment produced in a substance of 1 g is\[\text{8}\text{.3}\times \text{1}{{0}^{\text{6}}}\]. If its density is\[\text{1}.\text{2}\times \text{1}{{0}^{-\text{7}}}\], then the intensity of magnetisation in A/m will be
A)
\[\text{3}\times \text{1}{{0}^{-\text{6}}}\]
done
clear
B)
3.0
done
clear
C)
\[CaC{{l}_{2}}\]
done
clear
D)
\[\text{MgS}{{\text{O}}_{\text{4}}}\]
done
clear
View Answer play_arrow
-
question_answer52) A point P lies on the axis of a ring of mass M and radius R at a distance 2R from its centre O. A small particle starts from R and reaches Q under gravitational attraction only. Its speed of O will be
A)
zero
done
clear
B)
\[CaC{{l}_{2}}\]
done
clear
C)
\[CaC{{l}_{2}}\]
done
clear
D)
\[\text{MgS}{{\text{O}}_{\text{4}}}\]
done
clear
View Answer play_arrow
-
question_answer53) A lamp hanging 4 m above the table is lowered by 1m. The illumination on the table
A)
increase by 25%
done
clear
B)
decrease by 25%
done
clear
C)
increase by 77.7%
done
clear
D)
decrease by 77.7%
done
clear
View Answer play_arrow
-
question_answer54) The temperature coefficient of resistance of a wire is\[\text{MgS}{{\text{O}}_{\text{4}}}\], its resistance is \[\upsilon /\text{1}0\]. At what temperature, the resistance of the wire will be \[f\]?
A)
800K
done
clear
B)
\[1.11f\]
done
clear
C)
600 K
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer55) The capacity of a parallel plate capacitor depends on the
A)
type of metal used
done
clear
B)
thickness of plates
done
clear
C)
potential applied across the plates
done
clear
D)
separation between the plates
done
clear
View Answer play_arrow
-
question_answer56) Which of the following solutions are isotonic with one another?
A)
0.15 M urea and 0.05 M \[1.22f\]
done
clear
B)
0.15M urea and 0.1 M\[f\]
done
clear
C)
0.15 M urea and 0.15 M glucose
done
clear
D)
0.1\[1.27f\] and 0.05 M \[\text{1}.0\text{1}\times \text{1}{{0}^{\text{5}}}\text{ N}/{{\text{m}}^{\text{2}}}\]
done
clear
View Answer play_arrow
-
question_answer57)
Five moles of a gas is put through a series of changes as shown graphically in a cyclic process. The process during \[\text{9}.\text{13}\times \text{1}{{0}^{\text{4}}}\text{ N}/{{\text{m}}^{\text{2}}}\] and \[\text{9}.\text{13}\times \text{1}{{0}^{\text{3}}}\text{N}/{{\text{m}}^{\text{2}}}\] respectively are 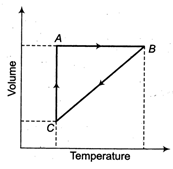
A)
isochoric, isobaric, isothermal
done
clear
B)
isobaricJsochoric, isothermal
done
clear
C)
isothermal, isobaric, isochoric
done
clear
D)
isochoric, isothermal, isobaric
done
clear
View Answer play_arrow
-
question_answer58) For the hypothetical reactions, the equilibrium constant (K) values are given \[AB,{{K}_{1}},=2.0\] \[BC,{{K}_{2}}=4.0\] \[CD;{{K}_{3}}=3.0\] The equilibrium constant (K), for the reaction \[9.13\times {{10}^{3}}N/{{m}^{2}}\]is
A)
48
done
clear
B)
6
done
clear
C)
2.7
done
clear
D)
24
done
clear
View Answer play_arrow
-
question_answer59) Standard electrode potential of half-cell reactions are given below \[C{{u}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Cu;\]\[{{E}^{o}}=0.34V\] \[Z{{n}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Zn;\]\[{{E}^{o}}=-0.76V\]What is the emf of the cell?
A)
\[\left[ \text{F}{{\text{L}}^{\text{2}}}{{T}^{-\text{2}}} \right]\]
done
clear
B)
\[\left[ \text{F}{{\text{L}}^{-\text{1}}}{{\text{T}}^{\text{2}}} \right]\]
done
clear
C)
\[\left[ {{\text{F}}^{2}}\text{L}{{\text{T}}^{\text{-2}}} \right]\]
done
clear
D)
\[-\text{273}.\text{15}{}^\circ \text{F}\]
done
clear
View Answer play_arrow
-
question_answer60) The product of reaction between aniline acetic anhydride is
A)
o-aminoacetophenone
done
clear
B)
m-aminoacetophenone
done
clear
C)
p-aminoacetophenone
done
clear
D)
acetanilide
done
clear
View Answer play_arrow
-
question_answer61) Anode reaction of a fuel cell is
A)
\[Zn(Hg)+2O{{H}^{-}}\xrightarrow{{}}ZnO(s)+{{H}_{2}}O+2{{e}^{-}}\]
done
clear
B)
\[Pb(s)+SO_{4}^{2-}(aq)\xrightarrow{{}}PbS{{O}_{4}}(s)+2{{e}^{-}}\]
done
clear
C)
\[2{{H}_{2}}(g)+4O{{H}^{-}}(aq)\xrightarrow{{}}4{{H}_{2}}O(l)+4{{e}^{-}}\]
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer62) 2.0 g of benzoic acid dissolved in 25.0 g benzene shows a depression in freezing poi equal to 1.62 K. Molal depression constant \[\text{Vc}=\text{1}.\text{5V}\] benzene is\[\text{1}00\text{ }\mu \text{A}\]. The percenta association of the acid is
A)
80%
done
clear
B)
99%
done
clear
C)
75%
done
clear
D)
100% \[1.5\mu \] \[\mu \] Normal molecular weight of \[W\] \[\frac{4W}{3}\]=122 \[\frac{5W}{2}\] \[\frac{\pi }{2}\] \[\sigma =\text{5}.\text{67}\times \text{1}{{0}^{-\text{8}}}\text{W}-{{\text{m}}^{\text{2}}}{{\text{K}}^{\text{-4}}}\] \[2{{C}_{6}}{{H}_{5}}COOH\xrightarrow{{}}{{({{C}_{6}}{{H}_{5}}COOH)}_{2}}\] \[t\] where, \[{{(Kg)}^{1/2}}\] \[{{(Kg)}^{-1/2}}\] \[{{(Kg)}^{2}}\] % association \[{{(Kg)}^{-2}}\] \[\frac{pV}{nT}\]
done
clear
View Answer play_arrow
-
question_answer63) The number of chloride ion/s produced complex tetraminedichloroplatinum chloride in a aqueous solution is
A)
four
done
clear
B)
two
done
clear
C)
one
done
clear
D)
three
done
clear
View Answer play_arrow
-
question_answer64) When \[\text{15}0\text{ }\mu \text{A}\]is added to phenol
A)
no reaction occurs
done
clear
B)
a coloured complex will be formed
done
clear
C)
\[\text{5 mA}\]will be oxidised to higher state
done
clear
D)
o-chlprophenol will be formed
done
clear
View Answer play_arrow
-
question_answer65) \[\text{10 mA}\]on treatment with \[\text{ }\!\!\beta\!\!\text{ }\]in aqueous medium gives
A)
no reaction
done
clear
B)
\[\left( \frac{1}{V(volume)} \right)\]
done
clear
C)
\[\frac{3}{4}\text{m}/\text{s}\]
done
clear
D)
isobutylene
done
clear
View Answer play_arrow
-
question_answer66) Conduction in a p-type semiconductor is increased by
A)
increasing the band gap
done
clear
B)
decreasing the temperature
done
clear
C)
adding appropriate electron deficient impurities
done
clear
D)
adding appropriate electron rich impurities
done
clear
View Answer play_arrow
-
question_answer67) \[\frac{1}{3}\text{m}/\text{s}\] and freons
A)
are green compounds because they are green coloured
done
clear
B)
deplete ozone
done
clear
C)
cause increase in ozone concentration
done
clear
D)
have no effect on ozone concentration
done
clear
View Answer play_arrow
-
question_answer68) Boron is unable to form\[BF_{6}^{3-}\] because of
A)
high electronegativity of boron
done
clear
B)
high electronegativity of fluorine
done
clear
C)
lack of d-orbitals in boron
done
clear
D)
less difference in electronegativity between B and F
done
clear
View Answer play_arrow
-
question_answer69) The blue colour obtained in the Lassaigne test is due to formation of the compound !m
A)
\[\frac{2}{3}\text{m}/\text{s}\]
done
clear
B)
\[{{\lambda }_{0}},\]
done
clear
C)
\[\frac{25}{16}{{\lambda }_{0}}\]
done
clear
D)
\[\frac{27}{20}{{\lambda }_{0}}\]
done
clear
View Answer play_arrow
-
question_answer70) A radioactive substance decays 20% in 10 min If at the start there are \[\frac{20}{27}{{\lambda }_{0}}\] atoms present after what time will the number of atoms be reduced to \[\frac{16}{25}{{\lambda }_{0}}\] atoms?
A)
5.65 h
done
clear
B)
4.65h
done
clear
C)
3.65 h
done
clear
D)
6.65 h
done
clear
View Answer play_arrow
-
question_answer71) \[3\Omega \]of gelatin is required to be added \[4\Omega \]of a standard gold solution to just prevent its precipitation by the addition \[4.5\Omega \]of 10% \[NaCl\] solution to it. Hence, the gold number of gelatin in milligram is
A)
\[5\Omega \]
done
clear
B)
\[\frac{\sqrt{3}}{1}\]
done
clear
C)
\[\frac{(\sqrt{3}+1)}{(\sqrt{3}-1)}\]
done
clear
D)
\[\frac{(\sqrt{3}+1)}{1}\]
done
clear
View Answer play_arrow
-
question_answer72) Which of the following are arranged in the decreasing order of dipole moment?
A)
\[\frac{4}{3}\]
done
clear
B)
\[4\mu F\]
done
clear
C)
\[10\mu F\]
done
clear
D)
\[8\mu F\]
done
clear
View Answer play_arrow
-
question_answer73) If a saturated solution prepared by dissolving \[120\mu F\]in water has \[\omega \] What is the value of \[R/2\] for\[\frac{4\omega }{5}\]?
A)
\[\frac{2\omega }{5}\]
done
clear
B)
\[\frac{3\omega }{5}\]
done
clear
C)
\[93.8\times {{10}^{-12}}\]
done
clear
D)
\[9.38\times {{10}^{-12}}\]
done
clear
View Answer play_arrow
-
question_answer74) Which one of the following molecules is achiral?
A)
1-bromo-2-butene
done
clear
B)
3-bromo-l-butene
done
clear
C)
2, 3-dihydroxy propanal
done
clear
D)
2-hydroxypropanoic acid
done
clear
View Answer play_arrow
-
question_answer75)
Consider the parallel reactions in the electrophilic addition of \[\mu =\frac{4}{3}\]to propene \[{{\sin }^{-1}}\left( \frac{9}{8} \right)\] the alternative pathways shown below 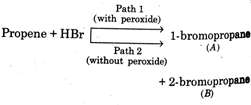 Identify the wrong statement with reference to Bafeabove
Identify the wrong statement with reference to Bafeabove
A)
Path1 has Predominance of (A) over (B)
done
clear
B)
Path-2 has predominance of (B) over (A)
done
clear
C)
Path-1 is in accordance with anti Markownikoff's rule
done
clear
D)
Both the paths afford 50% yield of (A) and (B)
done
clear
View Answer play_arrow
-
question_answer76) The raw materials for the commercial Manufacture of DDT are
A)
chlorobenzene and chloroform
done
clear
B)
chlorobenzene and chloromethane
done
clear
C)
chlorobenzene and chloral
done
clear
D)
chlorobenzene and iodoform
done
clear
View Answer play_arrow
-
question_answer77) The gas-phase reaction of nitric oxide and bromine yields nitrosyi bromide, \[2NO(g)+B{{r}_{2}}(g)\xrightarrow{{}}2NOBr(g)\] The rate law is, rate \[{{60}^{o}}\] What is the over all reaction order?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
-
question_answer78) Bakelite is formed by polymerisation between
A)
acrylonitrile molecules
done
clear
B)
tetrafluoroethene molecules
done
clear
C)
urea and formaldehyde molecules
done
clear
D)
phenol and formaldehyde molecules
done
clear
View Answer play_arrow
-
question_answer79) Chromatographic analysis is based on. The property of
A)
diffusion
done
clear
B)
absorption
done
clear
C)
adsorption
done
clear
D)
condensation
done
clear
View Answer play_arrow
-
question_answer80) Total number of metal atoms per unit cell in a face-centred 'cubic lattice is
A)
14
done
clear
B)
8
done
clear
C)
6
done
clear
D)
4
done
clear
View Answer play_arrow
-
question_answer81) The correct order of increasing oxidising power in the series is
A)
\[VO_{2}^{+}<C{{r}_{2}}O_{7}^{2-}<MnO_{4}^{-}\]
done
clear
B)
\[\beta =0.\text{1}\]
done
clear
C)
\[{{P}_{1}}\]
done
clear
D)
\[{{P}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer82) Normal human blood sugar range is \[{{P}_{1}}\]Considering density of human blood is 1.06 kg/L, if a patient's sugar level reads 720 ppm, his/her blood sugar at that time is
A)
normal
done
clear
B)
high
done
clear
C)
low
done
clear
D)
cannot say
done
clear
View Answer play_arrow
-
question_answer83) Which of the following statements is correct?
A)
The equivalent mass of \[{{P}_{2}}\]in alkaline medium is molar mass divided by five
done
clear
B)
The equivalent mass of \[\text{2}\times \text{1}{{0}^{\text{7}}}\text{m}/\text{s}\] in strongly alkaline medium is molar mass divided by three
done
clear
C)
The equivalent mass of \[\text{2}\times \text{1}{{0}^{-2}}T\] in neutral medium is molar mass divided by two
done
clear
D)
The equivalent mass of \[\left( \frac{e}{m} \right)\]in weakly acidic medium is molar mass divided by three
done
clear
View Answer play_arrow
-
question_answer84) The spin only magnetic moment of \[\text{1}.\text{76}\times \text{1}{{0}^{\text{11}}}\text{C}/\text{kg}\](at. no. for Cr is 24) is
A)
0
done
clear
B)
1.73BM
done
clear
C)
2.83BM
done
clear
D)
4.9BM
done
clear
View Answer play_arrow
-
question_answer85)
The correct relation between the following pair of compounds is 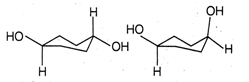
A)
constitutional isomers
done
clear
B)
enantiomers
done
clear
C)
diastereomers
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer86) The effective atomic number for\[2B\](at. no. for Rh is 45) is
A)
42
done
clear
B)
45
done
clear
C)
48
done
clear
D)
54
done
clear
View Answer play_arrow
-
question_answer87) The strongest acid among the choices is
A)
dichloroacetic acid
done
clear
B)
dimethylacetic acid
done
clear
C)
trifluoroacetic acid
done
clear
D)
triiodoacetic acid
done
clear
View Answer play_arrow
-
question_answer88) The correct order of leaving group ability in a nucleophilic substitution reaction is
A)
\[\frac{B}{4}\]
done
clear
B)
\[\frac{B}{2}\]
done
clear
C)
\[y=A\sin (Bx+Ct+D)\]
done
clear
D)
\[[{{m}^{0}}{{L}^{-1}}{{T}^{0}}]\]
done
clear
View Answer play_arrow
-
question_answer89) Glucose and fructose can be distinguished by (a) Lucas test
A)
Ninhydrin test
done
clear
B)
Benedict reagent test
done
clear
C)
All of the above
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer90) Which is the most stable compound among the following?
A)
done
clear
B)
done
clear
C)
done
clear
D)
All the compounds have same stability
done
clear
View Answer play_arrow
-
question_answer91) Entropy change in a process where 1 L of liquid He is poured into ice cold water is
A)
finite and positive
done
clear
B)
finite and negative
done
clear
C)
zero
done
clear
D)
infinity
done
clear
View Answer play_arrow
-
question_answer92) For an ideal system at thermal equilibrium, the velocity distribution of the constituent particles will be governed by
A)
Gaussian distribution
done
clear
B)
Maxwell-goltzmann distribution
done
clear
C)
Lorentzian distribution
done
clear
D)
Log-normal distribution
done
clear
View Answer play_arrow
-
question_answer93) Properties of elements are periodic function of number of...... present in the nucleus.
A)
protons
done
clear
B)
electrons
done
clear
C)
neutrons
done
clear
D)
mesons
done
clear
View Answer play_arrow
-
question_answer94)
Certain reactions follow the relation between concentrations of the reactant vs time as 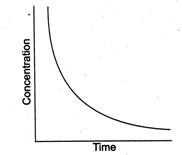 What is the expected order for such reactions?
What is the expected order for such reactions?
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
Infinity
done
clear
View Answer play_arrow
-
question_answer95) Maximum number of electrons in a shell with principal quantum number n is given by
A)
\[[{{m}^{0}}{{L}^{0}}{{T}^{-1}}]\]
done
clear
B)
\[[{{m}^{0}}{{L}^{-1}}{{T}^{-2}}]\]
done
clear
C)
\[[{{m}^{0}}{{L}^{0}}{{T}^{0}}]\]
done
clear
D)
\[1.5\mu \]
done
clear
View Answer play_arrow
-
question_answer96) The first step in the extraction of Cu from copper pyrites is
A)
reduction by carbon
done
clear
B)
electrolysis of ore
done
clear
C)
roasting of ore in\[\mu \]
done
clear
D)
magnetic separation
done
clear
View Answer play_arrow
-
question_answer97) A first order reaction has a rate constant \[W\]. How long it will take to decompose half of the reaction?
A)
\[\frac{4W}{3}\]
done
clear
B)
\[\frac{5W}{2}\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
\[\sigma =\text{5}.\text{67}\times \text{1}{{0}^{-\text{8}}}\text{W}-{{\text{m}}^{\text{2}}}{{\text{K}}^{\text{-4}}}\]
done
clear
View Answer play_arrow
-
question_answer98) \[y=5\sin \frac{\pi x}{3}\cos 40\pi t\] is a
A)
strong reducing agent
done
clear
B)
strong base
done
clear
C)
strong oxidising agent
done
clear
D)
weak base
done
clear
View Answer play_arrow
-
question_answer99) A ketone gives a yellow ppt when treated with \[t\] in an alkaline solution. Thus, the ketone is
A)
a cyclic ketone
done
clear
B)
a methyl ketone
done
clear
C)
an unsaturated ketone
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer100) The ore of magnetite is
A)
\[{{(Kg)}^{1/2}}\]
done
clear
B)
\[{{(Kg)}^{-1/2}}\]
done
clear
C)
\[{{(Kg)}^{2}}\]
done
clear
D)
\[{{(Kg)}^{-2}}\]
done
clear
View Answer play_arrow
-
question_answer101) A compound with nitro group was reduced by\[\frac{pV}{nT}\], followed by treatment with \[\frac{pV}{nT}\frac{pV}{nT}\upsilon ersus\] and followed by phenol. The chromophore group in the final compound is
A)
\[{{T}_{1}}>{{T}_{2}}\] group
done
clear
B)
\[\frac{pV}{nT}\] group
done
clear
C)
\[4\times {{10}^{3}}A{{m}^{-1}}\]group
done
clear
D)
\[\text{1}{{0}^{-\text{2}}}\]group
done
clear
View Answer play_arrow
-
question_answer102) The most stable oxidation state exhibited by thallium is
A)
0
done
clear
B)
+1
done
clear
C)
+2
done
clear
D)
+3
done
clear
View Answer play_arrow
-
question_answer103) Bohr model of hydrogen atom was unable to explain
A)
Rydberg's formula of atomic spectra
done
clear
B)
Heisenberg's uncertainty principle
done
clear
C)
Planck's law of energy quantization
done
clear
D)
Rutherford's model of atomic structure
done
clear
View Answer play_arrow
-
question_answer104) The order of basic strength for methyl substituted amine in aqueous solution is
A)
\[\text{1}{{0}^{-3}}\]
done
clear
B)
\[1\mu V\]
done
clear
C)
\[\text{1}.\text{96}\times \text{1}{{0}^{-\text{8}}}\text{ m}/\text{s}\]
done
clear
D)
\[\text{2}.\text{12}\times \text{1}{{0}^{\text{8}}}\text{ m}/\text{s}\]
done
clear
View Answer play_arrow
-
question_answer105) The crystal structure of solid Mn(II) oxide is
A)
\[\text{3}.\text{18}\times \text{1}{{0}^{8}}m/s\]structure
done
clear
B)
\[\text{3}.\text{33}\times {{10}^{\text{8}}}\text{ m}/\text{s}\]structure
done
clear
C)
\[\theta =\text{45}{}^\circ \] structure
done
clear
D)
\[\frac{1}{3}M{{L}^{2}}\] structure
done
clear
View Answer play_arrow
-
question_answer106) 2-bromobutane reacts with \[\frac{3}{2}M{{L}^{2}}\]in \[\frac{3}{4}M{{L}^{2}}\] to give 2-butanol. The reaction involves
A)
retention in configuration
done
clear
B)
inversion in configuration
done
clear
C)
racemization
done
clear
D)
mutarotation
done
clear
View Answer play_arrow
-
question_answer107) The compound used for gravimetric estimation of copper \[M{{L}^{2}}\] is
A)
\[{{R}_{1}}\]
done
clear
B)
\[{{R}_{2}}\]
done
clear
C)
\[{{Q}_{1}}\]
done
clear
D)
\[{{Q}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer108) Latent heat of vaporisation of water is \[{{Q}_{1}}{{R}_{2}}\ne {{Q}_{2}}{{R}_{1}}\] at\[{{Q}_{1}}{{R}_{2}}={{Q}_{2}}{{R}_{1}}\]. Calculate molal boiling point elevation constant of water.
A)
\[5.2{}^\circ \]
done
clear
B)
\[0.052{}^\circ \]
done
clear
C)
\[52.2{}^\circ \]
done
clear
D)
\[0.52{}^\circ \]
done
clear
View Answer play_arrow
-
question_answer109) The silver salt of an unknown monoacidic alkyne contains 67.08% silver. The structure of the alkyne is
A)
\[s=\frac{{{t}^{2}}}{4}\]
done
clear
B)
\[T\propto V\]
done
clear
C)
\[T\propto {{V}^{2}}\]
done
clear
D)
\[T\propto \frac{1}{{{V}^{2}}}\]
done
clear
View Answer play_arrow
-
question_answer110) Time required to deposit one millimole of As metal by the passage of 9.65A through aqueous solution of aluminium ion is
A)
30s
done
clear
B)
10s
done
clear
C)
3000s
done
clear
D)
10000s
done
clear
View Answer play_arrow
-
question_answer111) Matrix A is such that\[T\propto \frac{1}{V}\], where is the identity matrix. Then, for\[\text{6}\times \text{1}{{0}^{-\text{7}}}\text{A}-{{\text{m}}^{\text{2}}}\],\[{{A}^{n}}\] is equal to
A)
\[\text{5 g}/\text{c}{{\text{m}}^{\text{3}}}\]
done
clear
B)
\[nA-l\]
done
clear
C)
\[\text{1}.\text{2}\times \text{1}{{0}^{-\text{7}}}\]
done
clear
D)
\[\text{3}\times \text{1}{{0}^{-\text{6}}}\]
done
clear
View Answer play_arrow
-
question_answer112) The number of solutions of the system of equations \[CaC{{l}_{2}}\],\[\text{MgS}{{\text{O}}_{\text{4}}}\] and\[\text{MgS}{{\text{O}}_{\text{4}}}\] is
A)
zero
done
clear
B)
one
done
clear
C)
two
done
clear
D)
infinite
done
clear
View Answer play_arrow
-
question_answer113) Let\[CaC{{l}_{2}}\] be a function defined by \[x+3y-11=0\], where [ ] denotes the greatest integer function. Then, \[P({{x}_{1}},{{y}_{1}})\]is equal to
A)
\[Q\left( \text{4},-\text{3} \right)\]
done
clear
B)
\[\therefore \]
done
clear
C)
\[PQ\]
done
clear
D)
\[\frac{1}{x+\left[ \frac{\pi }{2} \right]}\]
done
clear
View Answer play_arrow
-
question_answer114) Let \[y=x\] be a function defined by \[\therefore \]The\[\frac{{{x}_{1}}+4}{2}=\frac{{{y}_{1}}-3}{2}\]is
A)
one-one onto
done
clear
B)
one-one but not onto
done
clear
C)
onto but not one-one
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer115) If the conjugate of \[\Rightarrow \]be \[{{x}_{1}}-{{y}_{1}}=-7\],then
A)
\[PQ=\frac{-3-{{y}_{1}}}{4-{{x}_{1}}}\]
done
clear
B)
\[y=x\]
done
clear
C)
\[\because \]
done
clear
D)
\[PQ\]
done
clear
View Answer play_arrow
-
question_answer116) In the argand plane, the complex number \[y=x\] is turned in the clockwise sense through 180° and stretched three times. The complex number represented by the new number is
A)
\[\therefore \]
done
clear
B)
\[\left( \frac{-3-{{y}_{1}}}{4-{{x}_{1}}} \right)(1)=-1\]
done
clear
C)
\[\Rightarrow \]
done
clear
D)
\[{{y}_{1}}+{{x}_{1}}=1\]
done
clear
View Answer play_arrow
-
question_answer117) If the sum of roots of equation \[{{x}_{1}}=-3\,\,\,and\,\,\,{{y}_{1}}=4\] is equal to sum of squares of their reciprocals, then \[\underset{x\to \infty }{\mathop{\lim }}\,{{\left( \frac{x+1}{x+2} \right)}^{2x+1}}=\underset{x\to \infty }{\mathop{\lim }}\,{{\left( 1-\frac{1}{x+2} \right)}^{2x+1}}\]and \[=\underset{x\to \infty }{\mathop{\lim }}\,{{\left[ {{\left( 1-\frac{1}{x+2} \right)}^{x+2}} \right]}^{\frac{2x+1}{x+2}}}\]are in
A)
GP
done
clear
B)
HP
done
clear
C)
AP
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer118) If the roots of the equation \[=\underset{x\to \infty }{\mathop{\lim }}\,\frac{2+1/x}{1+2/x}={{e}^{-2}}\] are real and less than 3,then
A)
\[[-1,\infty )-\{0\}\]
done
clear
B)
\[\text{x}=0\]
done
clear
C)
\[\therefore \]
done
clear
D)
\[Rf'(0)=\underset{h\to 0}{\mathop{\lim }}\,\frac{f(0+h)-f(0)}{h}\]
done
clear
View Answer play_arrow
-
question_answer119) If \[=\underset{h\to 0}{\mathop{\lim }}\,\frac{\sqrt{h+1}-1}{{{h}^{3/2}}}\times \frac{\sqrt{h+1}+1}{\sqrt{h+1}+1}\], y and z are in HP, then the value of expression \[=\underset{h\to 0}{\mathop{\lim }}\,\frac{h}{{{h}^{3/2}}(\sqrt{h+1}+1)}\] will be
A)
\[=\underset{h\to 0}{\mathop{\lim }}\,\frac{h}{\sqrt{h}(\sqrt{h+1}+1)}\]
done
clear
B)
\[=\frac{1}{0(\sqrt{0+1}+1)}=\frac{1}{0}=\infty \]
done
clear
C)
\[\underset{x\to 0}{\mathop{\lim }}\,\frac{\cos (\sin x)-1}{{{x}^{2}}}\]
done
clear
D)
\[\mu \]
done
clear
View Answer play_arrow
-
question_answer120) The term independent of. r in the expansion of \[W\]is
A)
\[\frac{4W}{3}\]
done
clear
B)
\[\frac{5W}{2}\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer121) The largest term in the expansion of\[\sigma =\text{5}.\text{67}\times \text{1}{{0}^{-\text{8}}}\text{W}-{{\text{m}}^{\text{2}}}{{\text{K}}^{\text{-4}}}\], where \[y=5\sin \frac{\pi x}{3}\cos 40\pi t\], is
A)
5th
done
clear
B)
3rd
done
clear
C)
7th
done
clear
D)
6th
done
clear
View Answer play_arrow
-
question_answer122) \[t\]is equal to
A)
0
done
clear
B)
\[{{(Kg)}^{1/2}}\]
done
clear
C)
\[{{(Kg)}^{-1/2}}\]
done
clear
D)
\[{{(Kg)}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer123) If A and B are square matrices of order 3 such that \[{{(Kg)}^{-2}}\]and\[\frac{pV}{nT}\], then \[\frac{pV}{nT}\frac{pV}{nT}\upsilon ersus\] is equal to
A)
-9
done
clear
B)
-81
done
clear
C)
-27
done
clear
D)
81
done
clear
View Answer play_arrow
-
question_answer124) If\[{{T}_{1}}>{{T}_{2}}\] , then r , is equal to
A)
3
done
clear
B)
4
done
clear
C)
8
done
clear
D)
6
done
clear
View Answer play_arrow
-
question_answer125) The number of ways in which \[\frac{pV}{nT}\] students can be distributed equal among n sections, is
A)
\[4\times {{10}^{3}}A{{m}^{-1}}\]
done
clear
B)
\[\text{1}{{0}^{-\text{2}}}\]
done
clear
C)
\[\text{1}{{0}^{-3}}\]
done
clear
D)
\[1\mu V\]
done
clear
View Answer play_arrow
-
question_answer126) The origin is shifted to (1, 2). The equation \[\text{1}.\text{96}\times \text{1}{{0}^{-\text{8}}}\text{ m}/\text{s}\] changes to \[\text{2}.\text{12}\times \text{1}{{0}^{\text{8}}}\text{ m}/\text{s}\] Then, \[\text{3}.\text{18}\times \text{1}{{0}^{8}}m/s\] is equal to
A)
1
done
clear
B)
2
done
clear
C)
-2
done
clear
D)
-1
done
clear
View Answer play_arrow
-
question_answer127) Given points are A (0,4) and\[\text{3}.\text{33}\times {{10}^{\text{8}}}\text{ m}/\text{s}\]. Then, locus of \[\theta =\text{45}{}^\circ \] such that \[\frac{1}{3}M{{L}^{2}}\]is
A)
\[\frac{3}{2}M{{L}^{2}}\]
done
clear
B)
\[\frac{3}{4}M{{L}^{2}}\]
done
clear
C)
\[M{{L}^{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer128) The equation of straight line perpendicular to a line \[{{R}_{1}}\] and passes through (5, 2) is
A)
\[{{R}_{2}}\]
done
clear
B)
\[{{Q}_{1}}\]
done
clear
C)
\[{{Q}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer129) The image of the point (4, -3) with respect to the line \[{{Q}_{1}}{{R}_{2}}\ne {{Q}_{2}}{{R}_{1}}\] is
A)
\[(-4,-3)\]
done
clear
B)
\[(3,4)\]
done
clear
C)
\[(-4,\text{ }3)\]
done
clear
D)
\[(-3,\text{ }4)\]
done
clear
View Answer play_arrow
-
question_answer130) \[{{Q}_{1}}{{R}_{2}}={{Q}_{2}}{{R}_{1}}\] is equal to
A)
\[s=\frac{{{t}^{2}}}{4}\]
done
clear
B)
e
done
clear
C)
\[T\propto V\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer131) The set of points of differentiability of the function \[T\propto {{V}^{2}}\] is
A)
R
done
clear
B)
\[T\propto \frac{1}{{{V}^{2}}}\]
done
clear
C)
\[T\propto \frac{1}{V}\]
done
clear
D)
\[\text{6}\times \text{1}{{0}^{-\text{7}}}\text{A}-{{\text{m}}^{\text{2}}}\]
done
clear
View Answer play_arrow
-
question_answer132) \[\text{5 g}/\text{c}{{\text{m}}^{\text{3}}}\]is equal to
A)
1
done
clear
B)
\[\text{8}\text{.3}\times \text{1}{{0}^{\text{6}}}\]
done
clear
C)
\[\text{1}.\text{2}\times \text{1}{{0}^{-\text{7}}}\]
done
clear
D)
\[\text{3}\times \text{1}{{0}^{-\text{6}}}\]
done
clear
View Answer play_arrow
-
question_answer133) Let \[CaC{{l}_{2}}\] and \[\text{MgS}{{\text{O}}_{\text{4}}}\]where \[CaC{{l}_{2}}\]is continuous. Then, \[CaC{{l}_{2}}\]is equal to
A)
\[f(x)g(0)\]
done
clear
B)
\[\text{MgS}{{\text{O}}_{\text{4}}}\]
done
clear
C)
\[\upsilon /\text{1}0\]
done
clear
D)
\[f\]
done
clear
View Answer play_arrow
-
question_answer134) The equation of a tangent parallel to\[1.11f\]drawn to\[1.22f\]is
A)
\[f\]
done
clear
B)
\[1.27f\]
done
clear
C)
\[\text{1}.0\text{1}\times \text{1}{{0}^{\text{5}}}\text{ N}/{{\text{m}}^{\text{2}}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer135) The lengths of the axes of the conic \[\text{9}.\text{13}\times \text{1}{{0}^{\text{4}}}\text{ N}/{{\text{m}}^{\text{2}}}\] are
A)
\[\text{9}.\text{13}\times \text{1}{{0}^{\text{3}}}\text{N}/{{\text{m}}^{\text{2}}}\]
done
clear
B)
\[\text{18}.\text{26 N}/{{\text{m}}^{\text{2}}}\]
done
clear
C)
\[\text{2}.\text{25}\times \text{1}{{0}^{\text{3}}}\text{min}\]
done
clear
D)
3,2
done
clear
View Answer play_arrow
-
question_answer136) If a chord which is normal to the parabola at one end, subtends a right angle at the vertex, then angle to the axis is
A)
\[\text{3}.\text{97}\times \text{1}{{0}^{\text{3}}}\text{min}\]
done
clear
B)
0
done
clear
C)
\[9.13\times {{10}^{3}}N/{{m}^{2}}\]
done
clear
D)
\[\text{5}.\text{25}\times \text{1}{{0}^{\text{3}}}\text{min}\]
done
clear
View Answer play_arrow
-
question_answer137) Two cards are drawn without replacement from a well-shuffled pack. The probability that one of them is an ace of heart, is
A)
\[\left[ \text{FL}{{\text{T}}^{-\text{2}}} \right]\]
done
clear
B)
\[\left[ \text{F}{{\text{L}}^{\text{2}}}{{T}^{-\text{2}}} \right]\]
done
clear
C)
\[\left[ \text{F}{{\text{L}}^{-\text{1}}}{{\text{T}}^{\text{2}}} \right]\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer138) If\[\left[ {{\text{F}}^{2}}\text{L}{{\text{T}}^{\text{-2}}} \right]\]and \[-\text{273}.\text{15}{}^\circ \text{F}\]then \[-\text{453}.\text{15}{}^\circ \text{F}\] is equal to
A)
\[-\text{459}.\text{67}{}^\circ \text{F}\]
done
clear
B)
\[-\text{491}.\text{67}{}^\circ \text{F}\]
done
clear
C)
\[\text{52}00\text{{ }\!\!\mathrm{\AA}\!\!\text{ }}\]
done
clear
D)
\[\text{Vc}=\text{1}.\text{5V}\]
done
clear
View Answer play_arrow
-
question_answer139) The value of\[[a-b\,b-c\,c-a]\] is
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
-
question_answer140) Let \[\text{15}0\text{ }\mu \text{A}\] and \[\text{5 mA}\]be unit vectors at an angle \[\text{10 mA}\] \[\text{ }\!\!\beta\!\!\text{ }\] from each other. Then, \[\left( \frac{1}{V(volume)} \right)\], if
A)
\[\frac{3}{4}\text{m}/\text{s}\]
done
clear
B)
\[\frac{1}{3}\text{m}/\text{s}\]
done
clear
C)
\[\frac{3}{2}\text{m}/\text{s}\]
done
clear
D)
\[\frac{2}{3}\text{m}/\text{s}\]
done
clear
View Answer play_arrow
-
question_answer141) The angle between the planes \[{{\lambda }_{0}},\]and \[\frac{25}{16}{{\lambda }_{0}}\] is
A)
\[\frac{27}{20}{{\lambda }_{0}}\]
done
clear
B)
\[\frac{20}{27}{{\lambda }_{0}}\]
done
clear
C)
\[\frac{16}{25}{{\lambda }_{0}}\]
done
clear
D)
\[3\Omega \]
done
clear
View Answer play_arrow
-
question_answer142) The equation of the plane which bisects the line joining (2, 3, 4) and (6, 7, 8), is
A)
\[4\Omega \]
done
clear
B)
\[4.5\Omega \]
done
clear
C)
\[5\Omega \]
done
clear
D)
\[\frac{\sqrt{3}}{1}\]
done
clear
View Answer play_arrow
-
question_answer143) The point on the line \[\frac{(\sqrt{3}+1)}{(\sqrt{3}-1)}\]at a distance of 6 from the point (2, -3, -5) is
A)
(3,-5,-3)
done
clear
B)
(4,-7.-9)
done
clear
C)
(0,2,-1)
done
clear
D)
(-3,5,3)
done
clear
View Answer play_arrow
-
question_answer144) The maximum value of \[\frac{(\sqrt{3}+1)}{1}\]is
A)
\[\frac{4}{3}\]
done
clear
B)
\[4\mu F\]
done
clear
C)
\[10\mu F\]
done
clear
D)
\[8\mu F\]
done
clear
View Answer play_arrow
-
question_answer145) If \[120\mu F\]then the value of \[\omega \] is
A)
1
done
clear
B)
2
done
clear
C)
0
done
clear
D)
\[R/2\]
done
clear
View Answer play_arrow
-
question_answer146) The number of solutions of the equation \[\frac{4\omega }{5}\] in \[\frac{2\omega }{5}\]is
A)
zero
done
clear
B)
one
done
clear
C)
two
done
clear
D)
three
done
clear
View Answer play_arrow
-
question_answer147) If in a \[\frac{3\omega }{5}\]\[\frac{2\omega }{3}\], then \[\mu =\frac{3}{2}\] is equal to
A)
\[30{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer148) In \[\mu =\frac{4}{3}\] then a, b and care in
A)
AP
done
clear
B)
GP
done
clear
C)
HP
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer149) \[{{\sin }^{-1}}\left( \frac{9}{8} \right)\] is equal to
A)
\[{{\cos }^{-1}}\left( \frac{x-4}{5} \right)+C\]
done
clear
B)
\[si{{n}^{-1}}\left( \frac{x-4}{5} \right)+C\]
done
clear
C)
\[si{{n}^{-1}}\left( \frac{5}{x-4} \right)+C\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer150) \[\beta =0.\text{1}\]is equal to
A)
\[\frac{{{x}^{2}}}{2}+C\]
done
clear
B)
\[-\frac{{{x}^{2}}}{2}+C\]
done
clear
C)
\[x|x|+C\]
done
clear
D)
\[\frac{x|x|}{2}+C\]
done
clear
View Answer play_arrow
-
question_answer151) \[\text{2}\times \text{1}{{0}^{\text{7}}}\text{m}/\text{s}\]is equal to
A)
\[\text{2}\times \text{1}{{0}^{-2}}T\]
done
clear
B)
\[\left( \frac{e}{m} \right)\]
done
clear
C)
\[\text{1}.\text{76}\times \text{1}{{0}^{\text{11}}}\text{C}/\text{kg}\]
done
clear
D)
\[2B\]
done
clear
View Answer play_arrow
-
question_answer152) \[\frac{B}{4}\] is equal to
A)
\[\frac{B}{2}\]
done
clear
B)
\[y=A\sin (Bx+Ct+D)\]
done
clear
C)
\[[{{m}^{0}}{{L}^{-1}}{{T}^{0}}]\]
done
clear
D)
\[[{{m}^{0}}{{L}^{0}}{{T}^{-1}}]\]
done
clear
View Answer play_arrow
-
question_answer153) The area bounded by \[[{{m}^{0}}{{L}^{-1}}{{T}^{-2}}]\] and X-axis is
A)
\[[{{m}^{0}}{{L}^{0}}{{T}^{0}}]\]
done
clear
B)
\[1.5\mu \]
done
clear
C)
\[\mu \]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer154) A man on the top of a cliff 100 m high observes the angles of depression of two points on the opposite sides of the cliff as 30° and 60°, respectively. Then, the distance between the two points is
A)
400 m
done
clear
B)
\[W\]
done
clear
C)
\[\frac{4W}{3}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer155) The solution set of the equation \[\frac{5W}{2}\]is
A)
[0,1]
done
clear
B)
[-1,1]
done
clear
C)
[1.3]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer156) If \[\frac{\pi }{2}\],then the value of \[\sigma =\text{5}.\text{67}\times \text{1}{{0}^{-\text{8}}}\text{W}-{{\text{m}}^{\text{2}}}{{\text{K}}^{\text{-4}}}\] will be
A)
2abc
done
clear
B)
abc
done
clear
C)
\[y=5\sin \frac{\pi x}{3}\cos 40\pi t\]
done
clear
D)
\[t\]
done
clear
View Answer play_arrow
-
question_answer157) The. solution of the differential equation \[{{(Kg)}^{1/2}}\] is
A)
\[{{(Kg)}^{-1/2}}\]
done
clear
B)
\[{{(Kg)}^{2}}\]
done
clear
C)
\[{{(Kg)}^{-2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer158) The integrating factor of the differential equation \[\frac{pV}{nT}\]is
A)
\[{{x}^{\log x}}\]
done
clear
B)
\[{{(\sqrt{x})}^{\log x}}\]
done
clear
C)
\[{{(\sqrt{e})}^{{{(\log x)}^{2}}}}\]
done
clear
D)
\[{{e}^{{{x}^{2}}}}\]
done
clear
View Answer play_arrow
-
question_answer159) If \[\text{1}{{0}^{-\text{2}}}\].then \[\text{1}{{0}^{-3}}\] is equal to
A)
\[1\mu V\]
done
clear
B)
\[\text{1}.\text{96}\times \text{1}{{0}^{-\text{8}}}\text{ m}/\text{s}\]
done
clear
C)
\[{{(\tan x)}^{\sin x}}[\sec x+\cos x\log \tan x]\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer160) If\[\text{3}.\text{18}\times \text{1}{{0}^{8}}m/s\] and \[\text{3}.\text{33}\times {{10}^{\text{8}}}\text{ m}/\text{s}\] then \[\theta =\text{45}{}^\circ \] is equal to
A)
\[\frac{-y}{x}\]
done
clear
B)
\[\frac{y}{x}\]
done
clear
C)
\[-\frac{x}{y}\]
done
clear
D)
\[\frac{x}{y}\]
done
clear
View Answer play_arrow
-
question_answer161) The equation of the tangent to the curve\[{{R}_{1}}\]at the point, where the ordinate and the abscissa are equal, is
A)
\[{{R}_{2}}\]
done
clear
B)
\[{{Q}_{1}}\]
done
clear
C)
\[{{Q}_{2}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer162) The function \[{{Q}_{1}}{{R}_{2}}\ne {{Q}_{2}}{{R}_{1}}\]has
A)
no maxima and minima
done
clear
B)
one maximum and one minimum
done
clear
C)
two maxima
done
clear
D)
two minima
done
clear
View Answer play_arrow
-
question_answer163) If \[{{Q}_{1}}{{R}_{2}}={{Q}_{2}}{{R}_{1}}\]and f(0) = 0, then the value of a for which Rolle's theorem can be applied in [0,1], is
A)
-2
done
clear
B)
-1
done
clear
C)
0
done
clear
D)
\[s=\frac{{{t}^{2}}}{4}\]
done
clear
View Answer play_arrow
-
question_answer164) The algebraic sum of the deviation of 20 observations measured from 30 is 2. Then mean of observations is
A)
28.5
done
clear
B)
30.1
done
clear
C)
30.5
done
clear
D)
29.6
done
clear
View Answer play_arrow
-
question_answer165) The standard deviation of 15 items is 6 and if each item is decreased by 1, then standard deviation will be
A)
5
done
clear
B)
7
done
clear
C)
\[T\propto V\]
done
clear
D)
6
done
clear
View Answer play_arrow
-
question_answer166) If \[T\propto {{V}^{2}}\]is equal to
A)
2
done
clear
B)
0
done
clear
C)
\[T\propto \frac{1}{{{V}^{2}}}\]
done
clear
D)
0
done
clear
View Answer play_arrow
-
question_answer167) The equation of the smallest circle passing through the intersection of the line \[T\propto \frac{1}{V}\] and the circle \[\text{6}\times \text{1}{{0}^{-\text{7}}}\text{A}-{{\text{m}}^{\text{2}}}\] is
A)
\[\text{5 g}/\text{c}{{\text{m}}^{\text{3}}}\]
done
clear
B)
\[\text{8}\text{.3}\times \text{1}{{0}^{\text{6}}}\]
done
clear
C)
\[\text{1}.\text{2}\times \text{1}{{0}^{-\text{7}}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer168) The complex number \[\text{3}\times \text{1}{{0}^{-\text{6}}}\] in polar form is
A)
\[CaC{{l}_{2}}\]
done
clear
B)
\[\text{MgS}{{\text{O}}_{\text{4}}}\]
done
clear
C)
\[\text{MgS}{{\text{O}}_{\text{4}}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer169) If \[CaC{{l}_{2}}\]and \[A\to B,B\to C\], then\[C\to A\]is equal to
A)
\[\upsilon /\text{1}0\]
done
clear
B)
\[f\]
done
clear
C)
\[1.11f\]
done
clear
D)
\[1.22f\]
done
clear
View Answer play_arrow
-
question_answer170) The orthocentre of the triangle formed by (0,0), (8, 0) and (4, 6) is
A)
\[f\]
done
clear
B)
\[1.27f\]
done
clear
C)
\[\text{1}.0\text{1}\times \text{1}{{0}^{\text{5}}}\text{ N}/{{\text{m}}^{\text{2}}}\]
done
clear
D)
\[\text{9}.\text{13}\times \text{1}{{0}^{\text{4}}}\text{ N}/{{\text{m}}^{\text{2}}}\]
done
clear
View Answer play_arrow
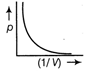

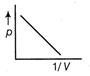
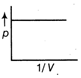


 Read the following statements concerning the above curves. (i) The dotted line corresponds to the ideal gas behavior . (ii) \[4\times {{10}^{3}}A{{m}^{-1}}\] (iii)The value of \[\text{1}{{0}^{-\text{2}}}\] at the point, where the curves meet on the y-axis is the same for all edges. Which of the above statement is true?
Read the following statements concerning the above curves. (i) The dotted line corresponds to the ideal gas behavior . (ii) \[4\times {{10}^{3}}A{{m}^{-1}}\] (iii)The value of \[\text{1}{{0}^{-\text{2}}}\] at the point, where the curves meet on the y-axis is the same for all edges. Which of the above statement is true?

 Identify the wrong statement with reference to Bafeabove
Identify the wrong statement with reference to Bafeabove

 What is the expected order for such reactions?
What is the expected order for such reactions?
