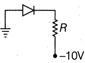-
question_answer1) A satellite in a circular orbit of radius R has a period of 4 h. Another satellite with orbital radius 3R around the same planet will have a period (in hours)
A)
16
done
clear
B)
4
done
clear
C)
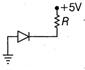
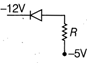
\[0.5\Omega \]
done
clear
D)
\[1\Omega \]
done
clear
View Answer play_arrow
-
question_answer2) The volume of a block of metal changes by 0.12% when heated through 20°C. Then, a is
A)
\[4\Omega \]
done
clear
B)
\[\frac{M{{R}^{2}}T}{2\pi }\]
done
clear
C)
\[\frac{M{{R}^{2}}T}{4\pi }\]
done
clear
D)
\[\frac{4\pi M{{R}^{2}}}{5T}\]
done
clear
View Answer play_arrow
-
question_answer3) In Young's double slit experiment, intensity at a point is (1 / 4) of the maximum intensity. Angular position of this point is
A)
\[\frac{2\pi M{{R}^{2}}}{5T}\]
done
clear
B)
\[U(X)=\left[ \frac{{{x}^{4}}}{4}-\frac{{{x}^{2}}}{2} \right]J\]
done
clear
C)
\[\sqrt{2}\]
done
clear
D)
\[\frac{1}{\sqrt{2}}\]
done
clear
View Answer play_arrow
-
question_answer4) A bar magnet is placed inside a non-uniform magnetic field. It experiences
A)
a torque but not a force
done
clear
B)
a force but not a torque
done
clear
C)
a force and a torque
done
clear
D)
neither a force nor a torque
done
clear
View Answer play_arrow
-
question_answer5) The potential to which a conductor is raised depends upon
A)
the amount of charges
done
clear
B)
geometry and size of the conductor
done
clear
C)
both (a) and (b)
done
clear
D)
Only on (a)
done
clear
View Answer play_arrow
-
question_answer6) The efficiency of a Carnot engine working between 800 K and 500 K is
A)
0.625
done
clear
B)
0.5
done
clear
C)
0.4.
done
clear
D)
0.375
done
clear
View Answer play_arrow
-
question_answer7)
From the following figures, choose the correct observation. 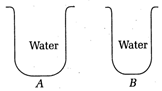
A)
The pressure depends on the shape of the container
done
clear
B)
The pressure on the bottoms of A and B is the same
done
clear
C)
The pressure on the bottom of tank A is greater than that at the bottom of B
done
clear
D)
The pressure on the bottom of the tank A is smaller than that at the bottom of B
done
clear
View Answer play_arrow
-
question_answer8) A ball is thrown upwards and it returns to ground describing a parabolic path. Which of the following remains constant?
A)
Vertical component of velocity
done
clear
B)
Speed of the ball
done
clear
C)
Kinetic energy of the ball
done
clear
D)
Horizontal component of velocity
done
clear
View Answer play_arrow
-
question_answer9)
Three blocks are placed at rest on a smooth inclined plane. Find the contact force between \[\frac{3}{\sqrt{2}}\] and \[\frac{45}{8}km/h\]. 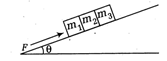
A)
\[\frac{25}{4}km/h\]
done
clear
B)
\[5km/h\]
done
clear
C)
\[\frac{30}{4}km/h\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer10) For a transistor amplifier in common emitter configuration for a load impedance of \[{{m}_{1}}\times {{m}_{2}}\]\[\frac{{{\lambda }_{1}}}{{{\lambda }_{2}}}\], the current gain is
A)
\[-48.78\]
done
clear
B)
\[-52\]
done
clear
C)
\[-24.8\]
done
clear
D)
\[-15.7\]
done
clear
View Answer play_arrow
-
question_answer11) In case of linearly polarised light, the magnitude of the electric field vector
A)
varies periodically with time
done
clear
B)
is parallel to the direction of propagation
done
clear
C)
does not change with time
done
clear
D)
increases and decreases linearly with time
done
clear
View Answer play_arrow
-
question_answer12) A moving coil galvanometer has 150 equal divisions. Its current sensitivity is 10 divisions \[\sqrt{\frac{{{m}_{2}}}{{{m}_{1}}}}\] and voltage sensitivity is 2 divisions \[\frac{{{m}_{2}}}{{{m}_{1}}}\]. In order that each division , reach IV, the resistance (in ohm) needed to be connected in series with the coil will be
A)
9995
done
clear
B)
99995
done
clear
C)
\[{{10}^{5}}\]
done
clear
D)
\[{{10}^{3}}\]
done
clear
View Answer play_arrow
-
question_answer13) The voltage of clouds is \[\frac{{{m}_{1}}}{{{m}_{2}}}\]with respect to the ground. In a lightning strike lasting 100 ms, a charge of 4 C is delivered to the ground. The power of the lightning strike is
A)
500MW
done
clear
B)
20MW
done
clear
C)
160MW
done
clear
D)
80MW
done
clear
View Answer play_arrow
-
question_answer14) The disc of a siren containing 60 holes rotates at a constant speed of 360 rpm. The emitted sound is in unison with a tuning fork of frequency
A)
60 Hz
done
clear
B)
360 Hz
done
clear
C)
10Hz
done
clear
D)
216 Hz
done
clear
View Answer play_arrow
-
question_answer15)
A force\[I\] acts on O, the origin of the coordinate system. The torque about the point (1,- 1) is 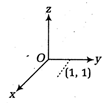
A)
\[5V\]
done
clear
B)
\[{{l}_{AC}}=\sqrt{2}{{l}_{EF}}\]
done
clear
C)
\[{{l}_{AC}}={{l}_{EF}}\]
done
clear
D)
\[\sqrt{2}{{l}_{AC}}={{l}_{EF}}\]
done
clear
View Answer play_arrow
-
question_answer16) A rope of length L and mass M are hanging from a rigid support. The tension in the rope at a distance x from the rigid support is
A)
\[{{l}_{AD}}=4{{l}_{EF}}\]
done
clear
B)
\[O=\overline{X+Y}\]
done
clear
C)
\[O=\overline{XY}\]
done
clear
D)
\[O=\overline{X}.\overline{Y}\]
done
clear
View Answer play_arrow
-
question_answer17) The half life of an element A is\[O=\overline{X}+\overline{Y}\]is. The time taken for the radioactivity of a sample of element A to decay to \[y=a\cos (\omega t-kx)\] of its intial value is
A)
\[[{{M}^{o}}LT]\]
done
clear
B)
\[[{{M}^{o}}{{L}^{-1}}{{T}^{o}}]\]
done
clear
C)
\[[{{M}^{o}}{{L}^{-1}}{{T}^{-1}}]\]
done
clear
D)
\[[{{M}^{o}}L{{T}^{-1}}]\]
done
clear
View Answer play_arrow
-
question_answer18) The Young's modulus of the material of a wire is\[{{t}^{-1}}\]. If the elongation strain is 2%, then the energy stored in the wire per unit volume is \[{{t}^{\frac{-1}{2}}}\]is
A)
\[{{t}^{\frac{1}{2}}}\]
done
clear
B)
\[t\]
done
clear
C)
\[[FL{{T}^{-2}}]\]
done
clear
D)
\[[F{{L}^{2}}{{L}^{-2}}]\]
done
clear
View Answer play_arrow
-
question_answer19) The speed of electromagnetic radiation in vacuum is
A)
\[[F{{L}^{-1}}{{T}^{2}}]\]
done
clear
B)
\[[{{F}^{2}}L{{T}^{-2}}]\]
done
clear
C)
\[2{{O}_{3}}\to 3{{O}_{2}}\]
done
clear
D)
\[{{O}_{3}}{{O}_{2}}+O......(fast)\]
done
clear
View Answer play_arrow
-
question_answer20) The ratio of amounts of scattering of two light waves is\[O+{{O}_{3}}\xrightarrow{{}}2{{O}_{2}}\,\,\,......(Slow)\]. The ratio of their wavelengths is
A)
\[r=k{{[{{O}_{3}}]}^{2}}\]
done
clear
B)
\[r=k{{[{{O}_{3}}]}^{2}}{{[{{O}_{2}}]}^{-1}}\]
done
clear
C)
\[r=k[{{O}_{3}}][{{O}_{2}}]\]
done
clear
D)
\[Mg(s)+Z{{n}^{2+}}(aq)(0.1M)\to M{{g}^{2+}}(aq)\]
done
clear
View Answer play_arrow
-
question_answer21) Coefficient of coupling between two coils of self- inductances\[(0.01M)+Zn(s)\] and \[{{E}_{(cell)}}\] is unity. It means
A)
100% flux of\[{{L}_{1}}\]is linked with \[E_{_{(cell)}}^{o}=1.61V\]
done
clear
B)
50% of\[{{L}_{1}}\]is linked with \[\Delta {{H}_{(mixing)}}>0\]
done
clear
C)
\[900K,{{S}_{8}}\]times of flux of \[{{S}_{2}}\] is Linked with 4
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer22) If in a circular coil A of radius R, current \[0.\text{75 at}{{\text{m}}^{\text{3}}}\] is flowing and in another coil B of radius 2R, a current 21 is flowing ; then the ratio of the magnetic fields\[{{B}_{A}}\]and\[{{B}_{B}}\]produced by them will be
A)
2
done
clear
B)
\[\text{2}.\text{55 at}{{\text{m}}^{\text{3}}}\]
done
clear
C)
3
done
clear
D)
1
done
clear
View Answer play_arrow
-
question_answer23) A\[10\mu F\]capacitor is charged to 500 V and then its plates are joined together through a resistance of \[100\Omega \]. The heat produced
A)
250J
done
clear
B)
\[\text{25}.0\text{ at}{{\text{m}}^{\text{3}}}\]
done
clear
C)
500J
done
clear
D)
\[\text{1}.\text{33 at}{{\text{m}}^{\text{3}}}\]
done
clear
View Answer play_arrow
-
question_answer24) An ideal gas is expanding such that \[\text{C}{{\text{H}}_{\text{3}}}\text{Cl}\]constant. The coefficient of volume expansion of the gas is
A)
\[\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\]
done
clear
B)
\[\text{CHC}{{\text{l}}_{\text{3}}}\]
done
clear
C)
\[\text{CC}{{\text{l}}_{\text{4}}}\]
done
clear
D)
\[\text{C}\text{N}\]
done
clear
View Answer play_arrow
-
question_answer25) Motion of liquid in a tube is best described by
A)
Bernoulli theorem
done
clear
B)
Archimedes' principle
done
clear
C)
Poiseuille's equation
done
clear
D)
Stoke's law
done
clear
View Answer play_arrow
-
question_answer26)
A simple pendulum oscillates in a vertical plane. When it passes through the mean position, the tension in the string is 3 times the weight of the pendulum bob. What is the maximum displacement of the pendulum of the string with respect to the vertical? 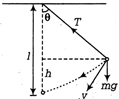
A)
\[60{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
\[30{}^\circ \]
done
clear
D)
\[45{}^\circ \]
done
clear
View Answer play_arrow
-
question_answer27) A ball is dropped from a height A on a floor of coefficient of restitution. The total distance covered by the ball just before second hit is
A)
\[\text{S}0\]
done
clear
B)
\[\text{Si}\text{F}\]
done
clear
C)
\[\text{P}\text{Cl}\]
done
clear
D)
\[O_{2}^{+}\]
done
clear
View Answer play_arrow
-
question_answer28) If\[O_{2}^{{}}\], then the angle between A and B is
A)
\[O_{2}^{+}\]
done
clear
B)
\[O_{2}^{{}}\]
done
clear
C)
\[O_{2}^{+}\]
done
clear
D)
\[O_{2}^{{}}\]
done
clear
View Answer play_arrow
-
question_answer29) The maximum distance upto which TV transmission from a TV tower of height A can be received is proportional to
A)
\[O_{2}^{+}\]
done
clear
B)
\[O_{2}^{{}}\]
done
clear
C)
\[\text{Xe}{{\text{F}}_{\text{2}}},\text{ Xe}{{\text{F}}_{\text{4}}}.\text{ Xe}{{\text{O}}_{\text{3}}}\]
done
clear
D)
\[\text{Xe}{{\text{F}}_{\text{2}}},\text{ Xe}{{\text{O}}_{\text{3}}},\text{Xe}{{\text{F}}_{6}}\text{ }\]
done
clear
View Answer play_arrow
-
question_answer30) The potential difference applied to an X-ray tube is 5 kV and the current through it is 3.2 mA. Then, the number of electrons striking the target per second is
A)
\[N{{H}_{3}},S{{O}_{2}},{{H}_{2}}O\]
done
clear
B)
\[\text{KMn}{{O}_{\text{4}}}\]
done
clear
C)
\[{{C}_{2}}O_{4}^{2-}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer31) The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4 eV. The stopping potential in volt is
A)
4
done
clear
B)
2
done
clear
C)
10
done
clear
D)
6
done
clear
View Answer play_arrow
-
question_answer32)
An equiconvex lens is cut into two halves along (i) POP9 and (ii) LOU as shown in figure. Let\[\text{KMn}{{O}_{\text{4}}}\]be the focal lengths of the complete lens of each half in case (i) of each half in case (ii) respectively. The correct statement from the following 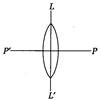
A)
\[\left( {{\text{H}}_{\text{2}}}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}-\text{2}{{\text{H}}_{\text{2}}}\text{O} \right)\]
done
clear
B)
\[-\text{ 3268 kJ mo}{{\text{l}}^{\text{-1}}}\]
done
clear
C)
\[-\text{3264}\,\text{kJmo}{{\text{r}}^{\text{-1}}}\]
done
clear
D)
\[-\text{ 326}.\text{4 kJ mo}{{\text{l}}^{\text{-1}}}\]
done
clear
View Answer play_arrow
-
question_answer33) For a transistor amplifier, the voltage gain
A)
remains constant for frequencies
done
clear
B)
is high at high and low frequencies and constant in the middle frequency range
done
clear
C)
is low at high and low frequencies
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer34) An astronomical telescope often fold angular magnification has a length of 44 cm. The focal length of the object is
A)
40 cm
done
clear
B)
4cm
done
clear
C)
440 cm
done
clear
D)
44 cm
done
clear
View Answer play_arrow
-
question_answer35) In a L-R circuit, \[-\text{ 32}.\text{64 kJ mo}{{\text{l}}^{\text{-1}}}\], and \[-\text{ 3264}0\text{ kJ mo}{{\text{l}}^{\text{-1}}}\]. Energy stored in the inductor is
A)
25J
done
clear
B)
50 J
done
clear
C)
\[{{H}_{2}}\]
done
clear
D)
75 J
done
clear
View Answer play_arrow
-
question_answer36) In a potentiometer experiment, the. Balancing with a cell is at length 240 cm on shutting the cell with a resistance of \[C{{l}_{2}}\], the balancing length becomes 120 cm. The internal resistance of the cell is
A)
\[\text{HCl}\]
done
clear
B)
\[\text{231 kJ mo}{{\text{l}}^{\text{-1}}}\]
done
clear
C)
\[\text{HCl}\]
done
clear
D)
\[\text{93 kJ mo}{{\text{l}}^{-\text{1}}}\]
done
clear
View Answer play_arrow
-
question_answer37) In the case of charged metallic sphere, potential (V) changes with respect to distance (r) from the centre as
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer38) Steam at\[100{}^\circ C\]is passed into 1.1 kg of water contained in a calorimeter of water equivalent 0.02 kg at\[15{}^\circ C,\]till the temperature of the calorimeter and its contents rises to\[80{}^\circ C\]. The mass of steam condensed (in kg) is
A)
0.195
done
clear
B)
0.065
done
clear
C)
0.130
done
clear
D)
0.260
done
clear
View Answer play_arrow
-
question_answer39) A rectangular vessel when full of water takes 10 min to be emptied through an orifice in its bottom. How much time will it take to be emptied when half filled with water?
A)
3 min
done
clear
B)
7 min
done
clear
C)
5 min
done
clear
D)
9 min
done
clear
View Answer play_arrow
-
question_answer40) If the earth is treated as a sphere of radius R and mass M having period of rotation T, then its angular momentum about its axis of rotation is
A)
\[-\text{ 245 kJ mo}{{\text{l}}^{-\text{1}}}\]
done
clear
B)
\[-\text{ 93 kJ mo}{{\text{l}}^{-\text{1}}}\]
done
clear
C)
\[\text{245 kJ mo}{{\text{l}}^{\text{-1}}}\]
done
clear
D)
\[2HI(g){{H}_{2}}(g)+{{I}_{2}}(g)\]
done
clear
View Answer play_arrow
-
question_answer41) The potential energy of 1 kg particle free to move along the x-axis is given by \[\text{1}.\text{4}\times \text{1}{{0}^{-\text{2}}}\]. The total mechanical energy of the particle is 2 J. Then, the maximum speed (in ms~1) is
A)
\[\text{n}=\text{4}\]
done
clear
B)
2
done
clear
C)
\[\text{n}=1\]
done
clear
D)
\[\text{H=2}.\text{18}\times \text{1}{{0}^{-\text{18}}}\text{J at}{{\text{m}}^{\text{-1}}}\]
done
clear
View Answer play_arrow
-
question_answer42) A man walks on a straight road from his home to the market 2.5 km away with a speed of 5 km/h. Finding the market closed, he instantly turns and walks back home with a speed of 7,5 km/h. The average speed of the man over the internal of time 0 to 40 min is
A)
\[h=6.625\times {{10}^{-34}}Js\]
done
clear
B)
\[\text{1}.\text{54}\times \text{1}{{0}^{\text{15}}}{{\text{s}}^{-\text{1}}}\]
done
clear
C)
\[\text{1}.0\text{3}\times \text{1}{{0}^{\text{15}}}\text{ J}{{\text{s}}^{-\text{1}}}\]
done
clear
D)
\[3.08\times \text{1}{{0}^{\text{15}}}{{\text{s}}^{-\text{1}}}\]
done
clear
View Answer play_arrow
-
question_answer43) The sky wave propagation is suitable for radio frequency
A)
from 2 MHz to 20 MHz
done
clear
B)
from 2 MHz to 30 MHz
done
clear
C)
from 2 MHz to 50 MHz
done
clear
D)
from 2 MHz to 8 MHz
done
clear
View Answer play_arrow
-
question_answer44) A particle of mass M at rest decays into two particles of masses\[\text{2}.0\times \text{1}{{0}^{\text{15}}}{{\text{s}}^{-\text{1}}}\], having non-zero velocities. The ratio of the de-Broglie wavelengths of the particles \[H{{e}^{+}}\] is
A)
\[{{n}_{2}}=3\xrightarrow{{}}{{n}_{1}}=1\]
done
clear
B)
\[{{n}_{2}}=4\xrightarrow{{}}{{n}_{1}}=1\]
done
clear
C)
\[{{n}_{2}}=2\xrightarrow{{}}{{n}_{2}}=1\]
done
clear
D)
1
done
clear
View Answer play_arrow
-
question_answer45) A choke coil has
A)
high resistance and low inductance
done
clear
B)
low resistance and high inductance
done
clear
C)
low resistance and low inductanpe
done
clear
D)
high resistance and high inductance
done
clear
View Answer play_arrow
-
question_answer46)
The current \[{{n}_{2}}=3\xrightarrow{{}}{{n}_{1}}=2\] drawn from the \[4p\] source will be 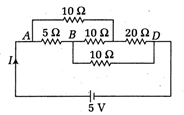
A)
0.67 A
done
clear
B)
0.5A
done
clear
C)
0.17 A
done
clear
D)
0.33 A
done
clear
View Answer play_arrow
-
question_answer47) A constant volume air thermometer works on
A)
Pascal's law
done
clear
B)
Gay Lussac's law
done
clear
C)
Arcihmedes' principle
done
clear
D)
Boyle's law
done
clear
View Answer play_arrow
-
question_answer48)
For the given uniform square lamina ABCD, with centre P 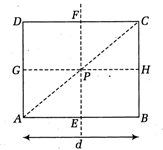
A)
\[4d\]
done
clear
B)
\[4f\]
done
clear
C)
\[\text{CsCl}\]
done
clear
D)
\[{{N}_{2}}{{O}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer49) A ball is projected vertically upwards with a certain initial speed. Another ball of the same mass is projected at an angle of 60 with the vertical with the same initial speed. At highest point of the journey, the ratio of their potential energies will be
A)
2:1
done
clear
B)
3:2
done
clear
C)
4:-1
done
clear
D)
1 :1
done
clear
View Answer play_arrow
-
question_answer50)
Identify the output of the following logic circuit. 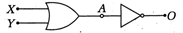
A)
NOR gate with output \[{{N}_{2}}{{O}_{4}}\]
done
clear
B)
NAND gate with output \[N{{O}_{2}}\]
done
clear
C)
NAND gate with output \[\text{IUPAC}\]
done
clear
D)
NAND gate with output \[C{{H}_{3}}-C\equiv CH\xrightarrow[1%HgS{{O}_{4}}]{40%{{H}_{2}}S{{O}_{4}}}A\xrightarrow{Isomerisation}\]
done
clear
View Answer play_arrow
-
question_answer51) In the relation\[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\],the dimensional formula for k is
A)
\[[{{M}^{0}}LT]\]
done
clear
B)
\[[{{M}^{0}}{{L}^{-1}}{{T}^{0}}]\]
done
clear
C)
\[[{{M}^{0}}{{L}^{-1}}{{T}^{-1}}]\]
done
clear
D)
\[[{{M}^{0}}L{{T}^{-1}}]\]
done
clear
View Answer play_arrow
-
question_answer52) The kinetic energy of a body varies directly as the time (t) elapse. The force acting varies directly as
A)
\[\overset{\bullet }{\mathop{OH}}\,\]
done
clear
B)
\[PC{{l}_{3}}(g)+C{{l}_{2}}(g)P{{C}_{5}}(g),\]
done
clear
C)
\[KI\]
done
clear
D)
\[{{C}_{6}}{{H}_{6}}+{{C}_{2}}{{H}_{5}}Cl\xrightarrow{AlC{{l}_{3}}}{{C}_{6}}{{H}_{5}}{{C}_{2}}{{H}_{5}}+HCl\]
done
clear
View Answer play_arrow
-
question_answer53) Which one is reverse biased diode?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer54) If there were a reduction in gravitational effect, which of the following forces do you think would change in some respect?
A)
Magnetic force
done
clear
B)
Electrostatic force
done
clear
C)
Viscous force
done
clear
D)
Archimedes' uplift
done
clear
View Answer play_arrow
-
question_answer55) If force (F), length (L) and time (T) be considered fundamental units, then units of mass will be
A)
\[{{C}_{2}}{{H}_{5}}OH+HCl\xrightarrow{ZnC{{l}_{2}}}{{C}_{2}}{{H}_{5}}Cl+{{H}_{2}}O\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}Cl+C{{H}_{3}}COCl\xrightarrow{AlC{{l}_{3}}}{{C}_{6}}{{H}_{5}}COCl+C{{l}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}Br+Mg\xrightarrow{Ether}{{C}_{5}}{{H}_{5}}MgBr\]
done
clear
D)
\[FeSi{{O}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer56) 400 mg of a capsule contains 100 mg of ferrous fumarate. The percentage of iron present in the capsule is approximately.
A)
8.2%
done
clear
B)
25%
done
clear
C)
16%
done
clear
D)
unpredictable
done
clear
View Answer play_arrow
-
question_answer57) The chemical reaction, \[MgSi{{O}_{3}}\], proceeds as follows: \[CaSi{{O}_{3}}\] \[N{{a}_{2}}C{{O}_{3}}\xrightarrow{s{{o}_{2}}}A\xrightarrow{Na{{ & }_{2}}C{{O}_{3}}}B\xrightarrow[\Delta ]{Elemental}\] The rate law expression should be
A)
\[C\xrightarrow{{{I}_{2}}}D\]
done
clear
B)
\[r={{k}^{1}}{{[{{O}_{3}}]}^{2}}{{[{{O}_{2}}]}^{-1}}\]
done
clear
C)
\[\text{N}{{\text{a}}_{\text{2}}}{{\text{S}}_{2}}{{\text{O}}_{3}}\]
done
clear
D)
unpredictable
done
clear
View Answer play_arrow
-
question_answer58) Represent the cell in which of the following reaction takes place? \[\text{N}{{\text{a}}_{\text{2}}}{{\text{S}}_{4}}{{\text{O}}_{6}}\] \[\text{NaHS}{{\text{O}}_{3}}\] Calculate,\[\text{Sr}\] if \[\text{Sr}\]
A)
1.6395V
done
clear
B)
0.6395V
done
clear
C)
0.06395V
done
clear
D)
-1.6395V
done
clear
View Answer play_arrow
-
question_answer59) \[\text{I}\] for a pair of liquids, it suggests that the liquid mixture
A)
is an ideal solution
done
clear
B)
shows positive deviation from Raoult's law
done
clear
C)
shows negative deviation from Raoult's law
done
clear
D)
release heat
done
clear
View Answer play_arrow
-
question_answer60) Heptane and octane form ideal solution at 37.3 K. The vapour pressure of them are 100 kPa and 50 kPa respectively. What will be total vapour pressure of a mixture containing 25g of heptane and 35 g of octane?
A)
150kPa
done
clear
B)
75 kPa
done
clear
C)
100.0kPa
done
clear
D)
72.5kPa
done
clear
View Answer play_arrow
-
question_answer61) When sulphur is heated at \[\text{C}{{\text{u}}^{\text{2}+}}\] is converted into \[\text{C}{{\text{d}}^{\text{2}+}}\]. What will be the equilibrium constant for the reaction if initial pressure of 1 atm falls by 25% at equilibrium?
A)
\[\left( \text{Z}=\text{23} \right)\]
done
clear
B)
\[HCl\]
done
clear
C)
\[\text{BeO}\]
done
clear
D)
\[\text{Ba}{{\text{O}}_{\text{2}}}\]
done
clear
View Answer play_arrow
-
question_answer62) Among the following, molecule with highest dipole moment is
A)
\[\text{MgO}\]
done
clear
B)
\[\text{CaO}\]
done
clear
C)
\[C{{H}_{3}}\,\,\overset{+}{\mathop{{{H}_{2}}}}\,\]
done
clear
D)
\[\text{C}{{\text{H}}_{\text{2}}}=\text{CH}\]
done
clear
View Answer play_arrow
-
question_answer63) Which one of the following bonds would be most polar?
A)
\[\text{CH}=\overset{+}{\mathop{\text{C}}}\,\]
done
clear
B)
\[S-O\]
done
clear
C)
\[{{\text{C}}_{6}}{{H}_{5}}C{{H}_{2}}\overset{\bullet \bullet }{\mathop{C{{H}_{2}}}}\,\]
done
clear
D)
\[{{\text{C}}_{6}}{{H}_{5}}\overset{\bullet \bullet }{\mathop{C{{H}_{2}}}}\,\]
done
clear
View Answer play_arrow
-
question_answer64) According to molecular orbital theory,
A)
\[{{(C{{H}_{3}})}_{2}}SiC{{l}_{2}}\] is paramagnetic and bond order is greater than that for \[{{(C{{H}_{3}})}_{4}}Si\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}SiCl\]is paramagnetic and bond order is lesser than that for\[C{{H}_{3}}SiC{{l}_{3}}\]
done
clear
C)
\[RN{{H}_{2}}+{{H}_{2}}OR-\overset{+}{\mathop{N{{H}_{3}}}}\,+O{{H}^{-}}\]is diamagnetic and bond order is lesser than that for \[K[{{H}_{2}}O]=\frac{[R-\overset{+}{\mathop{N{{H}_{3}}}}\,][O{{H}^{-}}]}{[RN{{H}_{2}}]}\]
done
clear
D)
\[{{K}_{b}}=\frac{[R-\overset{+}{\mathop{N{{H}_{3}}}}\,][O{{H}^{-}}]}{[R-N{{H}_{2}}]}\]is diamagnetic and bond order is more than that for \[p{{K}_{b}}=-\log {{K}_{b}}\]
done
clear
View Answer play_arrow
-
question_answer65) Which have distorted geometry based on VSEPR model?
A)
\[CaC{{l}_{2}}\]
done
clear
B)
\[XeO{{F}_{2}},Xe{{O}_{3}},Xe{{F}_{6}}\]
done
clear
C)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
-
question_answer66) \[C{{a}^{2+}}\]oxidises \[CaC{{O}_{3}}\] to \[CaO\] and each of the two molecules of \[NaCl\]gain 5 electrons during the process. The number of moles of \[KMn{{O}_{4}}\]required to oxidise 126 g of oxalic acid\[C{{O}_{2}}\] is
A)
0.2
done
clear
B)
0.4
done
clear
C)
0.6
done
clear
D)
1.0
done
clear
View Answer play_arrow
-
question_answer67) For the combustion of 1 mole of liquid benzene at 298K, the heat of reaction at constant pressure is \[{{H}_{2}}O\] What would be the heat of combustion of benzene at constant volume?
A)
\[C{{H}_{2}}\]
done
clear
B)
\[{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{4}}}\]
done
clear
C)
\[{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{3}}}\]
done
clear
D)
\[{{\text{C}}_{6}}{{\text{H}}_{8}}\]
done
clear
View Answer play_arrow
-
question_answer68) Bond dissociation enthalpy of \[5.0k\,mola{{l}^{-1}}\],\[Zn(s)+C{{u}^{2+}}(aq)\xrightarrow{{}}Z{{n}^{2+}}(aq)+Cu(s)\] and\[[E_{cell}^{o}=1.1\text{V}]\]are 406,242 and\[\text{2}\times \text{1}{{0}^{\text{32}}}\] respectively. Enthalpy of formation of\[\text{2}\times \text{1}{{0}^{\text{34}}}\]is
A)
\[\text{2}\times \text{1}{{0}^{\text{37}}}\]
done
clear
B)
\[\text{2}\times \text{1}{{0}^{\text{39}}}\]
done
clear
C)
\[LiCl,NaCl,KCl\]
done
clear
D)
\[\text{LiCl}>\text{NaCl}>\text{KCl}\]
done
clear
View Answer play_arrow
-
question_answer69) The equilibrium constant, K for the reaction \[\text{KCl}>\text{NaCl}>\text{LiCl}\]at room temperature is 2.85 and that at 698K is\[\text{NaCl}>\text{KCl}>\text{LiCl}\].This implies that
A)
\[HI\] is an exothermic compound
done
clear
B)
\[HI\]is very stable at room temperature
done
clear
C)
\[HI\]is relatively less stable than \[{{H}_{2}}\]and \[{{I}_{2}}\]
done
clear
D)
\[HI\] is resonance stabilized
done
clear
View Answer play_arrow
-
question_answer70) The frequency of the radiation emitted when the electron falls from \[\text{LiCl}>\text{KCl}>\text{NaCl}\]to \[{{C}_{2}}{{H}_{5}}OH\]in a hydrogen atom will be (Given ionization energy of \[C{{H}_{3}}OH\]and\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{COOH}\]
A)
\[{{\left( \text{C}{{\text{H}}_{\text{3}}} \right)}_{\text{2}}}\text{CHOH}\]
done
clear
B)
\[\int_{1}^{4}{{{\log }_{e}}[x]dx}\]
done
clear
C)
\[\text{lo}{{\text{g}}_{\text{e}}}\text{6}\]
done
clear
D)
\[\text{lo}{{\text{g}}_{\text{e}}}3\]
done
clear
View Answer play_arrow
-
question_answer71) What transition in the hydrogen spectrum would have the same wavelength as the Balmer transition from n = 4 to n = 2 of \[\text{lo}{{\text{g}}_{\text{e}}}2\]ion spectrum?
A)
\[a{{x}^{2}}+2hxy+b{{y}^{2}}=0\]
done
clear
B)
\[5{{h}^{2}}=ab\]
done
clear
C)
\[5{{h}^{2}}=9ab\]
done
clear
D)
\[9{{h}^{2}}=5ab\]
done
clear
View Answer play_arrow
-
question_answer72) The electron in which subshell experiences the largest effective nuclear charge in a multielectron atom?
A)
4s
done
clear
B)
\[{{h}^{2}}=ab\]
done
clear
C)
\[f(x)={{\sin }^{-1}}[2-4{{x}^{2}}]\]
done
clear
D)
\[[.]\]
done
clear
View Answer play_arrow
-
question_answer73) Langmuir adsorption isotherm is deduced using the assumption
A)
the adsorbed molecules interac with each other
done
clear
B)
the adsorption take place in multilayers
done
clear
C)
the adsorption sites are equivalent in their ability to adsorb the particle
done
clear
D)
the heat of adsorption varies with the coverage
done
clear
View Answer play_arrow
-
question_answer74) The number of \[\left[ -\frac{\sqrt{3}}{2},\frac{\sqrt{3}}{2} \right]\]formula units and the coordination number of each type of ion in it, respectively are
A)
4, 4
done
clear
B)
2, 8
done
clear
C)
1, 8
done
clear
D)
4, 8
done
clear
View Answer play_arrow
-
question_answer75) In a closed container, a certain amount of \[\left[ -\frac{\sqrt{3}}{2},0 \right]\] is maintained at\[0{}^\circ C\]. At\[273{}^\circ C,\] the \[\left[ -\frac{\sqrt{3}}{2},0 \right)\cup \left( 0,\frac{\sqrt{3}}{2} \right]\]is completely dissociated to \[\left[ -\frac{\sqrt{3}}{2},\infty \right)\] molecules. What will be its pressure as compared to initial pressure?
A)
Double
done
clear
B)
Three times
done
clear
C)
Four times
done
clear
D)
Same
done
clear
View Answer play_arrow
-
question_answer76) The \[f(x)=\left\{ \begin{matrix} \frac{1-\sqrt{2}\sin x}{\pi -4x}, & x\ne \frac{\pi }{4} \\ \frac{4a+1}{4}, & x=\frac{\pi }{4} \\ \end{matrix} \right.\]name of iso-octane is
A)
2, 2-dimethyl pentane
done
clear
B)
2, 3-dimethyl pentane
done
clear
C)
2, 3, 3-trimethyl pentane
done
clear
D)
2, 2, 4-trimethyl pentane
done
clear
View Answer play_arrow
-
question_answer77) Name the reaction which involves the conversion of benzaldehyde to cinnamic acid in the presence of acetic anhydride.
A)
Benzoin condensation
done
clear
B)
Reformatsky reaction
done
clear
C)
Knoevenagel reaction
done
clear
D)
Perkin's reaction
done
clear
View Answer play_arrow
-
question_answer78) \[x=\frac{\pi }{4}\] \[f(x)={{\cos }^{-1}}\left[ \frac{1-{{({{\log }_{e}}x)}^{2}}}{1+{{({{\log }_{e}}x)}^{2}}} \right],\] Structure of A and type of isomerism in the above reaction are respectively
A)
prop-1-en-2-ol, metamerism
done
clear
B)
prop-1 -en-1 -ol, tautomerism
done
clear
C)
prop-2-en-2-ol, geometrical isomerism
done
clear
D)
prop-1-en-2-ol, tautomerism
done
clear
View Answer play_arrow
-
question_answer79) Consider the following reactions, \[f'(e)\] The products X and V are respectively
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer80) Which of the following free radicals is responsible for causing break down of ozone into oxygen due to use of\[CFCs\]?
A)
\[\frac{-1}{e}\]
done
clear
B)
\[\frac{1}{e}\]
done
clear
C)
\[lx+my=1\]
done
clear
D)
\[{{x}^{2}}+{{y}^{2}}={{a}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer81) For the reaction, \[(1,m)\] the \[{{K}_{c}}\]value at\[250{}^\circ C\] is 26. The value of\[{{K}_{p}}\]at this temperature will be
A)
0.6
done
clear
B)
0.57
done
clear
C)
0.83
done
clear
D)
0.46
done
clear
View Answer play_arrow
-
question_answer82) Which one of these is not true for benzene?
A)
It forms only one type of mono substituted product
done
clear
B)
There are three carbon-carbon single bond and three carbon-carbon double bonds
done
clear
C)
The heat of hydrogenation of benzene is less than the theoretical value
done
clear
D)
The bond angle between the carbon-carbon bonds is 120°
done
clear
View Answer play_arrow
-
question_answer83) 10 g of bleaching powder on reaction with \[{{x}^{2}}+{{y}^{2}}={{a}^{-2}}\]required 100 mL of IN hypo. Thus, the percentage of pure bleaching powder in a given sample is
A)
100%
done
clear
B)
80%
done
clear
C)
63.5%
done
clear
D)
35.5%
done
clear
View Answer play_arrow
-
question_answer84) The example of Friedel-Craft's reaction is
A)
\[{{x}^{2}}+{{y}^{2}}={{a}^{2}}\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}={{a}^{-1}}\]
done
clear
C)
\[f:R\to A\]
done
clear
D)
\[f(x)=\frac{{{x}^{2}}}{{{x}^{2}}+1}\]
done
clear
View Answer play_arrow
-
question_answer85) The half-life period of a first order reaction is 6.93 min. The time required' for the completion of 99% of the chemical reaction will be
A)
230.3 min
done
clear
B)
23.03 min
done
clear
C)
46.06 min
done
clear
D)
4606.6 min
done
clear
View Answer play_arrow
-
question_answer86) In the extraction of iron, the slag formed is
A)
\[CO\]
done
clear
B)
\[R\]
done
clear
C)
\[\left[ 0,\text{1} \right]\]
done
clear
D)
\[\left( 0,\text{1} \right]\]
done
clear
View Answer play_arrow
-
question_answer87) Identify the product D in the following reaction sequence. \[\left[ 0,\text{1} \right)\]\[f(x)={{x}^{3}}-6{{x}^{2}}+ax+b\]
A)
\[c=\frac{2\sqrt{3}+1}{\sqrt{3}}\]
done
clear
B)
\[\text{a}=\text{11},\text{b}=\text{6}\]
done
clear
C)
\[a=-11,b\ne 6\]
done
clear
D)
\[a=11,b\in R\]
done
clear
View Answer play_arrow
-
question_answer88) The most electronegative element belongs to
A)
transition elements
done
clear
B)
nitrogen family
done
clear
C)
halogen group
done
clear
D)
chalcogens
done
clear
View Answer play_arrow
-
question_answer89) Of the metals\[Be,\text{ }Mg,\text{ }Ca\]and \[\sin x+\sin y=3(\cos y-\cos x),\]of group 2 in the periodic table, the least ionic chloride will be formed by
A)
Be
done
clear
B)
Ca
done
clear
C)
Mg
done
clear
D)
\[\frac{\sin 3x}{\sin 3y}\]
done
clear
View Answer play_arrow
-
question_answer90) Consider the following properties of the noble gases. \[\sqrt{8}\].They readily form compounds which are colourless. II. They generally do not form ionic compounds. II. They have variable oxidation states in their compounds. IV. Generally do not form covalent compounds. Select the correct properties.
A)
I, II, III
done
clear
B)
II, III
done
clear
C)
l, lll
done
clear
D)
Only I
done
clear
View Answer play_arrow
-
question_answer91) \[\text{\hat{j}}+\text{\hat{k}}\]and \[\text{\hat{i}+}\alpha \text{\hat{j}-\hat{k}}\]ion can be separated by their
A)
oxalate complexes
done
clear
B)
chelates
done
clear
C)
cyano complexes
done
clear
D)
sulphide formation
done
clear
View Answer play_arrow
-
question_answer92) A particular compound of vanadium shows a magnetic moment of 3.86 BM. The oxidation state of vanadium in the compound is \[\text{\hat{i}+\hat{j}}\]
A)
+ 3
done
clear
B)
0
done
clear
C)
+2
done
clear
D)
+4
done
clear
View Answer play_arrow
-
question_answer93) An amphoteric oxide dissolved in \[a=-\hat{i}+\hat{j}+2\hat{k},b=2\hat{i}-\hat{j}-\hat{k}\] to form a salt. The salt does not impart any colour to the Bunsen flame. The salt fumes in moist air. The oxide is
A)
\[\text{c}=-\text{2\hat{i}}+\text{\hat{j}}+\text{3\hat{k}}\]
done
clear
B)
\[\text{2a}-\text{c}\]
done
clear
C)
\[\text{a}+\text{b}\]
done
clear
D)
\[\frac{3\pi }{2}\]
done
clear
View Answer play_arrow
-
question_answer94) Which of the following carbocations is most stable?
A)
\[\frac{\pi }{2}\]
done
clear
B)
\[C{{H}_{2}}=\overset{+}{\mathop{C}}\,H\]
done
clear
C)
\[CH\equiv \overset{+}{\mathop{C}}\,\]
done
clear
D)
\[\text{5a}+\text{2b}\]
done
clear
View Answer play_arrow
-
question_answer95) Which of the following carbocations is resonance stabilised?
A)
done
clear
B)
done
clear
C)

\[\text{a}-\text{3b}\]
done
clear
D)
\[|a|=2\sqrt{2},|b|=3\]
done
clear
View Answer play_arrow
-
question_answer96) How many tripeptides can be prepared by linking the amino acids glycine, alanine and phenyl alanine?
A)
One
done
clear
B)
Three
done
clear
C)
Six
done
clear
D)
Twelve
done
clear
View Answer play_arrow
-
question_answer97) A straight chain polymer silicone is formed by the
A)
hydrolysis of\[\frac{\pi }{4}\]followed by condensation polymerization
done
clear
B)
hydrolysis of\[\sqrt{369}\]followed by addition polymerization
done
clear
C)
hydrolysis of\[\sqrt{593}\]followed by condensation polymerization
done
clear
D)
hydrolysis of\[\sqrt{113}\]followed by condensation polymerisation
done
clear
View Answer play_arrow
-
question_answer98) For the reaction,\[2x+y-7=0\]Select the correct expression(s) or formula (s) for the above reaction.
A)
\[\left( \frac{9}{5},\frac{17}{5} \right)\]
done
clear
B)
\[2x+y=1\]
done
clear
C)
\[3{{x}^{2}}+4yx-4x+1=0\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
-
question_answer99) A mixture of \[\frac{\pi }{2}\]and \[\frac{\pi }{3}\]of mass 4.44 g is treated with \[\frac{\pi }{4}\] solution to precipitate all \[\frac{\pi }{6}\] ions to \[\frac{{{x}^{2}}}{144}+\frac{{{y}^{2}}}{169}=1\]. It is heated strongly to get 0.56 g of \[\frac{{{x}^{2}}}{169}+\frac{{{y}^{2}}}{144}=1\]. The per cent of \[\frac{{{x}^{2}}}{12}+\frac{{{y}^{2}}}{13}=1\] in mixture (atomic mass Of\[Ca=40\])is
A)
75%
done
clear
B)
30.6%
done
clear
C)
25%
done
clear
D)
69.4%
done
clear
View Answer play_arrow
-
question_answer100) Which of the following is a heterocyclic alicyclic compound?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer101) Eclipsed and the staggered conformations can be represented as
A)
Sawhorse
done
clear
B)
Newmann projection
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer102) The\[p{{K}_{a}}\]f a weak acid is 4.0. What should be the [salt] to [acid] ratio, if we have to prepare a buffer with\[pH=5\]?
A)
\[10:1\]
done
clear
B)
\[1:10\]
done
clear
C)
\[4\text{ }:\text{ }5\]
done
clear
D)
\[5\text{ }:\text{ }4\]
done
clear
View Answer play_arrow
-
question_answer103) Complete combustion of a sample of a hydrocarbon gives 0.66g of \[{{x}^{2}}+{{y}^{2}}-2x+4y=0\] and 0.36 g of \[{{x}^{2}}+{{y}^{2}}-8x-6y=0\]. The empirical formula of compound is
A)
\[2{{x}^{2}}+2{{y}^{2}}-x-7y=0\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}-6x-10y=0\]
done
clear
C)
\[{{\left( px+\frac{1}{x} \right)}^{n}}\]
done
clear
D)
\[n\in N\]
done
clear
View Answer play_arrow
-
question_answer104) The presence of delocalised Ti-electrons in benzene indicates that it is
A)
less stable than cyclohexatriene
done
clear
B)
more stable than cyclohexatriene
done
clear
C)
more basic than cyclohexatriene
done
clear
D)
Both (b) and (c)
done
clear
View Answer play_arrow
-
question_answer105) Least stable conformer of cyclohexane is
A)
chair
done
clear
B)
boat
done
clear
C)
twist boat
done
clear
D)
planar hexagon
done
clear
View Answer play_arrow
-
question_answer106) 2.5g of benzoic acid dissolved in 25g of benzene shows a depression in freezing point of 2.25K. If \[{{K}_{f}}\]for benzene is \[\frac{5}{2}\] the per cent association of benzoic acid is
A)
90%
done
clear
B)
99%
done
clear
C)
99.9%
done
clear
D)
100%
done
clear
View Answer play_arrow
-
question_answer107) Calculate the equilibrium constant for the following reaction,\[{{y}^{2}}=x\] \[q=p\]
A)
\[q>p\]
done
clear
B)
\[q<p\]
done
clear
C)
\[pq=1\]
done
clear
D)
\[{{x}^{2}}=4ay\]
done
clear
View Answer play_arrow
-
question_answer108) Arrange \[{{m}_{PA}}\]in the decreasing order of their equivalent conductance.
A)
\[{{m}_{PB}}\]
done
clear
B)
\[m_{PA}^{2}+m_{_{PB}}^{2}=4\]
done
clear
C)
\[{{y}^{2}}=2x(x-a)\]
done
clear
D)
\[{{y}^{2}}=x(x-a)\]
done
clear
View Answer play_arrow
-
question_answer109) Name the product formed during the decarboxylation of malonic acid.
A)
Acetic acid
done
clear
B)
Ethanone
done
clear
C)
Propanone
done
clear
D)
Formic acid
done
clear
View Answer play_arrow
-
question_answer110) Which of the following alcohol is manufactured from the water gas?
A)
\[{{y}^{2}}=2x(2x-a)\]
done
clear
B)
\[{{y}^{2}}=2x(x-2a)\]
done
clear
C)
\[(x,y)\]
done
clear
D)
\[{{\tan }^{-1}}(2x+3y)\]
done
clear
View Answer play_arrow
-
question_answer111) \[6x+9y+2=26{{e}^{3(x-1)}}\] equals
A)
\[6x-9y+2=26{{e}^{3(x-1)}}\]
done
clear
B)
\[6x+9y-2=26{{e}^{3(x-1)}}\]
done
clear
C)
\[6x-9y-2=26{{e}^{3(x-1)}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer112) If the slope of one of the lines given by \[a=\hat{i}-k,b=x\hat{i}+\hat{j}+(1-x)\hat{k}\]is 5 times the other, then
A)
\[c=y\hat{i}-x\hat{j}+(1+x-y)\hat{k}\]
done
clear
B)
\[x\]
done
clear
C)
\[x\]
done
clear
D)
\[x\]
done
clear
View Answer play_arrow
-
question_answer113) The domain of definition of \[{{x}^{3}}-\left( \frac{9}{2}+\sqrt{5} \right){{x}^{2}}+\left( \frac{9\sqrt{5}}{2}+5 \right)x-5\sqrt{5}=0\], where \[{{x}^{3}}+\left( \frac{9}{2}+\sqrt{5} \right){{x}^{2}}-\left( \frac{9\sqrt{5}}{2}+5 \right)x-5\sqrt{5}=0\] denotes the greatest integer function, is
A)
\[{{x}^{3}}+\left( \frac{9}{2}-\sqrt{5} \right){{x}^{2}}+\left( \frac{9\sqrt{5}}{2}-5 \right)x+5\sqrt{5}=0\]
done
clear
B)
\[f\]
done
clear
C)
\[f(x)\]
done
clear
D)
\[f(1)=f(-1)\]
done
clear
View Answer play_arrow
-
question_answer114) If\[f'(a),f'(b)\] is continuous at \[f'(c)\], then a equals
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
-
question_answer115) If \[\text{a}=\text{lo}{{\text{g}}_{\text{24}}}\text{l2},\text{ b}=\text{lo}{{\text{g}}_{\text{36}}}\text{24}\]then \[\text{c }=\text{lo}{{\text{g}}_{\text{48}}}\text{36}\] is equal to
A)
\[e\]
done
clear
B)
\[-e\]
done
clear
C)
\[\text{2bc}-\text{1}\]
done
clear
D)
\[\text{2bc}+\text{1}\]
done
clear
View Answer play_arrow
-
question_answer116) If\[\text{bc}-\text{1}\] touches the circle \[\text{bc}+\text{1}\], then the point \[B\left( 5,\frac{1}{2} \right)\]lies on the circle
A)
\[B\left( 7,\frac{1}{2} \right)\]
done
clear
B)
\[\text{P}\left( \text{X}+\text{Y}=\text{3} \right)\]
done
clear
C)
\[\text{A}:\text{B}:\text{C}=\text{3}:\text{5}:\text{4}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer117) If the function \[\text{a}+\text{b}+\sqrt{2}c\] given by \[3b\]is a surjection, then A equals
A)
\[\angle A=\frac{\pi }{2},b=4\]
done
clear
B)
\[\text{c}=\text{3}\]
done
clear
C)
\[\frac{R}{r}\]
done
clear
D)
\[\frac{5}{2}\]
done
clear
View Answer play_arrow
-
question_answer118) If the function \[\frac{7}{2}\] defined on [1, 3] satisfies the Rolle's theorem for\[\frac{9}{2}\]then
A)
\[\frac{35}{24}\]
done
clear
B)
\[2{{\sin }^{3}}x+2{{\cos }^{3}}x-3\sin 2x+2=0\]
done
clear
C)
\[[0,4\pi ]\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer119) If\[3x+2y=26\], then the value or\[6x+y=31\]is
A)
1
done
clear
B)
\[-1\]
done
clear
C)
0
done
clear
D)
\[\pm \text{ }1\]
done
clear
View Answer play_arrow
-
question_answer120) If \[\frac{1}{2}\]is the magnitude of the moment about the point \[\frac{-1}{2}\]of a force \[\frac{1}{3}\]acting through the point \[\frac{-1}{3}\], then the value of a is
A)
\[\pm \text{ }1\]
done
clear
B)
\[\pm 2\]
done
clear
C)
\[\pm \text{ }3\]
done
clear
D)
\[\pm \text{ }4\]
done
clear
View Answer play_arrow
-
question_answer121) If \[y=f\left( \frac{2x+3}{3-2x} \right)\]and\[f(x)=\sin (\log x)\], then the angle between\[\frac{dy}{dx}\] and \[\frac{12}{9-4{{x}^{2}}}\cos \left\{ \log \left( \frac{2x+3}{3-2x} \right) \right\}\]is
A)
\[\frac{12}{9-4{{x}^{2}}}\cos \left\{ \log \left( \frac{2x+3}{2x-3} \right) \right\}\]
done
clear
B)
\[\frac{12}{4{{x}^{2}}-9}\cos \left\{ \log \left( \frac{2x+3}{3-2x} \right) \right\}\]
done
clear
C)
\[\frac{12}{9-4{{x}^{2}}}\cos \left\{ \log \left( \frac{3-2x}{2x+3} \right) \right\}\]
done
clear
D)
\[f(x)={{x}^{2}}{{e}^{-x}}\]
done
clear
View Answer play_arrow
-
question_answer122) The length of the longer diagonal of the parallelogram constructed on \[\int{\sqrt{\frac{x}{{{a}^{3}}-{{x}^{3}}}}}dx\] and\[\frac{2}{3}{{\cos }^{-1}}\left( \frac{{{x}^{2/3}}}{{{a}^{2/3}}} \right)+C\], if it is given that)\[\frac{2}{3}{{\tan }^{-1}}\left( \frac{{{x}^{2/3}}}{{{a}^{2/3}}} \right)+C\]and TT. angle between a and b is \[\frac{2}{3}{{\sin }^{-1}}\left( \frac{{{x}^{2/3}}}{{{a}^{2/3}}} \right)+C\], is
A)
\[\frac{2}{3}{{\sin }^{-1}}\left( \frac{{{x}^{3/2}}}{{{a}^{3/2}}} \right)+C\]
done
clear
B)
\[\int{\frac{dx}{({{x}^{2}}+1)({{x}^{2}}+4)}}=k{{\tan }^{-1}}x+1{{\tan }^{-1}}\tan \frac{x}{c}+C\]
done
clear
C)
\[k=\frac{1}{3},l=-\frac{1}{6}\]
done
clear
D)
15
done
clear
View Answer play_arrow
-
question_answer123) The coordinates of the foot of the perpendicular drawn from the point (3, 4) on the line \[k=\frac{2}{3},l=\frac{2}{3}\], is
A)
\[k=-\frac{1}{3},l=-\frac{1}{6}\]
done
clear
B)
\[(1,5)\]
done
clear
C)
\[(-5,1)\]
done
clear
D)
\[(1,-5)\]
done
clear
View Answer play_arrow
-
question_answer124) The angle between lines joining origin and intersection points of line \[k=-\frac{2}{3},l=-\frac{-2}{3}\] and curve \[a=2\hat{i}+\hat{j}-2\hat{k}\]is
A)
\[b=\hat{i}+\hat{j}\]
done
clear
B)
\[a.c=|c|,|c-a|=2\sqrt{2}\]
done
clear
C)
\[\text{a}\times \text{b}\]
done
clear
D)
\[|(a\times b)\times c|\]
done
clear
View Answer play_arrow
-
question_answer125) If vertices and foci of an ellipse are \[(0,\pm 13)\] and \[(0,\pm 5)\] respectively, then the equation of an ellipse is
A)
\[-\text{1}\le x\le \text{1}\]
done
clear
B)
\[f(x)=x\]
done
clear
C)
\[f(x)={{x}^{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer126) Equation of a circle through the origin and belonging to the coaxial system, of which the limiting points are (1, 2) and (4, 3), is
A)
\[f(x)=|x|\]
done
clear
B)
\[f(x)=2{{x}^{3}}+3\]
done
clear
C)
\[\frac{2\pi }{3}\]
done
clear
D)
\[\frac{\pi }{3}\]
done
clear
View Answer play_arrow
-
question_answer127) If the 4th term in the expansion of \[\pi \];\[\frac{\pi }{2}\], is\[\frac{13}{56}\] and three normals to the parabola\[\frac{3}{56}\]are drawn through a point (q,0), then
A)
\[\frac{1}{56}\]
done
clear
B)
\[\frac{14}{56}\]
done
clear
C)
\[\frac{91}{15}\]
done
clear
D)
\[(a-b){{x}^{2}}+(c-a)x+(b-c)=0\]
done
clear
View Answer play_arrow
-
question_answer128) Tangents PA and PB are drawn to \[A=\left[ \begin{matrix} 1 & 0 & -k \\ 2 & 1 & 3 \\ k & 0 & 1 \\ \end{matrix} \right]\]. If \[K=1\] and\[K=-1\]are the slopes of these tangents and\[|adj\,\text{A}|\], then locus of P is
A)
\[{{\text{n}}^{\text{2}}}\]
done
clear
B)
\[f(x)=\left| \begin{matrix} 0 & x-a & x-b \\ x+a & 0 & x-c \\ x+b & x+c & 0 \\ \end{matrix} \right|\]
done
clear
C)
\[f(a)=0\]
done
clear
D)
\[f(b)=0\]
done
clear
View Answer play_arrow
-
question_answer129) The equation of the curve whose tangent at any point \[f(0)=0\] makes an angle\[f(1)=0\]with X-axis and which passes through (1, 2), is
A)
\[^{47}{{C}_{4}}+\sum\limits_{r=1}^{5}{^{52-r}}{{C}_{3}}\]
done
clear
B)
\[^{47}{{C}_{6}}\]
done
clear
C)
\[^{52}{{C}_{4}}\]
done
clear
D)
\[^{52}{{C}_{5}}\]
done
clear
View Answer play_arrow
-
question_answer130) Let\[{{[({{t}^{-1}}-1)x+{{({{t}^{-1}}-1)}^{-1}}{{x}^{-1}}]}^{8}}\]and\[70{{\left( \frac{1+t}{1-t} \right)}^{4}}\]. Then [a b c] depends on
A)
only y
done
clear
B)
only \[70{{\left( \frac{1-t}{1+t} \right)}^{4}}\]
done
clear
C)
both \[56{{\left( \frac{1+t}{1-t} \right)}^{3}}\] and y
done
clear
D)
neither \[56{{\left( \frac{1-t}{1+t} \right)}^{3}}\] nor y
done
clear
View Answer play_arrow
-
question_answer131) If 3 arithmetic means, 3 geometric means and 3 harmonic means are inserted between and 5, then the cubic equation whose roots are first AM, second GM and third HM between 1 and 5, is
A)
\[f\]
done
clear
B)
\[{{f}^{-1}}\]
done
clear
C)
\[f(x)=\left\{ \begin{matrix} 2x & x<0 \\ 2x+1 & x\ge 0 \\ \end{matrix}, \right.\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer132) If \[f(x)\] is a periodic function and g is a non-periodic function, then
A)
fog is always periodic
done
clear
B)
gof is never periodic
done
clear
C)
gof is always periodic
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer133) Let\[x=0\]be a polynomial of second degree. If\[f(x)\]and a, b, c are in AP, then\[x=0\]and\[f(|x|)\]Mare in
A)
AGP
done
clear
B)
AP
done
clear
C)
GP
done
clear
D)
HP
done
clear
View Answer play_arrow
-
question_answer134) If \[x=0\]and\[\underset{x\to 1}{\mathop{\lim }}\,\frac{\tan ({{x}^{2}}-1)}{x-1}\], then abc equals
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{-1}{2}\]
done
clear
C)
\[y={{x}^{3}}-3{{x}^{2}}-9x+5\]
done
clear
D)
\[(1,2,\sqrt{3})\]
done
clear
View Answer play_arrow
-
question_answer135) If X and Y are independent binomial variates of\[\frac{{{x}^{2}}}{{{a}^{2}}}+\frac{{{y}^{2}}}{{{b}^{2}}}=1\]and\[y=x\]then \[\frac{{{x}^{2}}}{3}+\frac{{{y}^{2}}}{2}=1\] equals
A)
\[\frac{35}{47}\]
done
clear
B)
\[\frac{55}{1024}\]
done
clear
C)
\[\frac{220}{512}\]
done
clear
D)
\[\frac{11}{204}\]
done
clear
View Answer play_arrow
-
question_answer136) In \[\Delta ABC\],if\[x-y+1=0\],then \[x-y+2=0\]equals
A)
\[2b\]
done
clear
B)
\[2c\]
done
clear
C)
\[3a\]
done
clear
D)
\[x+y-1=0\]
done
clear
View Answer play_arrow
-
question_answer137) In MBC, if\[x+y-2=0\]and\[{{x}^{2}}+{{y}^{2}}-3x-6y+14=0\], then the value of \[{{x}^{2}}+{{y}^{2}}-x-4y+8=0\]is
A)
\[{{x}^{2}}+{{y}^{2}}+2x-6y+9=0\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}-2x-4y+1=0\]
done
clear
C)
\[{{x}^{2}}+{{y}^{2}}+2x+4x+1=0\]
done
clear
D)
\[{{x}^{2}}+{{y}^{2}}-2x+4y+1=0\]
done
clear
View Answer play_arrow
-
question_answer138) The number of solutions of the equation\[{{x}^{2}}+{{y}^{2}}-2x-4y-1=0\]in \[{{x}^{2}}+2{{y}^{2}}=6\],is
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
(d) 5
done
clear
View Answer play_arrow
-
question_answer139) The two lines of regression are given by \[{{x}^{2}}+4{{y}^{2}}=4\]and \[{{x}^{2}}+{{y}^{2}}=4\]. The coefficient of correlation between x and y is
A)
\[{{x}^{2}}+{{y}^{2}}=6\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}=9\]
done
clear
C)
\[({{x}^{2}}+1)y'+xy=0\]
done
clear
D)
\[zy'+({{x}^{2}}+1)y=0\]
done
clear
View Answer play_arrow
-
question_answer140) If \[({{x}^{2}}-1)y'-xy=0\]and \[({{x}^{2}}-1)y''+(x-1)y'=0\], then \[2\{{{(x-a)}^{2}}+{{(y-a)}^{2}}\}={{(x+y)}^{2}}\]is equal to
A)
\[2\sqrt{2}a\]
done
clear
B)
\[\sqrt{2}a\]
done
clear
C)
\[\text{si}{{\text{n}}^{\text{2}}}\beta =\text{ 3si}{{\text{n}}^{\text{2}}}\theta \]
done
clear
D)
\[\text{co}{{\text{s}}^{\text{2}}}\theta \]
done
clear
View Answer play_arrow
-
question_answer141) The function\[\frac{2}{3}\]increases in the interval
A)
(4. 5)
done
clear
B)
(0,2)
done
clear
C)
(2.3)
done
clear
D)
(3, 4)
done
clear
View Answer play_arrow
-
question_answer142) \[\frac{3}{5}\]is equal to
A)
\[\frac{1}{5}\]
done
clear
B)
\[\frac{2}{5}\]
done
clear
C)
\[\text{O}\left( 0,0,0 \right)\]
done
clear
D)
\[\text{A}\left( \text{1},\text{2},\text{1} \right),\text{B}\left( \text{2},\text{1},\text{3} \right)\]
done
clear
View Answer play_arrow
-
question_answer143) If\[\text{C}\left( -\text{1},\text{1},\text{2} \right)\]then
A)
\[{{\cos }^{-1}}\left( \frac{19}{35} \right)\]
done
clear
B)
\[{{\cos }^{-1}}\left( \frac{17}{31} \right)\]
done
clear
C)
\[100\pi c{{m}^{3}}/\min \]
done
clear
D)
\[{{T}^{2}}\propto {{R}^{3}}\Rightarrow \frac{{{T}_{2}}}{{{T}_{1}}}={{\left( \frac{{{R}_{2}}}{{{R}_{1}}} \right)}^{3/2}}={{\left( \frac{3R}{R} \right)}^{3/2}}\]
done
clear
View Answer play_arrow
-
question_answer144) Let\[\frac{{{T}_{2}}}{{{T}_{1}}}=\sqrt{27}\]and \[{{T}_{2}}=\sqrt{27}\,\,{{T}_{1}}\]. If c is a vector such that\[=\sqrt{27}\,\times 4=4\sqrt{27}h\]and the angle between \[\gamma =\frac{\Delta V}{V\Delta T}\]and c is\[30{}^\circ \], then\[=\frac{0.12}{100}\times \frac{1}{20}=6\times {{10}^{-5}}{{/}^{o}}c\]is equal to
A)
\[\frac{3}{2}\]
done
clear
B)
\[\frac{2}{3}\]
done
clear
C)
3
done
clear
D)
2
done
clear
View Answer play_arrow
-
question_answer145) The Rolle's theorem is applicable in the interval \[\alpha =\frac{\gamma }{3}=\frac{6\times {{10}^{-5}}}{3}=2\times {{10}^{-5}}{{/}^{o}}c\] for the function
A)
\[I=4{{I}_{0}}{{\cos }^{2}}(\phi /2)\]
done
clear
B)
\[\Rightarrow \]
done
clear
C)
\[\phi =2\pi /3\]
done
clear
D)
\[\Delta x\times (2\pi /\lambda )=2\pi /3\]
done
clear
View Answer play_arrow
-
question_answer146) The angle between two forces each equal to P when their resultant is also equal to P, is
A)
\[\Delta x=\lambda /3\]
done
clear
B)
\[\sin \theta =\frac{\Delta x}{d}\]
done
clear
C)
\[\Rightarrow \]
done
clear
D)
\[\sin \theta =\frac{\lambda }{3d}\]
done
clear
View Answer play_arrow
-
question_answer147) The chances to fail in Physics are 20% and the chances to fail in Mathematics are 10%. What are the chances to fail in atleast one subject?
A)
82%
done
clear
B)
38%
done
clear
C)
28%
done
clear
D)
72%
done
clear
View Answer play_arrow
-
question_answer148) A bag contains 3 black, 3 white and 2 red balls. Three balls are drawn without replacement one-by-one. The probability that the third ball is red, is
A)
\[\theta ={{\sin }^{-1}}\left[ \frac{\lambda }{3d} \right]\]
done
clear
B)
\[V=\frac{Q}{C}=Q.\frac{d}{{{\varepsilon }_{0}}A}\]
done
clear
C)
\[\eta =1-\frac{{{T}_{2}}}{{{T}_{1}}}=1-\frac{500}{800}\]
done
clear
D)
\[=\frac{3}{8}=0.375\]
done
clear
View Answer play_arrow
-
question_answer149) If runs of two players A and B in 10 cricket matches are such that player A has mean 50 and variance 36 and player B has mean 60 and variance 81 of runs, then the player more consistent in runs is
A)
A
done
clear
B)
B
done
clear
C)
both are equally consistent
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer150) The standard deviation of 15 items is 6 and if each item is decreased by 1, then standard deviation will be
A)
5
done
clear
B)
7
done
clear
C)
6
done
clear
D)
\[p=\rho hg\]
done
clear
View Answer play_arrow
-
question_answer151) A flagstaff is upon the top of a building. If at a distance of 40 m from the base of building, the angles of elevation of the top of the flagstaff and building' are 60° and 30° respectively, then the height of the flagstaff is
A)
50m
done
clear
B)
25m
done
clear
C)
46.19 m
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer152) If roots of the equation\[a=\frac{\text{Net pushing force}}{\text{Total mass}}\]are equal, then a, b and c are in
A)
AP
done
clear
B)
GP
done
clear
C)
HP
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer153) Matrix \[a=\frac{F-({{m}_{1}}+{{m}_{2}}+{{m}_{3}})g\,\sin \theta }{({{m}_{1}}+{{m}_{2}}+{{m}_{3}})}\]is invertible for
A)
\[{{m}_{3}}\]
done
clear
B)
\[N-{{m}_{3}}\,\,g\sin \theta ={{m}_{3}}a\]
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer154) If A is a skew-symmetric matrix of odd order, then \[N={{m}_{3}}\,g\sin \theta \]is equal to
A)
0
done
clear
B)
n
done
clear
C)
\[+{{m}_{3}}\left[ \frac{F-({{m}_{1}}+{{m}_{2}}+{{m}_{3}})g\,\sin \theta }{({{m}_{1}}+{{m}_{2}}+{{m}_{3}})} \right]\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer155) If\[=\frac{{{m}_{3}}\,\,\,F}{{{m}_{1}}+{{m}_{2}}+{{m}_{3}}}\], then
A)
\[{{A}_{i}}=-\frac{{{h}_{fe}}}{1+{{h}_{oe}}{{R}_{L}}}\]
done
clear
B)
\[{{h}_{fe}}=50,{{h}_{oe}}=25\mu A{{V}^{-1}}\]
done
clear
C)
\[=25\times {{10}^{-6}}A{{V}^{-1}}\]
done
clear
D)
\[{{R}_{L}}=1k\Omega ={{10}^{3}}\Omega \]
done
clear
View Answer play_arrow
-
question_answer156) The value of\[{{A}_{i}}=\frac{-50}{1+25\times {{10}^{-6}}\times {{10}^{3}}}\]is
A)
\[=-48.78\]
done
clear
B)
\[G=\frac{\text{Current sensitivity}}{\text{Voltage sensitivity}}\]
done
clear
C)
\[=\frac{10}{2}=5\Omega \]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer157) The term independent of x in the expansion of \[n=150\]is
A)
\[{{I}_{g}}=\frac{n}{\text{Current sensitivity}}=\frac{150}{10}\]
done
clear
B)
\[=15mA=15\times {{10}^{-3}}A\]
done
clear
C)
\[=\text{15}0\times \text{1}=\text{15}0\text{V}\]
done
clear
D)
\[R=\frac{V}{{{I}_{g}}}-G=\frac{150}{15\times {{10}^{-3}}}-5=9995\Omega \]
done
clear
View Answer play_arrow
-
question_answer158) If\[W=qV=4\times 4\times {{10}^{6}}\]is decreasing odd function, then \[=\text{ 16}\times \text{1}{{0}^{\text{6}}}\text{J}\] is
A)
odd and decreasing
done
clear
B)
even and decreasing
done
clear
C)
odd and increasing
done
clear
D)
even and increasing
done
clear
View Answer play_arrow
-
question_answer159) If\[P=\frac{W}{t}=\frac{16\times {{10}^{6}}J}{100\times {{10}^{-3}}s}\]then
A)
\[=160\times {{10}^{5}}W\] is discontinuous at \[=160MW\]
done
clear
B)
\[=\text{36}0\,\text{rpm}\]is continuous at \[=\frac{\text{36}0}{60}\,\text{rps}\]
done
clear
C)
\[=\text{ 6}0\text{ rps}\] is continuous at \[=\text{ 6}\times \text{number of holes}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer160) \[=\text{6}0\times \text{6}0\]is equal to
A)
\[-2\]
done
clear
B)
2
done
clear
C)
\[=\text{36}0\text{ Hz}\]
done
clear
D)
\[\tau =r\times F\]
done
clear
View Answer play_arrow
-
question_answer161) The abscissae of the points, where the E tangent to curve \[\tau =(\hat{i}-\hat{j})\times (-F\hat{k})\]is parallel to X-axis, are
A)
\[x=1\]and -1
done
clear
B)
\[x=1\] and 3
done
clear
C)
\[x=1\] and -3
done
clear
D)
\[x=0\]and 0
done
clear
View Answer play_arrow
-
question_answer162) If tangents drawn to the ellipse through point \[=F[(-\hat{i}\times \hat{k})+(\hat{j}\times \hat{k})]\]to the ellipse \[=F[\hat{j}+\hat{i}]=F[\hat{i}+\hat{j}]\]are at right angled, then value of b is
A)
1
done
clear
B)
4
done
clear
C)
2
done
clear
D)
None of these
done
clear
View Answer play_arrow
-
question_answer163) The equation of a tangent parallel to \[{{T}_{x}}=\]drawn to \[(L-x)\], is
A)
(a) \[=\frac{M}{k}(L-x)g\]
done
clear
B)
\[{{T}_{x}}=\frac{Mg(L-x)}{L}\]
done
clear
C)
\[\left( \frac{N}{{{N}_{0}}} \right)={{\left( \frac{1}{2} \right)}^{n}}\]
done
clear
D)
\[\frac{1}{16}={{\left( \frac{1}{2} \right)}^{n}}\]
done
clear
View Answer play_arrow
-
question_answer164) The equation of a circle which cuts the three circles \[{{\left( \frac{1}{2} \right)}^{4}}={{\left( \frac{1}{2} \right)}^{n}}\]\[n=4\]and\[t=n\times half-life\]orthogonally, is
A)
\[=4\times 100\mu s=400\mu s\]
done
clear
B)
\[=\frac{1}{2}\times Y\times {{(\text{Strain})}^{2}}\]
done
clear
C)
\[=\frac{1}{2}Y\times {{\left( \frac{l}{L} \right)}^{2}}\]
done
clear
D)
\[l=L\times 2%\]
done
clear
View Answer play_arrow
-
question_answer165) The locus of the points of intersection of the tangents at the extremities of the chords of the ellipse\[l=\frac{L}{200}\times stored\,\,energy\]which touches the ellipse \[Y=\frac{1}{2}\times 3\times {{10}^{10}}\times {{\left( \frac{L}{200L} \right)}^{2}}\], is
A)
\[Y=\frac{1}{2}\times 3\times {{10}^{10}}\times \frac{1}{4\times {{10}^{4}}}=\frac{3}{8}\times {{10}^{6}}\]
done
clear
B)
\[=0.375\times {{10}^{6}}\]
done
clear
C)
\[=3.75\times {{10}^{5}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
-
question_answer166) The differential equation of all ellipses with centres at the origin and the ends of one axis of symmetry is at (± 1,0), is
A)
\[c=\frac{1}{\sqrt{{{\mu }_{0}}{{\varepsilon }_{0}}}}\]
done
clear
B)
\[I\propto \frac{1}{{{\lambda }^{4}}}\]
done
clear
C)
\[\frac{{{\lambda }_{1}}}{{{\lambda }_{2}}}={{\left[ \frac{{{I}_{2}}}{{{I}_{1}}} \right]}^{\frac{1}{4}}}={{\left( \frac{4}{1} \right)}^{\frac{1}{4}}}=\sqrt{2}:1\]
done
clear
D)
\[K=\frac{M}{\sqrt{4{{L}_{2}}}}\]
done
clear
View Answer play_arrow
-
question_answer167) The length of the latusrectum of the parabola\[A,{{B}_{A}}=\frac{{{\mu }_{0}}I}{2R}\],is
A)
2a
done
clear
B)
\[B,{{B}_{B}}=\frac{{{\mu }_{0}}2I}{2\times 2R}\]
done
clear
C)
4a
done
clear
D)
\[\frac{{{B}_{A}}}{{{B}_{B}}}=1\]
done
clear
View Answer play_arrow
-
question_answer168) A line makes the same angle 0, with each of \[X\]and Z-axes. If the angle\[\beta \], which it makes with y-axis, is such that\[=\frac{1}{2}C{{V}^{2}}=\frac{1}{2}\times 10\times {{10}^{-6}}\times {{(500)}^{2}}\], then \[=1.25\text{J}\]equals
A)
\[\text{p}{{\text{T}}^{\text{2}}}=\text{constant}\]
done
clear
B)
\[\left[ \frac{nRT}{V} \right]{{T}^{2}}=\text{constant}\]
done
clear
C)
\[{{T}^{3}}{{V}^{-1}}=\text{constant}\]
done
clear
D)
\[\frac{3{{T}^{2}}}{V}dT-\frac{{{T}^{3}}}{{{V}^{2}}}dV=0\]
done
clear
View Answer play_arrow
-
question_answer169) If a tetrahedron has vertices at\[3dT=\frac{T}{V}dV\], \[dV=V\gamma dT\]and\[\gamma \text{=coefficient of volume expansion of gas=}\frac{dV}{VdT}\]. Then, the angle between the faces OAB and ABC will be
A)
\[90{}^\circ \]
done
clear
B)
\[\gamma \text{=}\frac{dV}{VdT}=\frac{3}{T}\]
done
clear
C)
\[Q=\frac{V}{t'}=\frac{\pi p{{r}^{4}}}{8\eta l}\]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
-
question_answer170) A spherical, iron ball of radius 10 cm, coated with a layer of ice of uniform thickness, melts at the rate of\[T=3mg\]. The rate at which the thickness of decreases when the thickness of ice is 5 .cm, is
A)
1 cm/min
done
clear
B)
2 cm/min
done
clear
C)
5 cm/min
done
clear
D)
3 cm/min
done
clear
View Answer play_arrow