A) diethyl ether
B) phenol
C) ethanol
D) aniline
Correct Answer: B
Solution :
\[\underset{diethyl\,\,ether}{\mathop{{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}}}\,+C{{H}_{3}}\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,Cl\xrightarrow{ZnC{{l}_{2}}}\]\[{{C}_{2}}{{H}_{5}}Cl+C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}\]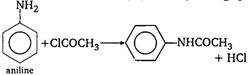 \[C{{H}_{3}}CO\underset{ethanol}{\mathop{O{{C}_{2}}{{H}_{5}}}}\,\xrightarrow{{}}\]\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+HCl\] Acetyl chloride does not react direct with phenol. Firstly phenol reacts with alkali to form sodium phenoxide which reacts with acetyl chloride to give phenyl acetate.
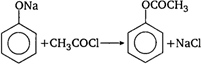
\[C{{H}_{3}}CO\underset{ethanol}{\mathop{O{{C}_{2}}{{H}_{5}}}}\,\xrightarrow{{}}\]\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+HCl\] Acetyl chloride does not react direct with phenol. Firstly phenol reacts with alkali to form sodium phenoxide which reacts with acetyl chloride to give phenyl acetate. 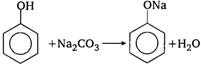
You need to login to perform this action.
You will be redirected in
3 sec
