A) colourless and diamagnetic
B) coloured and octahedral
C) colourless and paramagnetic
D) coloured and paramagnetic
Correct Answer: A
Solution :
In\[{{[Sc{{({{H}_{2}}O)}_{6}}]}^{3+}}\],oxidation state of\[Sc\]is\[+3\].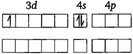 \[\because \]\[S{{c}^{3+}}\]has no unpaired electrons. \[\therefore \]\[{{[Sc{{({{H}_{2}}O)}_{6}}]}^{3+}}\]is diamagnetic and-colourless,
\[\because \]\[S{{c}^{3+}}\]has no unpaired electrons. \[\therefore \]\[{{[Sc{{({{H}_{2}}O)}_{6}}]}^{3+}}\]is diamagnetic and-colourless,
You need to login to perform this action.
You will be redirected in
3 sec
