A) 3-methl-2-butanol
B) 2-methyl-2-butanol
C) 2, 2-dimethyl-l-propanol
D) 2 methyl-1-butanol
Correct Answer: B
Solution :
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{C}}\,H-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}\xrightarrow[-HBr]{{{H}^{+}}}\]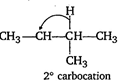 \[\xrightarrow{{{H}^{-}}\text{Rearrangement}}\underset{3{}^\circ carbocation(more\,\,stable)}{\mathop{C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{+}{\mathop{C}}}\,-C{{H}_{3}}}}\,\] \[\xrightarrow{O{{H}^{-}}}\underset{\text{2-methyl-2butanol}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}}}\,\]
\[\xrightarrow{{{H}^{-}}\text{Rearrangement}}\underset{3{}^\circ carbocation(more\,\,stable)}{\mathop{C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{+}{\mathop{C}}}\,-C{{H}_{3}}}}\,\] \[\xrightarrow{O{{H}^{-}}}\underset{\text{2-methyl-2butanol}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
