A) \[HCl{{O}_{3}}\]and \[C{{l}_{2}}O\]
B) \[HCl{{O}_{2}}\]and\[HCl{{O}_{4}}\]
C) \[HCl\]and \[C{{l}_{2}}O\]
D) \[HCl\]and \[HCl{{O}_{3}}\]
Correct Answer: D
Solution :
In disproportionation reactions, the same element is oxidised as well as reduced. In \[\text{HOCl,}\]the oxidation state of \[\text{Cl}\] is \[+\,1.\] In \[\text{HCl}{{\text{O}}_{\text{3}}}\text{,C}{{\text{l}}_{\text{2}}}\text{O,}\,\text{HCl}{{\text{O}}_{\text{2}}}\text{,}\,\text{HCl}{{\text{O}}_{\text{4}}}\]and \[\text{HCl,}\]the oxidation states of Cl are \[+\,5,+\,1,+\,3,+\,7\]and\[-1\] respectively. Thus, the disproportionation products are \[\text{HCl}\] and \[\text{HCl}{{\text{O}}_{3}}.\]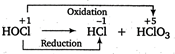
You need to login to perform this action.
You will be redirected in
3 sec
