question_answer 1) The direction of vector \[\overset{\to }{\mathop{\text{A}}}\,\] is reversed. What are the values \[\Delta \overset{\to }{\mathop{\text{A}}}\,\] and \[\Delta |\overset{\to }{\mathop{\text{A}}}\,|\] ?
A)
\[+\,2\vec{A},0\]
done
clear
B)
\[+\,\vec{A},0\]
done
clear
C)
\[-\,\vec{A},0\]
done
clear
D)
\[-2\,\vec{A},0\]
done
clear
View Answer play_arrow
question_answer 2) The velocity of a body moving with uniform acceleration at a given instant of time t is 10 m/s. After 5 s its velocity is 20 m/s. Distance travelled in that time is:
A)
300m
done
clear
B)
400m
done
clear
C)
150m
done
clear
D)
75m
done
clear
View Answer play_arrow
question_answer 3) A stone falls freely such that the distance covered by It in the last second of its motion is equal to the distance covered by it in the first 5 s. It is in air for:
A)
26s
done
clear
B)
25s
done
clear
C)
13s
done
clear
D)
12s
done
clear
View Answer play_arrow
question_answer 4) A child is swinging a swing. Minimum and maximum heights of swing from earths surface are 0.75 m and 2m respectively. The maximum velocity of this swing is:
A)
5 m/s
done
clear
B)
10 m/s
done
clear
C)
15 m/s
done
clear
D)
20 m/s
done
clear
View Answer play_arrow
question_answer 5) An object is thrown along a direction making an angle \[{{45}^{o}}\] with the horizontal direction. The horizontal range of the object is equal to:
A)
twice the vertical height
done
clear
B)
vertical height
done
clear
C)
four times the vertical height
done
clear
D)
three times the vertical height
done
clear
View Answer play_arrow
question_answer 6) A fireman wants to slide down a rope. The breaking load for the rope is\[\frac{\text{3}}{\text{4}}\text{th}\] of the weight of the man. With what acceleration should the fireman slide down? (Acceleration due to gravity is g)
A)
\[\frac{g}{2}\]
done
clear
B)
\[\frac{g}{4}\]
done
clear
C)
\[\frac{3g}{4}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 7) A body of mass 1 kg is rotating in a vertical circle of radius 1m. What will be (he difference in kinetic energy at the top and at the bottom of the circle? (Take\[g=10\,m/{{s}^{2}}\])
A)
50 J
done
clear
B)
30 J
done
clear
C)
20 J
done
clear
D)
10 J
done
clear
View Answer play_arrow
question_answer 8) A circular ring of mass m and radius r is rolling on a smooth horizontal surface with speed v. Its kinetic energy is:
A)
\[\frac{1}{8}m{{v}^{2}}\]
done
clear
B)
\[\frac{1}{4}m{{v}^{2}}\]
done
clear
C)
\[\frac{1}{3}m{{v}^{2}}\]
done
clear
D)
\[m{{v}^{2}}\]
done
clear
View Answer play_arrow
question_answer 9) A satellite is revolving around the earth in a circular orbit of radius 4 times that of the parking orbit. The time period of the .satellite
A)
16 days
done
clear
B)
2 days
done
clear
C)
4 days
done
clear
D)
8 days
done
clear
View Answer play_arrow
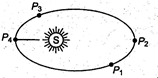
question_answer 10)
As shown in figure, a planet revolves in elliptical orbit around the sun. Where is KE of the planet maximum?
A)
\[At\,{{P}_{4}}\]
done
clear
B)
\[At\,{{P}_{1}}\]
done
clear
C)
\[At\,{{P}_{2}}\]
done
clear
D)
\[At\,{{P}_{3}}\]
done
clear
View Answer play_arrow
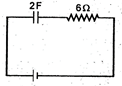
question_answer 11)
If the condenser shown in the circuit is charged to 5 V and left in the circuit, in 12 s the charge on the condenser will become:
A)
\[\frac{10}{e}\text{coulomb}\]
done
clear
B)
\[\frac{{{e}^{2}}}{10}\text{coulomb}\]
done
clear
C)
\[\frac{10}{{{e}^{2}}}\text{coulomb}\]
done
clear
D)
\[\frac{e}{10}\text{coulomb}\]
done
clear
View Answer play_arrow
question_answer 12) A sings with a frequency \[(n)\]and B sings with a frequency 1/8 that of A. If the energy remains the same and the amplitude of A is a, the amplitude of B will be:
A)
2a
done
clear
B)
8 a
done
clear
C)
4a
done
clear
D)
a
done
clear
View Answer play_arrow
question_answer 13) Reactance of a capacitor of capacitance \[C\,\mu F\]for AC frequency \[\frac{\text{400}}{\text{ }\!\!\pi\!\!\text{ }}\text{Hz}\]is \[25\,\Omega ,\] the value of C is:
A)
\[75\,\mu F\]
done
clear
B)
\[100\,\mu F\]
done
clear
C)
\[25\,\mu F\]
done
clear
D)
\[50\,\mu F\]
done
clear
View Answer play_arrow
question_answer 14) An electron and a proton have same de-Broglie wavelength, then kinetic energy of the electron is:
A)
greater than KE of proton
done
clear
B)
zero
done
clear
C)
equal to KE of proton
done
clear
D)
infinite
done
clear
View Answer play_arrow
question_answer 15) Relative permeability of iron is 5500. Its magnetic susceptibility is:
A)
5499
done
clear
B)
\[5500\times {{10}^{7}}\]
done
clear
C)
\[5500\times {{10}^{-7}}\]
done
clear
D)
5501
done
clear
View Answer play_arrow
question_answer 16) A force of \[7\hat{i}+6\hat{k}\]makes a body to move on a plane with the velocity \[3\hat{i}+4\hat{k}.\] The power developed is:
A)
\[\sqrt{45}\]
done
clear
B)
45
done
clear
C)
24
done
clear
D)
\[\sqrt{24}\]
done
clear
View Answer play_arrow
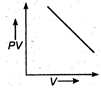
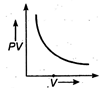
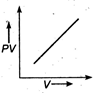
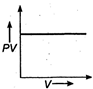
question_answer 17) The variation of PV with V of a fixed mass of an ideal gas at constant temperature is graphically represented by as shown in figure:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 18) The number of oxygen molecules in a cylinder of volume \[\text{1}{{\text{m}}^{\text{3}}}\]at a temperature of \[\text{27}{{\,}^{\text{o}}}\text{C}\]and pressure of 13.8 Pa is: (Boltzmanns constant\[k=1.38\times {{10}^{-23}}\,J{{K}^{-1}}\])
A)
\[6.23\times {{10}^{26}}\]
done
clear
B)
\[0.33\times {{10}^{28}}\]
done
clear
C)
\[3.3\times {{10}^{21}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 19) The minimum magnifying power of a telescope is M. If the focal length of the eye lens is halved, the magnifying power will become:
A)
\[\frac{M}{4}\]
done
clear
B)
\[3M\]
done
clear
C)
\[2M\]
done
clear
D)
\[4M\]
done
clear
View Answer play_arrow
question_answer 20) The surface temperature is maximum for:
A)
blue star
done
clear
B)
yellow star
done
clear
C)
green star
done
clear
D)
red star
done
clear
View Answer play_arrow
question_answer 21) A convergent doublet of separated lens, corrected for spherical aberration, are separated by 2 cm and has an equivalent focal length of 10 cm. The focal length of its component lenses are:
A)
\[{{f}_{1}}=18\,cm,\,{{f}_{2}}=10\,cm\]
done
clear
B)
\[{{f}_{1}}=20\,cm,\,{{f}_{2}}=28\,cm\]
done
clear
C)
\[{{f}_{1}}=20\,cm,\,{{f}_{2}}=18\,cm\]
done
clear
D)
\[{{f}_{1}}=24\,cm,\,{{f}_{2}}=18\,cm\]
done
clear
View Answer play_arrow
question_answer 22) In Youngs experiment using light of wavelength \[6000\overset{\text{o}}{\mathop{\text{A}}}\,,\] fringe width obtained at distance 2.5 m is 0.8 mm. If the entire apparatus is immersed in a liquid of refractive. index 1.6, the fringe width Will be:
A)
0.2 mm
done
clear
B)
0.4 mm
done
clear
C)
0.5mm
done
clear
D)
0.6 mm
done
clear
View Answer play_arrow
question_answer 23) In double slit experiment, the angular width of interference fringes with sodium light \[(\lambda =5890\,\overset{\text{o}}{\mathop{\text{A}}}\,)\]is \[\text{0}\text{.2}{{\text{0}}^{\text{o}}}\text{.}\] The change in wavelength required to increase the angular width by 10% will be:
A)
zero
done
clear
B)
increased by \[6479\text{ }\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
increased by \[589\text{ }\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
decreased by \[589\text{ }\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 24) SHM is executed by a particle of mass \[m.\]The displacement of the particle is \[\left( \frac{1}{\sqrt{2}} \right)\]times the amplitude. What fraction of the total energy is kinetic at this displacement?
A)
\[\frac{\sqrt{3}}{2}\]
done
clear
B)
\[\frac{1}{\sqrt{2}}\]
done
clear
C)
\[\frac{3}{4}\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 25) The period of SHM of a particle is 12 s. The phase difference between the positions at \[t=3\,s\]and \[t=4s\]will be:
A)
\[\pi /4\]
done
clear
B)
\[3\pi /5\]
done
clear
C)
\[\pi /6\]
done
clear
D)
\[\pi /=2\]
done
clear
View Answer play_arrow
question_answer 26) The volume of a metal sphere increases by \[\text{0}\text{.15 }\!\!%\!\!\text{ }\]when its temperature is raised by\[\text{24}{{\,}^{\text{o}}}\text{C}\text{.}\]The coefficient of linear expansion of metal is:
A)
\[2.5\times {{10}^{-5}}/{{\,}^{o}}C\]
done
clear
B)
\[2.0\times {{10}^{-5}}/{{\,}^{o}}C\]
done
clear
C)
\[1.5\times {{10}^{-5}}/{{\,}^{o}}C\,\]
done
clear
D)
\[1.2\times {{10}^{-5}}/{{\,}^{o}}C\,\]
done
clear
View Answer play_arrow
question_answer 27) The radii of two soap bubbles are \[{{r}_{1}}\]and\[{{r}_{2}}\]\[({{r}_{2}}>{{r}_{1}}).\]When they come into contact, the radius of their common interface is:
A)
\[{{r}_{2}}-{{r}_{1}}\]
done
clear
B)
\[\sqrt{r_{1}^{2}-r_{2}^{2}}\]
done
clear
C)
\[\frac{{{r}_{1}}+{{r}_{2}}}{2}\]
done
clear
D)
\[\frac{{{r}_{1}}{{r}_{2}}}{{{r}_{1}}-{{r}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 28) A ray of light is incident on the surface of a glass slab at \[\text{6}{{\text{0}}^{\text{o}}}\text{.}\] The refractive index of glass, if the reflected and refracted rays are mutually perpendicular to each other, will be:
A)
1.5
done
clear
B)
4.16
done
clear
C)
2.25
done
clear
D)
1.73
done
clear
View Answer play_arrow
question_answer 29) A stretched string is 1 m long. Its mass per unit length is 0.5 g/m. It is stretched with a force of 20 N. It is plucked at a distance of 25 cm from one end. The frequency of note emitted by it will be:
A)
400 m
done
clear
B)
300 Hz
done
clear
C)
200 Hz
done
clear
D)
100 Hz
done
clear
View Answer play_arrow
question_answer 30) A transverse wave is given by \[y=A\,\sin 2\pi (ft-x/\lambda ).\]The maximum particle velocity is 4 times the wave velocity when:
A)
\[\lambda =2\,\pi A\]
done
clear
B)
\[\lambda =\pi A\]
done
clear
C)
\[\lambda =\pi A/2\]
done
clear
D)
\[\lambda =\pi A/4\]
done
clear
View Answer play_arrow
question_answer 31) The energy in eV of red light of wavelength \[\lambda =6560\overset{\text{o}}{\mathop{\text{A}}}\,\] is:
A)
1.89 eV
done
clear
B)
2.89 eV
done
clear
C)
3.89 eV
done
clear
D)
4.89 Ev
done
clear
View Answer play_arrow
question_answer 32) The wavelength of first member of Lyman series is \[1215\overset{\text{o}}{\mathop{\text{A}}}\,.\] The wavelength of \[{{\text{H}}_{\text{ }\!\!\alpha\!\!\text{ }}}\]line is:
A)
\[6561\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[5464\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[800\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[4840\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 33) When silver is irradiated by ultraviolet light of \[1000\overset{\text{o}}{\mathop{\text{A}}}\,,\]potential of 7.7 V is required to stop the photo electrons. The work function of silver will be:
A)
3.72 eV
done
clear
B)
6.72 eV
done
clear
C)
5.72 eV
done
clear
D)
4.67eV
done
clear
View Answer play_arrow
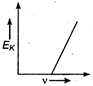
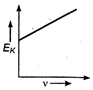
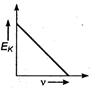
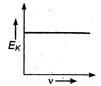
question_answer 34) Maximum kinetic energy\[{{E}_{k}}\]of a photoelectron varies with the frequency\[v\]of the incident radiation as:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 35) A radioactive element has half-life period 1600 yr. After 6400 yr, what part of element will remain?
A)
\[\frac{1}{4}\]
done
clear
B)
\[\frac{1}{8}\]
done
clear
C)
\[\frac{1}{16}\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 36) For a transistor\[{{I}_{E}}=25\,mA\] and \[{{I}_{B}}=1\,mA,\]the value of current gain \[\alpha \] will be:
A)
\[\frac{25}{24}\]
done
clear
B)
\[\frac{24}{25}\]
done
clear
C)
\[\frac{25}{26}\]
done
clear
D)
\[\frac{26}{25}\]
done
clear
View Answer play_arrow
question_answer 37) When two semiconductors of p and n type are brought into contact, they form a \[p-n\]junction which acts like a:
A)
rectifier
done
clear
B)
amplifier
done
clear
C)
oscillator
done
clear
D)
conductor
done
clear
View Answer play_arrow
question_answer 38) The de-Broglie wavelength of 1 kg mass moving with a velocity of 10 m/s, will be]:
A)
\[6.626\times {{10}^{-35}}m\]
done
clear
B)
\[6.626\times {{10}^{-34}}m\]
done
clear
C)
\[6.626\times {{10}^{-33}}m\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 39) Radioactive nuclei that are injected into a patient collected at certain sites within its body, undergoing radioactive decay and emitting electromagnetic radiation. These radiations can then be recorded by a detector. This procedure provides an important diagnostic tools called:
A)
gamma camera
done
clear
B)
CAT scan
done
clear
C)
radiotracer technique
done
clear
D)
gamma ray spectroscopy
done
clear
View Answer play_arrow
question_answer 40) If R, C and L denote resistance, capacitance and inductance. Which of the following will not have the dimensions of frequency?
A)
\[R{{L}^{-1}}\]
done
clear
B)
\[{{R}^{-1}}{{C}^{-1}}\]
done
clear
C)
\[{{L}^{-1/2}}{{C}^{-1/2}}\]
done
clear
D)
\[RCL\]
done
clear
View Answer play_arrow
question_answer 41) A \[10\,\mu F\]capacitor is connected across a 200 V, 50 Hz AC supply. The peak current through the circuit is:
A)
\[0.6\sqrt{2}A\]
done
clear
B)
\[0.6\,A\]
done
clear
C)
\[\frac{0.6\pi }{2}A\]
done
clear
D)
\[\frac{0.6}{\sqrt{2}}A\]
done
clear
View Answer play_arrow
question_answer 42) What is the energy stored in a 50 mH inductor carrying a current of 4A?
A)
0.2 J
done
clear
B)
0.4 J
done
clear
C)
0.05 J
done
clear
D)
0.1 J
done
clear
View Answer play_arrow
question_answer 43) In an ammeter, 4% of the main current is passing through galvanometer. If the galvanometer is shunted with a \[5\Omega \]resistance, the resistance of the galvanometer is:
A)
\[120\,\Omega \]
done
clear
B)
\[20\,\Omega \]
done
clear
C)
\[5\,\Omega \]
done
clear
D)
\[4\,\Omega \]
done
clear
View Answer play_arrow
question_answer 44) If 100 kWh of energy is consumed at 33 V in a copper voltameter, what is the mass of copper liberated? (Electrochemical equivalent of copper is\[0.33\times {{10}^{-6}}kg/C\])
A)
\[1\,mg\]
done
clear
B)
\[3.6\,kg\]
done
clear
C)
\[3.3\,kg\]
done
clear
D)
\[1\,kg\]
done
clear
View Answer play_arrow
question_answer 45) Four identical resistors when connected in series dissipate 5 W power. If they are connected in parallel, the power dissipated will be:
A)
80 W
done
clear
B)
60 W
done
clear
C)
40 W
done
clear
D)
20 W
done
clear
View Answer play_arrow
question_answer 46) What is the magnitude of the point charge due to which the electric field 30 cm away has the magnitude of 2N/C? \[\left( \frac{1}{4\pi {{\varepsilon }_{0}}}=9\times {{10}^{9}}N{{M}^{2}}/{{C}^{2}} \right)\]
A)
\[4\times {{10}^{-11}}C\]
done
clear
B)
\[2\times {{10}^{-11}}C\]
done
clear
C)
\[5.4\times {{10}^{-11}}C\]
done
clear
D)
\[7.5\times {{10}^{-11}}C\]
done
clear
View Answer play_arrow
question_answer 47) A parallel plate capacitor is immersed in an oil of dielectric constant 2. The field between the plates is:
A)
decreased, by a factor of\[\frac{1}{\sqrt{2}}\]
done
clear
B)
increased, by a factor of \[\sqrt{2}\]
done
clear
C)
increased, by a factor of\[\frac{1}{2}\]
done
clear
D)
decreased, by a factor of 2
done
clear
View Answer play_arrow
question_answer 48) A triode valve has an amplification factor of 20 and its plate is given a potential of 300 V. The grid-voltage to reduce the plate current to zero, is :
A)
25V
done
clear
B)
15V
done
clear
C)
12V
done
clear
D)
10 V
done
clear
View Answer play_arrow
question_answer 49) In an ideal parallel LC circuit, the capacitor is charged by connecting it to a DC source which is then disconnected. -The current in the circuit:
A)
becomes zero instantaneously
done
clear
B)
grows monotonically,
done
clear
C)
decays monotonically
done
clear
D)
oscillates instantaneously
done
clear
View Answer play_arrow
question_answer 50) For a satellite moving in an orbit around the earth, the ratio of kinetic to potential energy is:
A)
1 : 2
done
clear
B)
2 : 1
done
clear
C)
1:1
done
clear
D)
4:1
done
clear
View Answer play_arrow
question_answer 51) Which of the following Statements, about zero order reaction is not true?
A)
Its rate constant unit is \[{{s}^{-1}}\]
done
clear
B)
The graph between time and rate of reaction is a straight line
done
clear
C)
Rate of reaction is independent of concentration of reactants
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 52) The amount of\[{{\text{H}}_{\text{2}}}\text{S}\text{.}\]required to precipitate \[\text{1}\text{.69 g BaS}\]from\[\text{BaC}{{\text{l}}_{\text{2}}}\]solution is:
A)
3.4 g
done
clear
B)
0.034 g
done
clear
C)
0.34 g
done
clear
D)
0.17g
done
clear
View Answer play_arrow
question_answer 53) Which of the following is not a protein?
A)
Testosterone
done
clear
B)
Lipase
done
clear
C)
Keratin
done
clear
D)
Haemoglobin
done
clear
View Answer play_arrow
question_answer 54) The reaction, \[C{{H}_{3}}COC{{H}_{3}}\xrightarrow[(ii)\,\text{KOH},\text{glcol}]{(i)\,N{{H}_{2}}N{{H}_{2}}}C{{H}_{3}}C{{H}_{2}}C{{H}_{3}}\] is known as:
A)
Clemmensens reduction
done
clear
B)
Blancs reduction
done
clear
C)
Birch reduction
done
clear
D)
Wolff-Kishner reduction
done
clear
View Answer play_arrow
question_answer 55) pH-scale was proposed by:
A)
Arrhenius
done
clear
B)
Sorenson
done
clear
C)
Pierre de Coubertin
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 56) Thomas slag is:
A)
\[C{{a}_{3}}{{(P{{O}_{4}})}_{2}}.2{{H}_{2}}O\]
done
clear
B)
\[C{{a}_{3}}{{(P{{O}_{4}})}_{2}}.CaSi{{O}_{3}}\]
done
clear
C)
\[MgSi{{O}_{3}}\]
done
clear
D)
\[CaSi{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 57) Mohrs salt is
A)
\[N{{a}_{2}}S{{O}_{4}}.A{{l}_{2}}(S{{O}_{4}}).24{{H}_{2}}O\]
done
clear
B)
\[CuS{{O}_{4}}.A{{l}_{2}}{{(S{{O}_{4}})}_{3}}.24{{H}_{2}}O\]
done
clear
C)
\[FeS{{O}_{4}}.{{(N{{H}_{4}})}_{2}}S{{O}_{4}}.6{{H}_{2}}O\]
done
clear
D)
\[{{K}_{2}}S{{O}_{4}}.F{{e}_{2}}{{(S{{O}_{4}})}_{3}}.24{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 58) Which of the following has maximum ionisation energy?
A)
Caesium
done
clear
B)
Fluorine
done
clear
C)
Xenon
done
clear
D)
Nitrogen
done
clear
View Answer play_arrow
question_answer 59) Which of the following give ammonia, on heating with NaOH?
A)
Ethyl amine
done
clear
B)
Ethane nitrile
done
clear
C)
Nitroethane
done
clear
D)
Ethanamide
done
clear
View Answer play_arrow
question_answer 60) Which of the following is poisonous?
A)
Methanol
done
clear
B)
Ethanol
done
clear
C)
Glycerol
done
clear
D)
Castor oil
done
clear
View Answer play_arrow
question_answer 61) Which of the following fraction of coal tar distillation is obtained at \[{{270}^{o}}-{{360}^{o}}C\]?
A)
Light oil
done
clear
B)
Middle oil
done
clear
C)
Green oil
done
clear
D)
Heavy oil
done
clear
View Answer play_arrow
question_answer 62) The amount of oxalic acid, required to prepare 300 mL 2.5 M solution, is:
A)
67.5 g
done
clear
B)
9.45 g
done
clear
C)
6.75 g
done
clear
D)
94.5 g
done
clear
View Answer play_arrow
question_answer 63) The carbon di-oxide gas does not follow gaseous laws at all ranges of pressure and temperature because:
A)
it is triatpmic gas
done
clear
B)
its internal energy is quite high
done
clear
C)
there is attraction between its, molecules
done
clear
D)
it solidify at low temperature
done
clear
View Answer play_arrow
question_answer 64) The bond-angle in \[\text{As}{{\text{H}}_{\text{3}}}\]is greater than that in:
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[BC{{l}_{3}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 65) Which of the following gas, diffuse most slowly?
A)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
C)
\[C{{H}_{4}}\]
done
clear
D)
All of these diffuse at equal rate
done
clear
View Answer play_arrow
question_answer 66) Oxidation number of fluorine is+1 in:
A)
\[{{F}_{2}}O\]
done
clear
B)
\[N{{F}_{3}}\]
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 67) Which radioisotope is used in the treatment of Leukeamia?
A)
\[P-32\]
done
clear
B)
\[Na-24\]
done
clear
C)
\[1-128\]
done
clear
D)
\[Co-59\]
done
clear
View Answer play_arrow
question_answer 68) A metal having higher value of \[{{\text{E}}^{\text{o}}}\text{,}\]can:
A)
replace hydrogen from dilute \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]
done
clear
B)
replace hydrogen from concentrate \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]
done
clear
C)
act as oxidising agent
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 69) The method, which is used to purify colloidal solution is:
A)
electrophoresis
done
clear
B)
dialysis
done
clear
C)
chromatography
done
clear
D)
peptisation
done
clear
View Answer play_arrow
question_answer 70) Four gases ethane, ethene, ethyne and propene are passed into a wolf bottle containing ammoniacal \[\text{AgN}{{\text{O}}_{3}}\]solution. Which gas will come out of wolf bottle?
A)
Only ethyne
done
clear
B)
Ethane and ethyne
done
clear
C)
Ethane and ethene
done
clear
D)
Ethane, ethene and propene
done
clear
View Answer play_arrow
question_answer 71) How many conformers are known of ethane?
A)
Two
done
clear
B)
Three
done
clear
C)
\[>3\]
done
clear
D)
Ethane cannot show conformation
done
clear
View Answer play_arrow
question_answer 72) Interfering radicals interfere in the usual test of:
A)
acid radicals
done
clear
B)
basic radicals
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 73) Flux in the smelting process is added to:
A)
decrease the solubility of impurities
done
clear
B)
increase the fusion temperature of roasted ore
done
clear
C)
convert impurities into slag
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 74) The heat of formation of \[\text{MgO,A}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}\]and \[\text{Si}{{\text{O}}_{2}}\]are \[-692,\,-1676,-911,\,\text{kJ}\text{mo}{{\text{l}}^{-1}}.\] Most stable oxide is:
A)
\[MgO\]
done
clear
B)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
C)
\[Si{{O}_{2}}\]
done
clear
D)
cannot be predicted
done
clear
View Answer play_arrow
question_answer 75) Which of the following reaction, will be favoured by low pressure?
A)
\[PC{{l}_{5}}PC{{l}_{3}}+C{{l}_{2}}\]
done
clear
B)
\[{{N}_{2}}+3{{H}_{2}}2N{{H}_{3}}\]
done
clear
C)
\[{{H}_{2}}+\frac{1}{2}{{O}_{2}}{{H}_{2}}O\]
done
clear
D)
\[2NO+{{O}_{2}}2N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 76) In III group of basic radical analysis, \[N{{H}_{4}}Cl\]is added before group reagent \[N{{H}_{4}}OH.\]This is done to:
A)
increase the solubility product of III group salts
done
clear
B)
decrease the solubility product of III group salts
done
clear
C)
increase the ionic product of \[\text{O}{{\text{H}}^{-}}\]and III group radicals
done
clear
D)
decrease the degree of ionisation of group reagent
done
clear
View Answer play_arrow
question_answer 77) Representative elements are:
A)
\[s-\]block elements
done
clear
B)
p-block elements
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 78) Which of the following equation is used in Paulings scale of electronegativity?
A)
\[{{x}_{A}}=0.187(IE+EA)+0.17\]
done
clear
B)
\[{{x}_{A}}-{{x}_{B}}=0.182\]
done
clear
C)
\[{{[{{E}_{A-B}}-\sqrt{({{E}_{A-A}}\times {{E}_{B-B}})}]}^{1/2}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 79) Which hybridisation has sulphur in \[S{{O}_{2}}\]?
A)
\[s{{p}^{2}}\]
done
clear
B)
\[s{{p}^{3}}{{d}^{2}}\]
done
clear
C)
\[s{{p}^{3}}\]
done
clear
D)
\[sp\]
done
clear
View Answer play_arrow
question_answer 80) The energy of last electron of Li will be:
A)
\[-30.6\text{ }eV\]
done
clear
B)
\[~-13.6\text{ }eV\]
done
clear
C)
\[-24.6\text{ }eV\]
done
clear
D)
\[~-28.6\text{ }eV\]
done
clear
View Answer play_arrow
question_answer 81) Benzaldehyde, in presence of dil. NaOH, changes into benzyl alcohol and sodium benzoate. This reaction is an example of:
A)
rearrangement
done
clear
B)
oxidation
done
clear
C)
disproportionation
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 82) Which of the following is the strongest acid?
A)
o-nitro benzoic acid
done
clear
B)
\[m-\]nitro benzoic acid
done
clear
C)
\[m-\] chloro benzoic acid
done
clear
D)
\[m-\] cyano benzoic acid
done
clear
View Answer play_arrow
question_answer 83) What is the product X in the reaction, \[C{{H}_{3}}COCl\xrightarrow{C{{H}_{3}}COONa}X?\]
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[{{(C{{H}_{3}}CO)}_{2}}O\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 84) A 0.5 g/L solution of glucose is found to be isotonic with a 2.5 g/L solution of an organic compound. What will be the molecular weight of that organic compound?
A)
300
done
clear
B)
600
done
clear
C)
925
done
clear
D)
1200
done
clear
View Answer play_arrow
question_answer 85) Which of the following ion does not follow EAN rule?
A)
\[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
done
clear
B)
\[{{[Ag{{(CN)}_{2}}]}^{-}}\]
done
clear
C)
\[{{[PtC{{l}_{6}}]}^{2-}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 86) Potassium, on burning in oxygen atmosphere, gives:
A)
\[{{K}_{2}}O\]
done
clear
B)
\[{{K}_{2}}{{O}_{3}}\]
done
clear
C)
\[K{{O}_{2}}\]
done
clear
D)
\[{{K}_{2}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 87) Which method is employed to prepare copper sol?
A)
Peptisation
done
clear
B)
Bredigs arc method
done
clear
C)
Double decomposition
done
clear
D)
Reduction of \[\text{CuC}{{\text{l}}_{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 88) How much copper will be deposited at cathode, on passing 4 coulomb charge in 15 min?
A)
0.0195 g
done
clear
B)
0.0013 g
done
clear
C)
0.020 g
done
clear
D)
0.0020 g
done
clear
View Answer play_arrow
question_answer 89) If 50 % of a radioactive substance dissociates in 15 min, then the time taken by substance to dissociate 99% will be:
A)
50 min
done
clear
B)
100 min
done
clear
C)
99 min
done
clear
D)
150 min
done
clear
View Answer play_arrow
question_answer 90) Which of the following alcohol give positive iodoform test?
A)
Methanol
done
clear
B)
Ethyl carbinol
done
clear
C)
Pentanol-3
done
clear
D)
Methyl carbinol
done
clear
View Answer play_arrow
question_answer 91) Human body is an example of:
A)
open system
done
clear
B)
closed system
done
clear
C)
isolated system
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 92) The atomic size of cerium and promethium is quite close, due to:
A)
they are in same period in periodic table
done
clear
B)
their electronic configuration is same
done
clear
C)
\[f-\] electrons have poor shielding effect
done
clear
D)
nuclear charge is higher oil cerium than promethium
done
clear
View Answer play_arrow
question_answer 93) Brown glass and cement have/ which element common in them?
A)
\[Fe\]
done
clear
B)
\[Al\]
done
clear
C)
\[Na\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 94) The most common isomerism found in ethers, is:
A)
chain isomerism
done
clear
B)
tautomerism
done
clear
C)
metamerism
done
clear
D)
optical isomerism
done
clear
View Answer play_arrow
question_answer 95) \[t-\]butyl chloride preferrably undergo hydrolysis by:
A)
\[{{S}_{N}}1\] mechanism
done
clear
B)
\[{{S}_{N}}2\] mechanism
done
clear
C)
any of [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 96) Which of the following has lowest boiling point?
A)
\[NaCl\]
done
clear
B)
\[CuCl\]
done
clear
C)
\[CuC{{l}_{2}}\]
done
clear
D)
\[CsCl\]
done
clear
View Answer play_arrow
question_answer 97) Which of the following is true for the reaction \[CO(g)+\frac{1}{2}{{O}_{2}}(g)C{{O}_{2}}(g)\]
A)
\[{{K}_{p}}>{{k}_{c}}\]
done
clear
B)
\[{{K}_{p}}<{{k}_{c}}\]
done
clear
C)
\[{{K}_{p}}={{k}_{c}}\]
done
clear
D)
\[{{K}_{p}}\ge {{k}_{c}}\]
done
clear
View Answer play_arrow
question_answer 98) Which of the following is not a buffer solution?
A)
\[C{{H}_{3}}COOH+C{{H}_{3}}COONa\]
done
clear
B)
\[{{H}_{3}}B{{O}_{3}}+N{{a}_{3}}B{{O}_{3}}\]
done
clear
C)
\[HCl{{O}_{4}}+NaCl{{O}_{4}}\]
done
clear
D)
\[N{{H}_{4}}OH+{{(N{{H}_{4}})}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 99) Which of the following ore is concentrated by both, magnetic and gravimetric separation?
A)
Dolomite
done
clear
B)
Tinstone
done
clear
C)
Galena
done
clear
D)
Bauxite
done
clear
View Answer play_arrow
question_answer 100) Which of the following is not a polymer?
A)
RNA
done
clear
B)
Oxytocin
done
clear
C)
Cholesterol
done
clear
D)
Amylose
done
clear
View Answer play_arrow
question_answer 101) The first codon discovered by Nirenberg and Mathaei was:
A)
CCC
done
clear
B)
GGG
done
clear
C)
UUU
done
clear
D)
AAA
done
clear
View Answer play_arrow
question_answer 102) Maximum amount of energy/ATP is liberated on oxidation of:
A)
fats
done
clear
B)
proteins
done
clear
C)
starch
done
clear
D)
vitamins
done
clear
View Answer play_arrow
question_answer 103) The thermoregulatory centre in the body is:
A)
spinal cord
done
clear
B)
hypothalamus
done
clear
C)
cerebellum
done
clear
D)
pituitary
done
clear
View Answer play_arrow
question_answer 104) Which is not cancer?
A)
Leukemia
done
clear
B)
Trachoma
done
clear
C)
Carcinoma
done
clear
D)
Sarcoma
done
clear
View Answer play_arrow
question_answer 105) Bio gas production from waste bio mass with the help of methanogenic bacteria is:
A)
multi step process
done
clear
B)
one step process
done
clear
C)
two step process
done
clear
D)
three step process
done
clear
View Answer play_arrow
question_answer 106) Pace-maker in heart is situated:
A)
in the wall of left atrium
done
clear
B)
in the wall of right atrium
done
clear
C)
on inter-auricular septum
done
clear
D)
on inter-ventricular septum
done
clear
View Answer play_arrow
question_answer 107) In the animal body blood bank is:
A)
heart
done
clear
B)
spleen
done
clear
C)
lungs
done
clear
D)
liver
done
clear
View Answer play_arrow
question_answer 108) Addisons disease results from:
A)
hypertrophy of gonads
done
clear
B)
hypo-secretion of adrenal cortex
done
clear
C)
hyperactivity of cells of Leydig
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 109) LSD is obtained from:
A)
Claviceps purpurea
done
clear
B)
Rauwolffia serpentina
done
clear
C)
Pdpayer somniferum
done
clear
D)
Cannabis sativa
done
clear
View Answer play_arrow
question_answer 110) Australopithecus existed in:
A)
Pliocene
done
clear
B)
Miocene
done
clear
C)
Pleistocene
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 111) who proved that oxygen evolved in photosynthesis comes from water?
A)
Calvin
done
clear
B)
Mayer
done
clear
C)
Blackman
done
clear
D)
Ruben, Hassid and Kamen
done
clear
View Answer play_arrow
question_answer 112) The process in which water is split during photosynthesis is:
A)
plasmolysis
done
clear
B)
photolysis
done
clear
C)
hydrolysis
done
clear
D)
haemolysis
done
clear
View Answer play_arrow
question_answer 113) Heroin is obtained from plant of family:
A)
Papaveraceae
done
clear
B)
Leguminosae
done
clear
C)
Cruciferae
done
clear
D)
Liliaceae
done
clear
View Answer play_arrow
question_answer 114) HIV has a protein coat and a genetic material which is:
A)
ss-RNA
done
clear
B)
ds-RNA
done
clear
C)
ds-DNA
done
clear
D)
ss-DNA
done
clear
View Answer play_arrow
question_answer 115) More\[\text{C}{{\text{O}}_{\text{2}}}\]is evolved than the volume of oxygen consumed. when the respiratory substrate is:
A)
fat
done
clear
B)
sucrose
done
clear
C)
glucose
done
clear
D)
organic acid
done
clear
View Answer play_arrow
question_answer 116) Immunoglobin found abundantly in serum is:
A)
IgM
done
clear
B)
IgA
done
clear
C)
IgN
done
clear
D)
IgG
done
clear
View Answer play_arrow
question_answer 117) During the process of blood coagulation vitamin-K helps in the:
A)
formation of thromboplastin
done
clear
B)
formation of prothrombin
done
clear
C)
conversion of prothrombin to thrombin
done
clear
D)
conversion of fibrinogen to fibrin
done
clear
View Answer play_arrow
question_answer 118) Krebs cycle begins with the reaction:
A)
citric acid+ acetyl Co-A
done
clear
B)
oxaloacetic acid + pyruvic acid
done
clear
C)
oxaloacetic acid + Citric acid
done
clear
D)
oxaloacetic + acetyl Co-A
done
clear
View Answer play_arrow
question_answer 119) Contraction of gall bladder is carried by:
A)
rennin
done
clear
B)
gastrin
done
clear
C)
cholecystokinin
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 120) Foetal sex is determined by examining cells from amniotic fluid looking for:
A)
chiasmata
done
clear
B)
Ban-bodies
done
clear
C)
sex-chromosomes
done
clear
D)
drumsticks
done
clear
View Answer play_arrow
question_answer 121) Theory of inheritance of acquired characters was given by:
A)
Darwin
done
clear
B)
Wallace
done
clear
C)
Lamarck
done
clear
D)
de Vries
done
clear
View Answer play_arrow
question_answer 122) Removal of anthers of, some flowers during plant breeding is:
A)
emasculation
done
clear
B)
anthesis
done
clear
C)
pollination
done
clear
D)
for collection of pollen
done
clear
View Answer play_arrow
question_answer 123) Transgenic crops are modified through genetic engineering to develop natural resistance to insect pests. Which one is a transgenic plant?
A)
Tobacco and cotton
done
clear
B)
Tomato and rice
done
clear
C)
Maize and sugarcane
done
clear
D)
Tomato and wheat
done
clear
View Answer play_arrow
question_answer 124) Neo-Darwinism believes that new species develops through:
A)
mutations
done
clear
B)
hybridization
done
clear
C)
mutations with natural selection
done
clear
D)
continuous variations with natural selection
done
clear
View Answer play_arrow
question_answer 125) Most hazardous metal pollutant of automobile exhaust is:
A)
cadmium
done
clear
B)
lead
done
clear
C)
mercury
done
clear
D)
copper
done
clear
View Answer play_arrow
question_answer 126) VAM is useful for:
A)
phosphate nutrition
done
clear
B)
breaking of dormancy
done
clear
C)
decrease in diseases
done
clear
D)
retarding flowering
done
clear
View Answer play_arrow
question_answer 127) Parathyroid hormone is a:
A)
peptide
done
clear
B)
carbohydrate
done
clear
C)
lipid
done
clear
D)
steroid
done
clear
View Answer play_arrow
question_answer 128) A clone is:
A)
heterozygote. obtained asexually
done
clear
B)
homozygote obtained asexually
done
clear
C)
heterozygote produced by sexual methods
done
clear
D)
homozygote produced by sexual reproduction
done
clear
View Answer play_arrow
question_answer 129) A petroleum plant is:
A)
Euphorbia lathyrus
done
clear
B)
Acaciaarabica
done
clear
C)
Pinus roxburghii
done
clear
D)
Prosopis cineraria
done
clear
View Answer play_arrow
question_answer 130) Progesterone hormone is secreted by:
A)
corpus albicans
done
clear
B)
corpus callosum
done
clear
C)
corpus luteum in ovaries
done
clear
D)
corpus uteri
done
clear
View Answer play_arrow
question_answer 131) Triploid endosperm occurs only in:
A)
angiosperms
done
clear
B)
gymnosperms
done
clear
C)
pteridophytes
done
clear
D)
spermatophytes
done
clear
View Answer play_arrow
question_answer 132) Cinchona offlcinalis belongs to family:
A)
Cruciferae,
done
clear
B)
Malvaceae
done
clear
C)
Rubiaceae
done
clear
D)
Leguminosae
done
clear
View Answer play_arrow
question_answer 133) Meiosis in AaBb will produce gametes:
A)
AB, aB, Ab, ab
done
clear
B)
AB, ab
done
clear
C)
Aa, bb
done
clear
D)
Aa, Bb
done
clear
View Answer play_arrow
question_answer 134) Respiratory centre in brain occurs in:
A)
medulla oblongata
done
clear
B)
cerebellum
done
clear
C)
hypothalamus
done
clear
D)
pericardium
done
clear
View Answer play_arrow
question_answer 135) Capitulum inflorescence is found in:
A)
Liliaceae
done
clear
B)
Papilionaceae
done
clear
C)
Compositae
done
clear
D)
Rosaceae
done
clear
View Answer play_arrow
question_answer 136) Mutations are generally induced by means of:
A)
\[\alpha -rays\]
done
clear
B)
\[\beta -rays\]
done
clear
C)
\[\gamma -rays\]
done
clear
D)
UV radiations
done
clear
View Answer play_arrow
question_answer 137) Pollen grains are shed at:
A)
1-celled stage
done
clear
B)
2-3- celled stage
done
clear
C)
4- celled stage
done
clear
D)
5- celled stage
done
clear
View Answer play_arrow
question_answer 138) Turners syndrome is caused by:
A)
polyploidy
done
clear
B)
autosomal aneuploidy
done
clear
C)
sex-chromosome aneuploidy
done
clear
D)
trisomy
done
clear
View Answer play_arrow
question_answer 139) Phrase Survival of the Fittest was used by:
A)
Hugo de Vries
done
clear
B)
Charles Darwin
done
clear
C)
Herbert Spencer
done
clear
D)
Jean Baptiste Lamarck
done
clear
View Answer play_arrow
question_answer 140) Entry of pollen tube through micropyle is:
A)
porogamy
done
clear
B)
mesogamy
done
clear
C)
pseudogamy
done
clear
D)
chalazogamy
done
clear
View Answer play_arrow
question_answer 141) Micropyle helps in:
A)
germination of pollen grain
done
clear
B)
growth of pollen tube
done
clear
C)
coming out of pollen tube from pollen grain
done
clear
D)
allowing entry of pollen tube
done
clear
View Answer play_arrow
question_answer 142) In mature RBC, nucleus is present in:
A)
frog
done
clear
B)
rabbit
done
clear
C)
both [a] and [b]
done
clear
D)
neither in frog noir in rabbit
done
clear
View Answer play_arrow
question_answer 143) Microbial insecticide is:
A)
Bacillus polymixa
done
clear
B)
Bacillus brevis
done
clear
C)
Bacillus subtilis
done
clear
D)
Bacillus thuringiensis
done
clear
View Answer play_arrow
question_answer 144) Gas released during Bhopal tragedy was:
A)
sodium isothiocyanate
done
clear
B)
methyl isocyanate
done
clear
C)
potassium isothiocyanate
done
clear
D)
ethyl isothiocyanate
done
clear
View Answer play_arrow
question_answer 145) banana, which of the following part is edible?
A)
Epicarp
done
clear
B)
Mesocarp
done
clear
C)
Endocarp
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 146) In pond ecosystem, diatoms represent:
A)
producers
done
clear
B)
primary consumers
done
clear
C)
secondary consumers
done
clear
D)
tertiary consumers
done
clear
View Answer play_arrow
question_answer 147) Sorosis is found in:
A)
jack fruit
done
clear
B)
mulberry
done
clear
C)
fig
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 148) Sertoli cells are involved in:
A)
respiration
done
clear
B)
nutrition of sperms
done
clear
C)
excretion
done
clear
D)
development of sex organs
done
clear
View Answer play_arrow
question_answer 149) The myocardium is found in :
A)
heart of mammals
done
clear
B)
brain of mammals
done
clear
C)
lungs of mammals
done
clear
D)
testes of mammals
done
clear
View Answer play_arrow
question_answer 150) Plasmid is:
A)
plastid
done
clear
B)
algae
done
clear
C)
fungus
done
clear
D)
extra nuclear DNA of bacterial cell
done
clear
View Answer play_arrow