question_answer 1) The dimension of \[\frac{P}{a}\]in the equation \[p=\frac{b-{{t}^{2}}}{ax}\] where p is pressure, \[x\]is distance and t is time are
A)
\[[ML{{T}^{-2}}]\]
done
clear
B)
\[[M{{T}^{-2}}]\]
done
clear
C)
\[[M{{L}^{3}}{{T}^{-2}}]\]
done
clear
D)
\[[L{{T}^{-3}}]\]
done
clear
View Answer play_arrow
question_answer 2) A body moving with uniform acceleration describes 12 m in the third second of its motion and 20 m in the 5th second. Find the velocity after 10 th second.
A)
40 m/s
done
clear
B)
42 m/s
done
clear
C)
52 m/s
done
clear
D)
4m/s
done
clear
View Answer play_arrow
question_answer 3) A ball rolls of the top of a stair way with a horizontal velocity\[u\,m{{s}^{-1}}.\] If the steps are \[h\] metre high and b metre wide, the ball will hit the edge of the nth step, where \[n\] is
A)
\[\frac{2hu}{g{{b}^{2}}}\]
done
clear
B)
\[\frac{2h{{u}^{2}}}{g{{b}^{2}}}\]
done
clear
C)
\[\frac{2h{{u}^{2}}}{gb}\]
done
clear
D)
\[\frac{h{{u}^{2}}}{g{{b}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 4) A man slides down a light rope whose breaking strength is \[\eta \]times his weight. What should be his maximum acceleration so that the rope just not breaks?
A)
\[g(1-\eta )\]
done
clear
B)
\[\eta g\]
done
clear
C)
\[\frac{g}{1+\eta }\]
done
clear
D)
\[\frac{g}{1-\eta }\]
done
clear
View Answer play_arrow
question_answer 5) The motor of an engine is rotating about its axis with an angular velocity of 100 rev/m. If conies to rest in 15s, after being switched off. Assuming constant angular deceleration. What are the numbers of revolutions made by it before coming to rest?
A)
12.5
done
clear
B)
40
done
clear
C)
32.6
done
clear
D)
15.6
done
clear
View Answer play_arrow
question_answer 6) By what percent the energy of a satellite has to be increased to shift it from an orbit of radius r to 3r?
A)
22.3%
done
clear
B)
33.3%
done
clear
C)
66.7%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 7) To maintain a rotar at uniform angular speed of 200 rad/s, an engine needs to transmit a torque of 180 Nm. What is the power required by engine? (Assume efficiency of engine to be 80%)
A)
36kW
done
clear
B)
18 kW
done
clear
C)
45kW
done
clear
D)
54kW
done
clear
View Answer play_arrow
question_answer 8) Two pendulum have time period T and\[\frac{5T}{4}\] they start SHM at the same time form the mean position. What will be the phase difference between them after the bigger pendulum completed one oscillation
A)
\[{{45}^{o}}\]
done
clear
B)
\[{{90}^{o}}\]
done
clear
C)
60°
done
clear
D)
\[~{{30}^{o}}\]
done
clear
View Answer play_arrow
question_answer 9) An open pipe of length 33 cm resonates with frequency of 1000 Hz. If the speed of sound is \[333\,m{{s}^{-1}},\]then this frequency is
A)
fundamental frequency of the pipe
done
clear
B)
third harmonic of the pipe
done
clear
C)
second harmonic of the pipe
done
clear
D)
fourth harmonic of the pipe
done
clear
View Answer play_arrow
question_answer 10) Water rises to a height h in a capillary at the surface of earth on the surface of the moon the height of water column in the same capillary will be
A)
\[6h\]
done
clear
B)
\[h/6\]
done
clear
C)
\[h\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 11) The molar heat capacity in a process of a diatomic gas, if it does a work of Q/4 when heat Q is supplied to it, is
A)
\[\frac{2}{5}R\]
done
clear
B)
\[\frac{10}{3}R\]
done
clear
C)
\[\frac{5}{3}R\]
done
clear
D)
\[\frac{6}{5}R\]
done
clear
View Answer play_arrow
question_answer 12) A black body has maximum energy at wavelength\[{{\lambda }_{m}}\]at temperature 2000 K. The corresponding wavelength at a temperature of 3000 K will be
A)
\[\frac{3}{2}{{\lambda }_{m}}\]
done
clear
B)
\[\frac{2}{3}{{\lambda }_{m}}\]
done
clear
C)
\[\frac{4}{9}{{\lambda }_{m}}\]
done
clear
D)
\[\frac{9}{4}{{\lambda }_{m}}\]
done
clear
View Answer play_arrow
question_answer 13) The potential field of an electric field \[E=(yi+xj)\]is
A)
\[V=-(x+y)+\]constant
done
clear
B)
\[V=\text{constant}\]
done
clear
C)
\[V=-({{x}^{2}}+{{y}^{2}})+cons\tan t\]
done
clear
D)
\[V=-xy+\text{constant}\]
done
clear
View Answer play_arrow
question_answer 14) The \[80\,\Omega \] galvanometer, deflects full scale for a potentials of 20 mV. A voltmeter deflecting full scale of 5V is to made using this galvanometer. We must connect
A)
a resistance of \[19.92\,k\Omega \]parallel to the galvanometer
done
clear
B)
a resistance of \[19.92\,k\Omega \]in series with the galvanoyneter
done
clear
C)
a resistance of \[20\,k\Omega \]parallel to the galvanometer
done
clear
D)
a resistance of \[20\,k\Omega \]in series with galvanometer
done
clear
View Answer play_arrow
question_answer 15) A current of 1 A is passed through a straight wire of length 20 m. The magnetic field at a point in air at a distance of 3 m from either end of wire and lying on the axis of wire will be
A)
\[\frac{{{\mu }_{0}}}{2\pi }\]
done
clear
B)
\[\frac{{{\mu }_{0}}}{4\pi }\]
done
clear
C)
\[\frac{{{\mu }_{0}}}{8\pi }\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 16) short bar magnet placed with its axis- at \[{{30}^{o}}\] with a uniform external magnetic field of 0.16 T experience a torque of magnitude 0.032 J. The magnetic moment of the bar magnet will be
A)
\[0.23\,J{{T}^{-1}}\]
done
clear
B)
\[0.40\,J{{T}^{-1}}\]
done
clear
C)
\[0.80\,J{{T}^{-1}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 17) A coil has an area of \[0.05\,{{m}^{2}}\]and has 800 turns. After placing the coil in a magnetic field of strength\[4\times {{10}^{-5}}Wb{{m}^{-2}}\]perpendicular to the field, the coil is rotated through \[{{90}^{o}}\] in 0. Is. The average emf induced is
A)
zero
done
clear
B)
0.016V
done
clear
C)
0.01V
done
clear
D)
0.032V
done
clear
View Answer play_arrow
question_answer 18) An alternating voltage (in volt) given by \[V=200\sqrt{2}\sin (100t)\] is connected to \[1\mu F\]capacitor through an AC ammeter. The reading of the ammeter will be
A)
10 mA
done
clear
B)
20 mA
done
clear
C)
40 mA
done
clear
D)
80 mA
done
clear
View Answer play_arrow
question_answer 19) Instantaneous displacement current of 1.0 A in the space between the parallel plates of \[1\mu F\]capacitor can be established by changing potential difference of
A)
\[{{10}^{-6\text{ }}}V/s\]
done
clear
B)
\[{{10}^{6}}\text{ }V/s\]
done
clear
C)
\[{{10}^{-8}}\text{ }V/s\]
done
clear
D)
\[~{{10}^{8}}\text{ }V/s\]
done
clear
View Answer play_arrow
question_answer 20) The maximum magnification that can be obtained with a convex lens of focal length 2.5 cm is least distance of distinct vision is 25 cm
A)
10
done
clear
B)
\[0.1\]
done
clear
C)
62.5
done
clear
D)
11
done
clear
View Answer play_arrow
question_answer 21) The magnifying power of an astronomical telescope is 8 and the distance between the two lenses is 54 cm. The focal length of eye lens and objective lens will be respectively
A)
6 cm and 48 cm
done
clear
B)
48 cm and 6 cm
done
clear
C)
8 cm and 64 cm
done
clear
D)
6 cm and 60 cm
done
clear
View Answer play_arrow
question_answer 22) The path difference between two wavefronts emitted by coherent sources of wavelength \[5460\overset{\text{o}}{\mathop{\text{A}}}\,\]is 2.1 micron The phase difference between the wave fronts at that point is
A)
7.692 rad
done
clear
B)
\[7.692\text{ }\pi \text{ }rad\]
done
clear
C)
\[\frac{7.692}{\pi }rad\]
done
clear
D)
\[\frac{7.692}{3\pi }rad\]
done
clear
View Answer play_arrow
question_answer 23) A photocell with a constant potential difference of V volt across it is illuminated by a point source from a distance of 25 cm. When the source is moved to a distance of 1 m, the electrons emitted by the photocell
A)
carry 1/4th their previous energy
done
clear
B)
are 1/6th as numerous as before
done
clear
C)
are 1/4th as numerous as before
done
clear
D)
carry 1/4th their previous momentum
done
clear
View Answer play_arrow
question_answer 24) When an electron in hydrogen atom is excited from its 4th to 5th stationary orbit, the change in angular momentum of electron is (Plancks constant \[h=6.6\times {{10}^{-34}}\,Js\])
A)
\[4.16\times {{10}^{-34}}Js\]
done
clear
B)
\[3.32\times {{10}^{-34}}Js\]
done
clear
C)
\[1.05\times {{10}^{-34}}Js\]
done
clear
D)
\[2.08\times {{10}^{-34}}Js\]
done
clear
View Answer play_arrow
question_answer 25) Let T be the mean life of a radioactive sample. 75% of the active nuclei present in the sample initially will decay in time
A)
\[2T\]
done
clear
B)
\[\frac{1}{2}(ln2)T\]
done
clear
C)
\[4T\]
done
clear
D)
\[2(ln2)T\]
done
clear
View Answer play_arrow
question_answer 26) A potential barrier of 0.50 V exists across a p-n junction. If the deplection region is \[5.0\times {{10}^{-7}}m\]wide, the strength of electric field in this region is
A)
\[1.0\times {{10}^{6}}V/m\]
done
clear
B)
\[1.0\times {{10}^{5}}V/m\]
done
clear
C)
\[2.0\times {{10}^{5}}V/m\]
done
clear
D)
\[2.0\times {{10}^{6}}V/m\]
done
clear
View Answer play_arrow
question_answer 27) What is an AND gate?
A)
It has not equivalence to switching circuit
done
clear
B)
It is equivalent to series switching circuit
done
clear
C)
It is equivalent to parallel switching circuit
done
clear
D)
It is a mixture of series and parallel switching
done
clear
View Answer play_arrow
question_answer 28) A parachutist drops first freely from an aero plane for 10 s and then parachute opens out. Now he descends with a net retardation of \[2.5\,m/{{s}^{2}}.\] If he bails out of the plane at a height of 2495 m and\[g=10\,m/{{s}^{2}},\] his velocity on reaching the ground will be
A)
5 m/s
done
clear
B)
10 m/s
done
clear
C)
15 m/s
done
clear
D)
20 m/s
done
clear
View Answer play_arrow
question_answer 29) A particle is moving along a circular path with uniform speed. Through what angle does it angular velocity change when it completes half of the circular path?
A)
\[{{0}^{o}}\]
done
clear
B)
\[{{45}^{o}}\]
done
clear
C)
\[{{180}^{o}}\]
done
clear
D)
\[~{{360}^{o}}\]
done
clear
View Answer play_arrow
question_answer 30) Tick out the wrong statement.
A)
Transverse waves can be generated in solids.
done
clear
B)
A system having ice floating on water has the same volume even after the ice is melted.
done
clear
C)
Heat radiations have the velocity of light.
done
clear
D)
Phase will not change when sound or light waves are reflected back.
done
clear
View Answer play_arrow
question_answer 31) Two particles P and Q describe SHM of same amplitude a, frequency v along the same straight line. The maximum distance between the two particle is \[a\sqrt{2}.\]The initial phase difference between the particles is
A)
zero
done
clear
B)
\[\pi /2\]
done
clear
C)
\[\pi /6\]
done
clear
D)
\[\pi /3\]
done
clear
View Answer play_arrow
question_answer 32) The magnitude of electric intensity E is such that an electron placed in it would experience an electrical force equal to its weight. E is given by
A)
\[mge\]
done
clear
B)
\[\frac{e}{mg}\]
done
clear
C)
\[\frac{mg}{e}\]
done
clear
D)
\[\frac{{{e}^{2}}g}{{{m}^{2}}}\]
done
clear
View Answer play_arrow
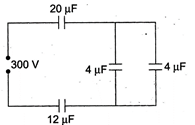
question_answer 33)
In the figure, charge and the potential difference across the \[4\mu F\] capacitor will be nearly
A)
\[600\,\mu C,150\,V\]
done
clear
B)
\[300\,\mu C,75\,V\]
done
clear
C)
\[800\,\mu C,200\,V\]
done
clear
D)
\[580\,\mu C,145\,V\]
done
clear
View Answer play_arrow
question_answer 34) A dip circle lies initially in the magnetic meridian. If it is now rotated, through angle\[\theta \] in the horizontal plane, then tangent of the angle of dip is changed in the ratio
A)
\[1:\cos \theta \]
done
clear
B)
\[\cos \theta :1\]
done
clear
C)
\[1:\sin \theta \]
done
clear
D)
\[\sin \theta :1\]
done
clear
View Answer play_arrow
question_answer 35) A 5 cm long solenoid having \[10\,\,\Omega \] resistance and 5 mH inductance is joined to a 10 V battery, At steady state, the current through the solenoid (in ampere) will be
A)
5
done
clear
B)
2
done
clear
C)
1
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 36) A convex lens makes a real image 4 cm long on a screen. When the lens is shifted to a new position without disturbing the object, we again get a real image on the screen which is 16 cm tall. The length of the object must be
A)
\[\frac{1}{4}cm\]
done
clear
B)
\[8\,cm\]
done
clear
C)
\[12\,cm\]
done
clear
D)
\[20\,cm\]
done
clear
View Answer play_arrow
question_answer 37) For a particle of mass m enclosed in a one-dimensional box of length L, the de-Broglie concept would lead to stationary waves, with nodes at the two ends. The energy values allowed for such a system (with n as integer) will be
A)
\[\frac{{{h}^{2}}}{8\,m{{L}^{2}}}{{n}^{2}}\]
done
clear
B)
\[\frac{{{h}^{2}}}{4mL}{{n}^{2}}\]
done
clear
C)
\[\frac{h}{4mL}n\]
done
clear
D)
\[\frac{{{h}^{2}}}{4m{{L}^{2}}}{{n}^{2}}\]
done
clear
View Answer play_arrow
question_answer 38) If \[{{N}_{0}}\]is the original mass of the substance of half-life period \[{{t}_{1/5}}=5yrs,\] then the amount of substance left after 15 days is
A)
\[\frac{{{N}_{0}}}{8}\]
done
clear
B)
\[\frac{{{N}_{0}}}{16}\]
done
clear
C)
\[\frac{{{N}_{0}}}{2}\]
done
clear
D)
\[\frac{{{N}_{0}}}{4}\]
done
clear
View Answer play_arrow
question_answer 39) A doubled layered wall has layer A, 10 cm thick and B, 20 cm thick. The thermal conductivity of A is thrice that of B. In the steady state, the temperature difference across the wall is \[35{{\,}^{o}}C.\] The temperature difference across the layer A is
A)
\[28{{\,}^{o}}C\]
done
clear
B)
\[14{{\,}^{o}}C\]
done
clear
C)
\[7{{\,}^{o}}C\]
done
clear
D)
\[5{{\,}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 40) The mass of a planet is six times that of the earth. The radius of the planet is twice that of the earth. If the escape velocity from the earth is \[v,\]then the escape velocity from the planet is
A)
\[\sqrt{3}\,v\]
done
clear
B)
\[\sqrt{2}\,v\]
done
clear
C)
\[\sqrt{5}\,v\]
done
clear
D)
\[\sqrt{12}\,v\]
done
clear
View Answer play_arrow
question_answer 41) Power supplied to a particle of mass 2 kg varies with time as \[P=\frac{3{{t}^{2}}}{2}W.\]Here t is in second. If velocity of particle at \[t=0\]is \[v=0,\]the velocity of particle at time \[t=2\,s\]will be
A)
1 m/s
done
clear
B)
4 m/s
done
clear
C)
2 m/s
done
clear
D)
\[2\sqrt{2}\,m/s\]
done
clear
View Answer play_arrow
question_answer 42) A particle is projected from the ground with an initial speed of\[v\]at an angle\[\theta \]with horizontally. The average velocity of the particle between its point of projection and highest point of trajectory is
A)
\[\frac{v}{2}\sqrt{1+2{{\cos }^{2}}\theta }\]
done
clear
B)
\[\frac{v}{2}\sqrt{1+2{{\cos }^{2}}\theta }\]
done
clear
C)
\[\frac{v}{2}\sqrt{1+3{{\cos }^{2}}\theta }\]
done
clear
D)
\[v\cos \theta \]
done
clear
View Answer play_arrow
question_answer 43) Given\[\sigma \] is the compressibility of water, \[\rho \] is the density of water arid k is the bulk modulus of water. What is the energy density of water at the bottom of a lake \[h\] metre deep?
A)
\[\frac{1}{2}\sigma {{(h\rho g)}^{2}}\]
done
clear
B)
\[\frac{1}{2}\sigma (h\rho g)\]
done
clear
C)
\[\frac{1}{2}\frac{h\rho g}{\sigma }\]
done
clear
D)
\[\frac{h\rho g}{\sigma }\]
done
clear
View Answer play_arrow
question_answer 44) An ideal gas heat engine operates in a Carnot cycle between \[227{{\,}^{o}}C\]and \[127{{\,}^{o}}C.\] It absorbs \[6.0\times {{10}^{4}}\,cal\]at the higher temperature. The amount of heat converted into work is equal to
A)
\[4.8\times {{10}^{4}}cal\]
done
clear
B)
\[3.5\times {{10}^{4}}cal\]
done
clear
C)
\[1.6\times {{10}^{4}}cal\]
done
clear
D)
\[1.2\times {{10}^{4}}cal\]
done
clear
View Answer play_arrow
question_answer 45) The latent heat of vaporisation of water is \[2240~J.\] If the work done in the process of vaporisation of 1 g is 168 J, then increases in internal energy is
A)
2408 J
done
clear
B)
2240 J
done
clear
C)
2072 J
done
clear
D)
1904 J
done
clear
View Answer play_arrow
question_answer 46) A body dropped from the top of a tower covers a distance \[7x\]in the last second of its journey, where \[x\] is the distance covered in first second. How much time does it take to reach the ground?
A)
3s
done
clear
B)
4s
done
clear
C)
5s
done
clear
D)
6s
done
clear
View Answer play_arrow
question_answer 47) A body just dropped from a tower explodes into two pieces of equal mass in mid-air. Which of the following is not possible?
A)
Each part will follow parabolic path
done
clear
B)
Only one part will follow parabolic path
done
clear
C)
Both parts move along a vertical line
done
clear
D)
One part reaches the ground earlier than the other
done
clear
View Answer play_arrow
question_answer 48) Moment of inertia of a uniform circular disc about a diameter is \[I.\]Its moment of inertia about an axis perpendicular to its plane and passing through a point on its rim will be
A)
57
done
clear
B)
37
done
clear
C)
6J
done
clear
D)
47
done
clear
View Answer play_arrow
question_answer 49) Two sources A and B are sounding notes of frequency 680 Hz. A listener moves from A to B with a constant velocity \[\upsilon .\]If the speed of sound 340 m/s, what must be the value of \[\upsilon .\]so that he hears 10 beats per second?
A)
2.0 m/s
done
clear
B)
2.5 m/s
done
clear
C)
3.0 m/s
done
clear
D)
3.5 m/s
done
clear
View Answer play_arrow
question_answer 50) A current of 1 A flows in a circular area of wire which subtends an angle of \[\left( \frac{3\pi }{2} \right)\text{rad}\]at its centre, whose radius is R. The magnetic induction B at the centre is
A)
\[\frac{{{\mu }_{0}}I}{R}\]
done
clear
B)
\[\frac{{{\mu }_{0}}I}{2R}\]
done
clear
C)
\[\frac{2{{\mu }_{0}}I}{R}\]
done
clear
D)
\[\frac{3{{\mu }_{0}}I}{8R}\]
done
clear
View Answer play_arrow
question_answer 51) Which of the following compounds corresponds to vant Hoff factor \[(i)\] to be equal to 2 for dilute solution?
A)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[NaHS{{O}_{4}}\]
done
clear
C)
Sugar
done
clear
D)
\[MgS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 52) \[\text{0}\text{.1 M NaCl}\]and \[\text{0}\text{.1 M C}{{\text{H}}_{\text{3}}}\text{COOH}\]are kept in separate containers. If their osmotic pressures are \[{{p}_{1}}\]and \[{{p}_{2}}\]respectively then what is the correct statement?
A)
\[{{p}_{1}}>{{p}_{2}}\]
done
clear
B)
\[{{p}_{1}}={{p}_{2}}\]
done
clear
C)
\[{{p}_{1}}<{{p}_{2}}\]
done
clear
D)
\[{{p}_{1}}={{p}_{2}}=0\,\text{atm}\]
done
clear
View Answer play_arrow
question_answer 53) Primary, secondary and tertiary alcohols may be distinguished by
A)
Fehling solution
done
clear
B)
Victor-Meyertest
done
clear
C)
Hofmanntest
done
clear
D)
Beilstein test
done
clear
View Answer play_arrow
question_answer 54) Which of the following will respond to Cannizaros reaction?
A)
2, 2-dimethylpropanal
done
clear
B)
Acetaldehyde
done
clear
C)
Propionaldehyde
done
clear
D)
Cinnamaldehyde
done
clear
View Answer play_arrow
question_answer 55) Which one of the following will increase the voltage of the cell? \[Sn(s)+2A{{g}^{+}}(aq)\xrightarrow{{}}S{{n}^{2+}}+(aq)+2Ag(s)\]
A)
Increase in the size of silver rod
done
clear
B)
Increasing the size of plate
done
clear
C)
Increase in the concentration of \[A{{g}^{+}}\]ions
done
clear
D)
Increase in the concentration of\[S{{n}^{2+}}\] ions
done
clear
View Answer play_arrow
question_answer 56) Which among the following can be purified by steam distillation?
A)
Phenol
done
clear
B)
Aniline
done
clear
C)
Benzoic acid
done
clear
D)
p-nitrophenol
done
clear
View Answer play_arrow
question_answer 57) For a chemical reaction \[A+BC,\]the thermodynamic equilibrium constant \[{{K}_{p}}\] is
A)
in \[\text{at}{{\text{m}}^{-2}}\]
done
clear
B)
in \[\text{at}{{\text{m}}^{-3}}\]
done
clear
C)
in \[\text{at}{{\text{m}}^{-1}}\]
done
clear
D)
dimensionless
done
clear
View Answer play_arrow
question_answer 58) If the equivalent weight of a trivalent metal is 32,7, the molecular weight of its chloride is
A)
68.2
done
clear
B)
103.7
done
clear
C)
204.6
done
clear
D)
32.7
done
clear
View Answer play_arrow
question_answer 59) Conjugate base of hydrazoic acid is
A)
\[HN_{3}^{-}\]
done
clear
B)
\[N_{2}^{-}\]
done
clear
C)
\[N_{3}^{-}\]
done
clear
D)
\[{{N}^{3-}}\]
done
clear
View Answer play_arrow
question_answer 60) The geometrical arrangement and shape of \[I_{3}^{-}\] are respectively
A)
trigonal bipyramidal geometry, linear shape
done
clear
B)
hexagonal geometry, T-shape
done
clear
C)
triangular planar geometry, triangular shape
done
clear
D)
tetrahedral geometry, pyramidal shape
done
clear
View Answer play_arrow
question_answer 61) Sodium reacts with water more vigorously than Li because it has
A)
higher atomic mass
done
clear
B)
more electropositive character
done
clear
C)
metallic nature
done
clear
D)
more electronegative character
done
clear
View Answer play_arrow
question_answer 62) When sodium is treated with sufficient oxygen/air, the product obtained is
A)
\[N{{a}_{2}}O\]
done
clear
B)
\[N{{a}_{2}}{{O}_{2}}\]
done
clear
C)
\[Na{{O}_{2}}\]
done
clear
D)
\[NaO\]
done
clear
View Answer play_arrow
question_answer 63) Gallium arsenide is purified by
A)
froth floatation process
done
clear
B)
van-Arkel method
done
clear
C)
zone refining method
done
clear
D)
electrolytic method
done
clear
View Answer play_arrow
question_answer 64) Which ion has the lowest radius from the. following ions?
A)
\[N{{a}^{+}}\]
done
clear
B)
\[M{{g}^{2+}}\]
done
clear
C)
\[A{{l}^{3+}}\]
done
clear
D)
\[S{{i}^{4+}}\]
done
clear
View Answer play_arrow
question_answer 65) The extraction of which of the following metals involves bessemerisation?
A)
Iron
done
clear
B)
Copper
done
clear
C)
Aluminium
done
clear
D)
Silver
done
clear
View Answer play_arrow
question_answer 66) Terylene is made by polymerisation of terephthalic acid with
A)
ethylene glycol
done
clear
B)
phenol
done
clear
C)
ethanol
done
clear
D)
catechol
done
clear
View Answer play_arrow
question_answer 67) Treatment of ammonia with excess of ethyl iodide will yield
A)
diethylamine
done
clear
B)
ethylamine
done
clear
C)
triethylamine
done
clear
D)
tetraethylammonium iodide
done
clear
View Answer play_arrow
question_answer 68) In the combustion of 2.0 g of methane, 25 kcal heat is liberated. Heat of combustion of methane would be
A)
150 kcal
done
clear
B)
200 kcal
done
clear
C)
250 kcal
done
clear
D)
350 kcal
done
clear
View Answer play_arrow
question_answer 69) Arsenic sulphide is a negative sol. The reagent with least precipitating power is
A)
\[AlC{{l}_{3}}\]
done
clear
B)
\[NaCl\]
done
clear
C)
\[Ca{{F}_{2}}\]
done
clear
D)
glucose
done
clear
View Answer play_arrow
question_answer 70) Which of the following salt when dissolved in water gets hydrolysed?
A)
\[NaCl\]
done
clear
B)
\[N{{H}_{4}}Cl\]
done
clear
C)
\[KCl\]
done
clear
D)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 71) 20 mL of a \[\text{HCl}\]solution exactly neutralizes 40 mL of 0.005 N NaOH solution. The pH of \[\text{HCl}\] solution is
A)
2.5
done
clear
B)
2.0
done
clear
C)
1.5
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 72) Which of the following is used for inducing sleep?
A)
Paracetamol
done
clear
B)
Chloroquine
done
clear
C)
Bithional
done
clear
D)
Barbituric acid derivatives
done
clear
View Answer play_arrow
question_answer 73) Which of the following acids has the smallest dissociation constant?
A)
\[C{{H}_{3}}CHFCOOH\]
done
clear
B)
\[FC{{H}_{2}}C{{H}_{2}}COOH\]
done
clear
C)
\[BrC{{H}_{2}}C{{H}_{2}}COOH\]
done
clear
D)
\[C{{H}_{3}}CHBrCOOH\]
done
clear
View Answer play_arrow
question_answer 74) Hydrolysis of benzonitrile by dilute \[\text{HCl}\]yields
A)
aniline
done
clear
B)
benzoic acid
done
clear
C)
benzamide
done
clear
D)
benzaldehyde
done
clear
View Answer play_arrow
question_answer 75) Anti-Markowhikoffs addition of HBr is not observed in
A)
propene
done
clear
B)
1-butene
done
clear
C)
but-2-ene
done
clear
D)
pent-2-ene
done
clear
View Answer play_arrow
question_answer 76) Kjeldahls method can be used for estimation of nitrogen in
A)
\[{{C}_{6}}{{H}_{5}}N{{O}_{2}}\]
done
clear
B)
pyridine
done
clear
C)
\[{{C}_{6}}{{H}_{5}}-N=N-{{C}_{6}}{{H}_{5}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 77) How many electrons in 19 K have\[n=3;l=0\]?
A)
1
done
clear
B)
2
done
clear
C)
4
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 78) Metallic bond is
A)
similar to ionic bond
done
clear
B)
similar to covalent bond
done
clear
C)
neither similar to ionic nor covalent bond
done
clear
D)
formed by the movement of positively charged spheres in a sea of electrons
done
clear
View Answer play_arrow
question_answer 79) Which of the following is not a Lewis base?
A)
\[C{{N}^{-}}\]
done
clear
B)
\[ROH\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[AlC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 80) Hydrogen can have oxidation number/s of
A)
\[-1\]only
done
clear
B)
\[+\,1\]only
done
clear
C)
0 only
done
clear
D)
\[-1,0,+1\]
done
clear
View Answer play_arrow
question_answer 81) 250 mL of a sodium carbonate solution contains 2.65 g of \[\text{N}{{\text{a}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}\text{.}\]If 10 mL of this solution is diluted to 1 L, what is the concentration of the resultant solution? (Mol. wt. of\[\text{N}{{\text{a}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}\text{=106}\])
A)
0.1 M
done
clear
B)
0.001 M
done
clear
C)
0.01 M
done
clear
D)
\[{{10}^{-4}}\,M\]
done
clear
View Answer play_arrow
question_answer 82) At constant temperature, in a given mass of an ideal gas
A)
the ratio of pressure and volume always remains constant
done
clear
B)
volume always remains constant
done
clear
C)
pressure always remains constant
done
clear
D)
the product of pressure and volume always remains constant
done
clear
View Answer play_arrow
question_answer 83) The rate of the reaction intermediates can be determined by the study of
A)
catalyst effects
done
clear
B)
concentration of the reactants
done
clear
C)
temperature effects
done
clear
D)
solvent effects
done
clear
View Answer play_arrow
question_answer 84) Order of reaction is decided by
A)
temperature
done
clear
B)
mechanism of reaction
done
clear
C)
molecularity
done
clear
D)
pressure
done
clear
View Answer play_arrow
question_answer 85) Which of the following conditions will always lead to a non-spontaneous change?
A)
\[+\,ve\text{ }\Delta H\]and \[+\,ve\text{ }\Delta S\]
done
clear
B)
\[-\,ve\text{ }\Delta H\] and \[-\,ve\text{ }\Delta S\]
done
clear
C)
\[+\,ve\text{ }\Delta H\]and \[-\,ve\text{ }\Delta S\]
done
clear
D)
\[-\,ve\text{ }\Delta H\] and\[+\,ve\text{ }\Delta S\]
done
clear
View Answer play_arrow
question_answer 86) The passage of current liberates \[{{\text{H}}_{\text{2}}}\]at cathode and \[\text{C}{{\text{l}}_{\text{2}}}\]at anode. The solution is
A)
copper chloride in water
done
clear
B)
\[\text{NaCl}\] in water
done
clear
C)
ferric chloride in water
done
clear
D)
\[\text{AuC}{{\text{l}}_{3}}\] in water
done
clear
View Answer play_arrow
question_answer 87) Compounds with \[{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{11}}}\text{N}\]as molecular formula can exhibit
A)
position isomerism
done
clear
B)
metamerism
done
clear
C)
functional isomerism
done
clear
D)
All the three
done
clear
View Answer play_arrow
question_answer 88) Which of the following species is a nucleophile?
A)
\[\overset{+}{\mathop{N}}\,{{O}_{2}}\]
done
clear
B)
\[_{.}^{.}C{{X}_{2}}\]
done
clear
C)
\[_{\centerdot }^{\centerdot }\overset{\centerdot \centerdot }{\mathop{N}}\,H_{2}^{-}\]
done
clear
D)
\[\centerdot C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 89) Which of the following carbocation is most stable?
A)
\[C{{H}_{3}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
B)
\[C{{H}_{2}}=\overset{+}{\mathop{C}}\,H\]
done
clear
C)
\[CH={{C}^{+}}\]
done
clear
D)
\[{{C}_{6}}H_{5}^{+}\]
done
clear
View Answer play_arrow
question_answer 90) Brown colour in \[\text{HN}{{\text{O}}_{\text{3}}}\]can be removed by
A)
adding Mg powder
done
clear
B)
boiling the acid
done
clear
C)
passing \[\text{N}{{\text{H}}_{\text{3}}}\]through acid
done
clear
D)
passing air through warm acid
done
clear
View Answer play_arrow
question_answer 91) What products are expected from the disproportionation reaction of hypochlorous acid?
A)
\[HCl{{O}_{3}}\]and \[C{{l}_{2}}O\]
done
clear
B)
\[HCl{{O}_{2}}\]and\[HCl{{O}_{4}}\]
done
clear
C)
\[HCl\]and \[C{{l}_{2}}O\]
done
clear
D)
\[HCl\]and \[HCl{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 92) Which of the following is not a wax?
A)
Myricyl palmitate
done
clear
B)
Tripalmitin
done
clear
C)
Myricyl cerotate
done
clear
D)
Cetyl palmitate
done
clear
View Answer play_arrow
question_answer 93) Which of the following ions forms most stable complex compound?
A)
\[F{{e}^{3+}}\]
done
clear
B)
\[M{{n}^{2+}}\]
done
clear
C)
\[N{{i}^{2+}}\]
done
clear
D)
\[C{{u}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 94) Silver plating is carried out from which of the following?
A)
\[AgN{{O}_{3}}\]
done
clear
B)
\[AgCl\]
done
clear
C)
\[K[Ag{{(CN)}_{2}}]\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 95) Which one of the following is an example of non-typical transition elements?
A)
\[Li,K,Na\]
done
clear
B)
\[Be,Al,Pb\]
done
clear
C)
\[Zn,Cd,Hg\]
done
clear
D)
\[Ba,Ga,Sr\]
done
clear
View Answer play_arrow
question_answer 96) In the reaction, \[{{C}_{6}}{{H}_{6}}\xrightarrow[AlC{{l}_{3}}]{C{{H}_{3}}Cl}A\xrightarrow{KMn{{O}_{4}}}B,\]B is
A)
benzoic acid
done
clear
B)
benzoyl chloride
done
clear
C)
benzaldehyde
done
clear
D)
chlorobenzene
done
clear
View Answer play_arrow
question_answer 97) The reaction, \[{{C}_{6}}{{H}_{5}}OH\xrightarrow[Pyridine]{C{{H}_{3}}COCl}{{C}_{6}}{{H}_{5}}OCOC{{H}_{3}}\] is called
A)
Reimer-Tiemann reaction
done
clear
B)
Schotten-Baumann reaction
done
clear
C)
acetylation
done
clear
D)
benzoylation
done
clear
View Answer play_arrow
question_answer 98) Which of the following enzymes is not useful in the digestion of proteins?
A)
Chymotripsin
done
clear
B)
Pepsin
done
clear
C)
Tripsin
done
clear
D)
Lipase
done
clear
View Answer play_arrow
question_answer 99) Which are isomers?
A)
Ethyl alcohol and dimethyl ether
done
clear
B)
Acetone and acetaldehyde
done
clear
C)
Propionic acid and propanone
done
clear
D)
Methyl alcohol and dimethyl ether
done
clear
View Answer play_arrow
question_answer 100) The dative bond is present in
A)
\[N{{H}_{3}}\]
done
clear
B)
\[S{{O}_{3}}\]
done
clear
C)
\[PC{{l}_{5}}\]
done
clear
D)
\[B{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 101) Who has developed the concept of phagocytosis in immunity?
A)
TH Huxley
done
clear
B)
E Strasburger
done
clear
C)
Ernst Haeckel
done
clear
D)
E Metchnikoff
done
clear
View Answer play_arrow
question_answer 102) Changes in the body form of some planktonic animals with seasonal changes in temperature are grouped under
A)
anamorphosis
done
clear
B)
cyclomorphosis
done
clear
C)
metamorphosis
done
clear
D)
heteromorphosis
done
clear
View Answer play_arrow
question_answer 103) Smallpox is a
A)
hereditary disease
done
clear
B)
viral disease
done
clear
C)
deficiency disease
done
clear
D)
bacterial disease
done
clear
View Answer play_arrow
question_answer 104) An animal with same generic, specific and subspecific name is
A)
man
done
clear
B)
gorilla
done
clear
C)
rabbit
done
clear
D)
elephant
done
clear
View Answer play_arrow
question_answer 105) Which of the following pairs correctly represent the grouping spermatophyta according to one of the schemes of classifying plants?
A)
Pinus, Cycas
done
clear
B)
Ginkgo, Pisum
done
clear
C)
Acacia, Casuarina
done
clear
D)
Rhizopus, Triticum
done
clear
View Answer play_arrow
question_answer 106) Trichodesmium erythreum shows phenomenon of
A)
red snow formation
done
clear
B)
red tide formation
done
clear
C)
red sea colouration
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 107) Euglena is a Protozoa because it possesses
A)
flagella
done
clear
B)
chloroplast
done
clear
C)
cell wall
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 108) Potato Dextrose Agar (PDA) medium is best for culturing
A)
saprophytic fungi
done
clear
B)
obligate fungi
done
clear
C)
aquatic fungi
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 109) Cycas differs from Pinus in producing
A)
porous wood
done
clear
B)
manoxylic wood
done
clear
C)
pycnoxylic wood
done
clear
D)
female cone
done
clear
View Answer play_arrow
question_answer 110) Red snow formation is associated with
A)
Chlamydomonas nivalis
done
clear
B)
Chlamydomonas longistigma
done
clear
C)
Chlamydomonas coccifera
done
clear
D)
Chlamydomona ooganus
done
clear
View Answer play_arrow
question_answer 111) Symmetry exhibited by sea walnuts is
A)
radial
done
clear
B)
spherical
done
clear
C)
bilateral
done
clear
D)
biradial
done
clear
View Answer play_arrow
question_answer 112) Cells not found in sponges are
A)
porocytes
done
clear
B)
myocytes
done
clear
C)
cnidoblasts
done
clear
D)
calcoblasts
done
clear
View Answer play_arrow
question_answer 113) Nematocvsts occur in
A)
ectosarc
done
clear
B)
cnidocytes
done
clear
C)
endosarc
done
clear
D)
epithelial cells
done
clear
View Answer play_arrow
question_answer 114) Precious red coral used in jewellery is
A)
Favia
done
clear
B)
Astraea
done
clear
C)
Coraltium
done
clear
D)
Meandrina
done
clear
View Answer play_arrow
question_answer 115) Which one is not a parasite?
A)
Schistosoma
done
clear
B)
Dugesia
done
clear
C)
Wuchereria
done
clear
D)
Echinococcus
done
clear
View Answer play_arrow
question_answer 116) Adult Wuchereria bancrofti lives in
A)
musdesofCizfey
done
clear
B)
human lymph nodes
done
clear
C)
salivary glands of Culex
done
clear
D)
human subdermal spaces
done
clear
View Answer play_arrow
question_answer 117) Pheretimd is
A)
volant
done
clear
B)
abyssal
done
clear
C)
arborea}
done
clear
D)
fossorial
done
clear
View Answer play_arrow
question_answer 118) Biramous appendages are present in
A)
Insecta
done
clear
B)
Crustacea
done
clear
C)
Onychophora
done
clear
D)
Cephalopoda
done
clear
View Answer play_arrow
question_answer 119) Which one of the following is correct?
A)
Arthropoda-Arachnida-Grasshopper
done
clear
B)
Mollusca-Cephalopoda-Octopus
done
clear
C)
Annelida-Hirudinea-Silverfish
done
clear
D)
Mollusca-Bivalvia-Pila
done
clear
View Answer play_arrow
question_answer 120) Select the one with incorrect class.
A)
(Asteroidea-Sea star
done
clear
B)
Ophiuroida-Brittle star
done
clear
C)
Echinoided-Sea urchin
done
clear
D)
Holothuroidea-Sea squid
done
clear
View Answer play_arrow
question_answer 121) Which one of the following is not a part of the chordate body?
A)
Urostyle
done
clear
B)
Pygostyle
done
clear
C)
Anal style
done
clear
D)
Endostyle
done
clear
View Answer play_arrow
question_answer 122) Whales are included in the same taxonomic class as a
A)
shark
done
clear
B)
sea horse
done
clear
C)
gorilla
done
clear
D)
crocodile
done
clear
View Answer play_arrow
question_answer 123) Which of the following can be seen only under the electron microscope?
A)
Ribosomes
done
clear
B)
Leucoplasts
done
clear
C)
Chloroplasts
done
clear
D)
Chromosomes
done
clear
View Answer play_arrow
question_answer 124) Desmosomes are concerned with
A)
cytolysis
done
clear
B)
cell division
done
clear
C)
cell adherence
done
clear
D)
cellular excretion
done
clear
View Answer play_arrow
question_answer 125) Centromere is required for
A)
replication of DNA
done
clear
B)
cytdplasmic cleavage
done
clear
C)
chromosome segregation
done
clear
D)
poleward movement of chromosomes
done
clear
View Answer play_arrow
question_answer 126) Chemical nature of silk is
A)
chitin
done
clear
B)
lipid
done
clear
C)
carbohydrate
done
clear
D)
protein
done
clear
View Answer play_arrow
question_answer 127) DNA strands are antiparallel because of
A)
hydrogen bonds
done
clear
B)
glycosidic bonds
done
clear
C)
disulphide bonds
done
clear
D)
phosphodiester bonds
done
clear
View Answer play_arrow
question_answer 128) Heat-resistant enzymes are known to occur in
A)
camel
done
clear
B)
viruses
done
clear
C)
kangaroo rat
done
clear
D)
blue-green algae
done
clear
View Answer play_arrow
question_answer 129) Genes A and B are necessary for normal hearing, What is the possible genotype of a normal child of deaf mother/father?
A)
AaBb
done
clear
B)
aaBB
done
clear
C)
aabb
done
clear
D)
Aabb
done
clear
View Answer play_arrow
question_answer 130) Chromosomal imbalance is most frequent during which of the following stages of human development?
A)
Foetal
done
clear
B)
Embryonic
done
clear
C)
Adult
done
clear
D)
Childhood
done
clear
View Answer play_arrow
question_answer 131) Which of the following enzymes is not required for DNA synthesis?
A)
Ligase
done
clear
B)
DNAse
done
clear
C)
DNA polymerase
done
clear
D)
RNA polymerase
done
clear
View Answer play_arrow
question_answer 132) A potent inhibitor of protein synthesis that acts as an analogue of aminoacyl tRNA is
A)
rifampicin
done
clear
B)
puromycin
done
clear
C)
mitomyocin
done
clear
D)
streptomycin
done
clear
View Answer play_arrow
question_answer 133) The immediate product of transcription in eukaryotes will be
A)
hn-RNA
done
clear
B)
mRNA
done
clear
C)
cDNA
done
clear
D)
sn-RNA
done
clear
View Answer play_arrow
question_answer 134) How many chromosomes are there in a spermatid of man?
A)
24
done
clear
B)
23
done
clear
C)
48
done
clear
D)
46
done
clear
View Answer play_arrow
question_answer 135) Which of the arbitrary values of DPD, OP and TP appear correct for a turgid cell?
A)
\[\text{DPD 02 atm; OP 07; TP 5 atm}\]
done
clear
B)
\[\text{DPD}\,\text{00}\,\text{atm;}\,\text{OP}\,\text{15}\,\text{atm;TP15}\,\text{atm}\]
done
clear
C)
\[\text{OPD}\,\text{10}\,\text{atm;}\,\text{OP}\,\text{15}\,\text{atm;TP}\,\text{6}\,\text{atm}\]
done
clear
D)
\[\text{ }\!\!~\!\!\text{ DPD 05 atm; OP 12}\,\text{atm; TP 7 atm}\]
done
clear
View Answer play_arrow
question_answer 136) The sarcoplasmic reticulum of skeletal muscle is a
A)
form of rough endoplasmic reticulum
done
clear
B)
site of glycogen storage and degradation to glucose
done
clear
C)
site into which calcium is released during muscle relaxation
done
clear
D)
site of a calcium-binding protein and a calcium-activated ATPase ,
done
clear
View Answer play_arrow
question_answer 137) Which of the following inhibit gastric \[\text{HCl}\]secretion during a meal?
A)
pistension of stomach
done
clear
B)
Distension of duodenum
done
clear
C)
Sight and smell of food
done
clear
D)
Presence of peptides in the stomach
done
clear
View Answer play_arrow
question_answer 138) As the \[{{\text{p}}_{\text{C}{{\text{O}}_{\text{2}}}}}\]of the venous blood increases the
A)
volume of the RBCs increases
done
clear
B)
concentration of \[\text{HCO}_{3}^{-}\]decreases
done
clear
C)
amount of chloride in RBCs decreases
done
clear
D)
affinity of the haemoglobin for\[{{\text{O}}_{\text{2}}}\] increases
done
clear
View Answer play_arrow
question_answer 139) Stroke volume can be decreased by
A)
increasing heart rate
done
clear
B)
decreasing blood pressure
done
clear
C)
increasing venous pressure
done
clear
D)
decreasing peripheral resistance
done
clear
View Answer play_arrow
question_answer 140) Glomerular filtration rate would be decreased by
A)
an increase in renal blood flow
done
clear
B)
compression of the renal capsule
done
clear
C)
constriction of the efferent arteriole
done
clear
D)
an increase in afferent arteriole pressure
done
clear
View Answer play_arrow
question_answer 141) Which of the following depresses heart beat?
A)
Vagus
done
clear
B)
Pericardial
done
clear
C)
Trigeminal
done
clear
D)
Spinal accessory
done
clear
View Answer play_arrow
question_answer 142) Glaucoma is due to
A)
increase in intraatrial pressure
done
clear
B)
increase in intraocular pressure
done
clear
C)
increase in intravesical pressure
done
clear
D)
increase in intraventricular pressure
done
clear
View Answer play_arrow
question_answer 143) The effect of insulin on glucose transport is to
A)
permit transport against a concentration gradient
done
clear
B)
enhance transport through ^the intestinal mucosa
done
clear
C)
enhance transport across the cell membrane
done
clear
D)
enhance transport into the brain
done
clear
View Answer play_arrow
question_answer 144) Ethylenegas
A)
slows down ripening of apples
done
clear
B)
speeds up maturation and ripening of fruits
done
clear
C)
is saturated hydrocarbon
done
clear
D)
retards ripening of tomatoes
done
clear
View Answer play_arrow
question_answer 145) The fertilized egg in human female is implanted in the uterus after
A)
one month of fertilization
done
clear
B)
two months of fertilization
done
clear
C)
three weeks of fertilization
done
clear
D)
about seven days of fertilization
done
clear
View Answer play_arrow
question_answer 146) Haustoria producing plants are
A)
commensals
done
clear
B)
ephimerals
done
clear
C)
parasites
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 147) When death rate declines, the population of
A)
old age persons increase
done
clear
B)
children increase
done
clear
C)
middle aged persons increase
done
clear
D)
children and middle aged person decrease
done
clear
View Answer play_arrow
question_answer 148) Astromatiferous leaves are seen in
A)
free-floating hydrophytes
done
clear
B)
submerged plants
done
clear
C)
emergent plants
done
clear
D)
marshy plants
done
clear
View Answer play_arrow
question_answer 149) Gas gangrene is caused by
A)
Clostridium difficile
done
clear
B)
Clostridium perfringens
done
clear
C)
Clostridium botulinum
done
clear
D)
Streptococcus pyrogenes
done
clear
View Answer play_arrow
question_answer 150) Synthesized vaccines are also called
A)
first generation vaccines
done
clear
B)
second and third generation vaccines
done
clear
C)
monoclonal vaccines
done
clear
D)
None of the above
done
clear
View Answer play_arrow