-
question_answer1) An experiment is performed to obtain the value of acceleration due to gravity g by using a simple pendulum of length L. In this experiment time for 100 oscillations is measured by using a watch of 1 second least count and the value is 90.0 seconds. The length L is measured by using a meter scale of least count 1 mm and the value is 20.0 cm. The error in the determination of g would be:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
1.7%
done
clear
B)
2.7%
done
clear
C)
4.4%
done
clear
D)
2.27%
done
clear
View Answer play_arrow
-
question_answer2) The position of a projectile launched from the origin at t = 0is given by \[\vec{r}=\left( 40\hat{i}+50\hat{j} \right)m\]at \[t=2s.\]If the projectile was launched at an angle \[\theta \] from the horizontal, then \[\theta \] is (take \[g=10\,m{{s}^{-2}}\])
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[{{\tan }^{-1}}\frac{2}{3}\]
done
clear
B)
\[{{\tan }^{-1}}\frac{3}{2}\]
done
clear
C)
\[{{\tan }^{-1}}\frac{7}{4}\]
done
clear
D)
\[{{\tan }^{-1}}\frac{4}{5}\]
done
clear
View Answer play_arrow
-
question_answer3) Water is flowing at a speed of 1.5 \[\text{m}{{\text{s}}^{\text{-1}}}\] through a horizontal tube of cross-sectional area \[\text{1}{{\text{0}}^{\text{-2}}}{{\text{m}}^{\text{2}}}\]and you are trying to stop the flow by your palm. Assuming that the water stops immediately after hitting the palm, the minimum force that you must exert should be (density of water \[\text{=1}{{\text{0}}^{3}}\text{kg}{{\text{m}}^{-3}}\])
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
15 N
done
clear
B)
22.5 N
done
clear
C)
33.7 N
done
clear
D)
45 N
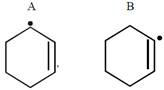
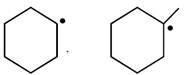
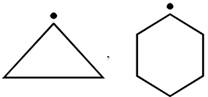
done
clear
View Answer play_arrow
-
question_answer4) A block A of mass 4 kg is placed on another block B of mass5 kg, and the block B rests on a smooth horizontal table. If the minimum force that can be applied on A so that both the blocks move together is 12 N, the maximum force that can be applied to B for the blocks to move together will be:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
30 N
done
clear
B)
25 N
done
clear
C)
27 N
done
clear
D)
48 N
done
clear
View Answer play_arrow
-
question_answer5)
Two bodies of masses 1 kg and 4 kg are connected to a vertical spring, as shown in the figure. The smaller mass executes simple harmonic motion of angular frequency 25rad/s, and amplitude 1.6 cm while the bigger mass remains stationary on the ground. The maximum force exerted by the system on the floor is (take \[g=10m{{s}^{-2}}\]) 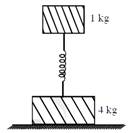 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
20 N
done
clear
B)
10 N
done
clear
C)
60 N
done
clear
D)
40 N
done
clear
View Answer play_arrow
-
question_answer6)
A cylinder of mass \[\text{Mc}\]and sphere of mass \[\text{Ms}\] are placed at points A and B of two inclines, respectively (See Figure). If they roll on the incline without sipping such that their accelerations are the same, then the ratio\[\frac{\sin {{\theta }_{c}}}{\sin {{\theta }_{s}}}\]is? 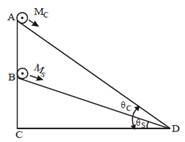 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[\sqrt{\frac{8}{7}}\]
done
clear
B)
\[\sqrt{\frac{15}{14}}\]
done
clear
C)
\[\frac{8}{7}\]
done
clear
D)
\[\frac{15}{14}\]
done
clear
View Answer play_arrow
-
question_answer7)
India's Mangalyan was sent to the Mars by launching it into a transfer orbit EOM around the sun. It leaves the ear that E and meets Mars at M. If the semi-major axis of Earth?s orbit is \[{{a}_{e}}=1.5\times {{10}^{11}}m,\] that of Mars orbit \[{{a}_{m}}=2.28\times {{10}^{11}}m,\]m, taken Kepler?s laws give the estimate of time for Mangalyan to reach Mars from Earth to be close to: 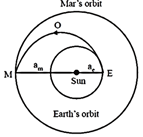 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
500 days
done
clear
B)
320 days
done
clear
C)
260 days
done
clear
D)
220 days
done
clear
View Answer play_arrow
-
question_answer8) In materials like aluminium and copper, the correct order of magnitude of various elastic modulii is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
Young's modulus < shear modulus < bulk modulus.
done
clear
B)
Bulk modulus < shear modulus < Young's modulus
done
clear
C)
Shear modulus < Young's modulus < bulk modulus.
done
clear
D)
Bulk modulus < Young's modulus < shear modulus.
done
clear
View Answer play_arrow
-
question_answer9) The amplitude of a simple pendulum, oscillating in air with a small spherical bob, decreases from 10 cm to 8 cm in 40seconds. Assuming that Stokes law is valid, and ratio of the coefficient of viscosity of air to that of carbon dioxide is 1.3. The time in which amplitude of this pendulum will reduce from 10 cm to 5 cm in carbon dioxide will be close to (In 5 =1.601, In 2 = 0.693).
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
231 s
done
clear
B)
208 s
done
clear
C)
161 s
done
clear
D)
142 s
done
clear
View Answer play_arrow
-
question_answer10) A capillary tube is immersed vertically in water and the height of the water column is x. When this arrangement is taken into a mine of depth d, the height of the water column is y. If R is the radius of earth, the ratio\[\frac{x}{y}\]is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[\left( 1-\frac{d}{R} \right)\]
done
clear
B)
\[\left( 1-\frac{2d}{R} \right)\]
done
clear
C)
\[\left( \frac{R-d}{R+d} \right)\]
done
clear
D)
\[\left( \frac{R+d}{R-d} \right)\]
done
clear
View Answer play_arrow
-
question_answer11) Water of volume 2 L in a closed container is heated with a coil of 1 kW. While water is heated, the container loses energy at a rate of 160 J/s. In how much time will the temperature of water rise from \[27{}^\circ C\] to \[77{}^\circ C\]? (Specific heat of water is 4.2 kJ/kg and that of the container is negligible).
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
8 min 20 s
done
clear
B)
6 min 2 s
done
clear
C)
7 min
done
clear
D)
14 min
done
clear
View Answer play_arrow
-
question_answer12) The equation of state for a gas is given by PV = nRT + aV, where n is the number of moles and a is a positive constant. The initial temperature and pressure of one mole of the gas contained in a cylinder are \[{{T}_{o}}\]and \[{{P}_{o}}\]respectively. The work done by the gas when its temperature doubles isobaric ally will be:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[\frac{{{P}_{o}}{{T}_{o}}R}{{{P}_{o}}-\alpha }\]
done
clear
B)
\[\frac{{{P}_{o}}{{T}_{o}}R}{{{P}_{o}}+\alpha }\]
done
clear
C)
\[{{P}_{o}}{{T}_{o}}R\ln 2\]
done
clear
D)
\[{{P}_{o}}{{T}_{o}}R\]
done
clear
View Answer play_arrow
-
question_answer13) Modern vacuum pumps can evacuate a vessel down to a pressure of \[4.0\times {{10}^{-15}}\]atm. at room temperature (300 K). Taking \[\text{R=8}\text{.0J}{{\text{K}}^{\text{-1}}}\text{mol}{{\text{e}}^{\text{-1}}}\text{,1atm=1}{{\text{0}}^{\text{5}}}\text{Pa}\]and\[{{\text{N}}_{\text{Avogadro}}}=6\times {{10}^{23}}\text{mol}{{\text{e}}^{-1}},\]the mean distance between molecules of gas in an evacuated vessel will be of the order of:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
0.2 mm
done
clear
B)
0.2 mm
done
clear
C)
0.2 cm
done
clear
D)
0.2 nm
done
clear
View Answer play_arrow
-
question_answer14) A particle which is simultaneously subjected to two perpendicular simple harmonic motions represented by\[;x={{a}_{1}}\cos \omega t\]and\[y={{a}_{2}}\cos 2\omega t\]traces a curve given by:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer15) A transverse wave is represented by\[y=\frac{10}{\pi }\sin \left( \frac{2\pi }{T}t-\frac{2\pi }{\lambda }x \right)\] For what value of the wavelength the wave velocity is twice the maximum particle velocity?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
40 cm
done
clear
B)
20 cm
done
clear
C)
10 cm
done
clear
D)
60 cm
done
clear
View Answer play_arrow
-
question_answer16) The magnitude of the average electric field normally present in the atmosphere just above the surface of the Earth is about 150 N/C, directed inward towards the center of the Earth. This gives the total net surface charge carried by the Earth to be: [Given \[{{\varepsilon }_{o}}=8.85\times {{10}^{-12}}{{C}^{2}}/N-{{m}^{2}},{{R}_{E}}=6.37\times {{10}^{6}}m\]]
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
+ 670 kC
done
clear
B)
- 670 kC
done
clear
C)
680 kC
done
clear
D)
+ 680 kC
done
clear
View Answer play_arrow
-
question_answer17) Three capacitors, each of \[3\mu F,\]are provided. These cannot be combined to provide the resultant capacitance of:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[1\mu F\]
done
clear
B)
\[2\mu F\]
done
clear
C)
\[4.5\mu F\]
done
clear
D)
\[6\mu F\]
done
clear
View Answer play_arrow
-
question_answer18) A d.c. main supply of e.m.f. 220 V is connected across a storage battery of e.m.f. 200 V through a resistance of \[1\Omega .\] The battery terminals are connected to an external resistance ?R?. The minimum value of 'R', so that a current passes through the battery to charge it is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[7\Omega \]
done
clear
B)
\[9\Omega \]
done
clear
C)
\[11\Omega \]
done
clear
D)
Zero
done
clear
View Answer play_arrow
-
question_answer19)
The mid points of two small magnetic dipoles of length d in end-on positions, are separated by a distance x, (x >> d). The force between them is proportional to \[{{x}^{-n}}\] where n is: 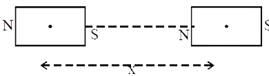 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
-
question_answer20) The magnetic field of earth at the equator is approximately \[4\times {{10}^{-5}}T.\]The radius of earth is \[6.4\times {{10}^{6}}m.\] Then the dipole moment of the earth will be nearly of the order of:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[{{10}^{23}}A{{m}^{2}}\]
done
clear
B)
\[{{10}^{20}}A{{m}^{2}}\]
done
clear
C)
\[{{10}^{16}}A{{m}^{2}}\]
done
clear
D)
\[{{10}^{10}}A{{m}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer21) When the rms voltages \[{{V}_{L}},{{V}_{C}}\]and \[{{V}_{R}}\] are measured respectively across the inductor L, the capacitor C and the resistor R in a series LCR circuit connected to an AC source, it is found that the ratio \[{{V}_{L}}:{{V}_{C}}:{{V}_{R}}=1:2:3.\]If the rms voltage of the AC sources is 100 V, the \[{{V}_{R}}\] is close to:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
50 V
done
clear
B)
70 V
done
clear
C)
90 V
done
clear
D)
100 V
done
clear
View Answer play_arrow
-
question_answer22)
Match List I (Wavelength range of electromagnetic spectrum)with List II (Method of production of these waves) and select the correct option from the options given below the lists.
| List I |
List II |
| (1) 700 nm to 1 mn |
(i) Vibration of atoms and molecules. |
| (2) 1 nm to 400 nm |
(ii) Inner shell electrons in atoms moving from one energy level to a lower level. |
| (3)\[<{{10}^{-3}}nm\] |
(iii) Radioactive decay of the nucleus. |
| (4) 1 mm to 0.1 m |
(iv) Magnetron valve. |
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
(1)-(iv), (2)-(iii), (3)-(ii), (4)-(i)
done
clear
B)
(1)-(iii), (2)-(iv), (3)-(i), (4)-(ii)
done
clear
C)
(1)-(ii), (2)-(iii), (3)-(iv), (4)-(i)
done
clear
D)
(1)-(i), (2)-(ii), (3)-(iii), (4)-(iv)
done
clear
View Answer play_arrow
-
question_answer23) A diver looking up through the water sees the outside world contained in a circular horizon. The refractive index of water is\[\frac{4}{3},\] and the diver?s eyes are 15 cm below the surface of water. Then the radius of the circle is:
A)
\[15\times 3\times \sqrt{5}cm\]
done
clear
B)
\[15\times 3\sqrt{7}cm\]
done
clear
C)
\[\frac{15\times \sqrt{7}}{3}cm\]
done
clear
D)
\[\frac{15\times 3}{\sqrt{7}}cm\]
done
clear
View Answer play_arrow
-
A)
520 nm
done
clear
B)
540 nm
done
clear
C)
560 nm
done
clear
D)
580 nm
done
clear
View Answer play_arrow
-
question_answer25) The focal lengths of objective lens and eye lens of a Galilean telescope are respectively 30 cm and 3.0 cm. telescope produces virtual, erect image of an object situated far away from it at least distance of distinct vision from the eye lens. In this condition, the magnifying power of the Galilean telescope should be:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
+ 11.2
done
clear
B)
- 11.2
done
clear
C)
8.8
done
clear
D)
+ 8.8
done
clear
View Answer play_arrow
-
question_answer26) For which of the following particles will it be most difficult to experimentally verify the de-Broglie relationship?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
an electron
done
clear
B)
a proton
done
clear
C)
an\[\text{ }\!\!\alpha\!\!\text{ }\]particle
done
clear
D)
a dust particle
done
clear
View Answer play_arrow
-
question_answer27) The binding energy of the electron in a hydrogen atom is13.6 eV, the energy required to remove the electron from the first excited state of \[\text{L}{{\text{i}}^{\text{++}}}\] is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
122.4 eV
done
clear
B)
30.6 eV
done
clear
C)
13.6 eV
done
clear
D)
3.4 eV
done
clear
View Answer play_arrow
-
question_answer28)
Identify the gate and match A, B, Y in bracket to check. 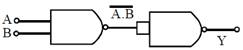 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
AND (A = 1, B = 1, Y = 1)
done
clear
B)
OR (A = 1, B = 1, Y = 0)
done
clear
C)
NOT (A = 1, B = 1, Y = 1)
done
clear
D)
XOR (A = 0, B = 0, Y = 0)
done
clear
View Answer play_arrow
-
question_answer29) A transmitting antenna at the top of a tower has height 32 m and height of the receiving antenna is 50 m. What is the maximum distance between them for satisfactory communication in line of sight (LOS) mode?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
55.4 km
done
clear
B)
45.5 km
done
clear
C)
54.5 km
done
clear
D)
455 km
done
clear
View Answer play_arrow
-
question_answer30) An n-p-n transistor has three leads A, B and C. Connecting B and C by moist fingers, A to the positive lead of an ammeter, and C to the negative lead of the ammeter, one finds large deflection. Then, A, B and C refer respectively to:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
Emitter, base and collector
done
clear
B)
Base, emitter and collector
done
clear
C)
Base, collector and emitter
done
clear
D)
Collector, emitter and base.
done
clear
View Answer play_arrow
-
question_answer31) In a face centered cubic lattice atoms A are at the corner points and atoms B at the face centered points. If atom B is missing from one of the face centered points, the formula of the ionic compound is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[A{{B}_{2}}\]
done
clear
B)
\[{{A}_{5}}{{B}_{2}}\]
done
clear
C)
\[{{A}_{2}}{{B}_{3}}\]
done
clear
D)
\[{{A}_{2}}{{B}_{5}}\]
done
clear
View Answer play_arrow
-
question_answer32) Van der Waal's equation for a gas is stated as, \[p=\frac{nRT}{V-nb}-a{{\left( \frac{n}{V} \right)}^{2}}.\] This equation reduces to the perfect gas equation,\[p=\frac{nRT}{V}\]when,
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
temperature is sufficient high and pressure is low.
done
clear
B)
temperature is sufficient low and pressure is high.
done
clear
C)
both temperature and pressure are very high.
done
clear
D)
both temperature and pressure are very low.
done
clear
View Answer play_arrow
-
question_answer33) The standard electrode potentials \[\left( E_{{{M}^{+}}/M}^{0} \right)\] of four metals A, B, C and D are - 1.2 V, 0.6 V, 0.85 V and - 0.76 V, respectively. The sequence of deposition of metals on applying potential is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
A, C, B, D
done
clear
B)
B, D, C, A
done
clear
C)
C, B, D, A
done
clear
D)
D, A, B, C
done
clear
View Answer play_arrow
-
question_answer34) At a certain temperature, only 50% HI is dissociated into \[{{H}_{2}}\]and \[{{I}_{2}}\] at equilibrium. The equilibrium constant is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
1.0
done
clear
B)
3.0
done
clear
C)
0.5
done
clear
D)
0.25
done
clear
View Answer play_arrow
-
question_answer35) Dissolving 120 g of a compound of (mol. wt. 60) in 1000 g of water gave a solution of density 1.12 g/mL. The molarity of the solution is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
1.00M
done
clear
B)
2.00 M
done
clear
C)
2.50 M
done
clear
D)
4.00 M
done
clear
View Answer play_arrow
-
question_answer36) The half-life period of a first order reaction is 15 minutes. The amount of substance left after one hour will be:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[\frac{1}{4}\]of the original amount
done
clear
B)
\[\frac{1}{8}\]of the original amount
done
clear
C)
\[\frac{1}{16}\]of the original amount
done
clear
D)
\[\frac{1}{32}\]of the original amount
done
clear
View Answer play_arrow
-
question_answer37) A current of 10.0 A flows for 2.00 h through an electrolytic cell containing a molten salt of metal X. This results in the decomposition of 0.250 mol of metal X at the cathode. The oxidation state of X in the molten salt is: (F = 96,500 C)
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
1+
done
clear
B)
2 +
done
clear
C)
3+
done
clear
D)
4 +
done
clear
View Answer play_arrow
-
question_answer38) The energy of an electron in first Bohr orbit of H-atom is -13.6 eV. The energy value of electron in the excited state of\[L{{i}^{2+}}\]is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
27.2 eV
done
clear
B)
30.6 eV
done
clear
C)
30.6 eV
done
clear
D)
27.2 eV
done
clear
View Answer play_arrow
-
question_answer39) The temperature at which oxygen molecules have the same root mean square speed as helium atoms have at 300 K is: Atomic masses: He = 4 u, O = 16 u)
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
300 K
done
clear
B)
600 K
done
clear
C)
1200 K
done
clear
D)
2400 K
done
clear
View Answer play_arrow
-
question_answer40) The standard enthalpy of formation of \[N{{H}_{3}}\] is -46.0 kJ/mol. If the enthalpy of formation of \[{{H}_{2}}\] from its atoms is -436 kJ/mol and that of \[{{N}_{2}}\] is - 712 kJ/mol, the average bond enthalpy of N - H bond in \[N{{H}_{3}}\] is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
-1102 kJ/mol
done
clear
B)
- 964 kJ/mol
done
clear
C)
+ 352 kJ/mol
done
clear
D)
+ 1056 kJ/mol
done
clear
View Answer play_arrow
-
question_answer41) The amount of oxygen in 3.6 moles of water is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
115.2 g
done
clear
B)
57.6 g
done
clear
C)
28.8 g
done
clear
D)
18.4 g
done
clear
View Answer play_arrow
-
question_answer42) The gas evolved on heating \[Ca{{F}_{2}}\]and \[Si{{O}_{2}}\] with concentrated\[{{H}_{2}}S{{O}_{4}},\]on hydrolysis gives a white gelatinous precipitate. The precipitate is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
Hydro fluosilicic acid
done
clear
B)
silica gel
done
clear
C)
silicic acid
done
clear
D)
calcium fluorosilicate
done
clear
View Answer play_arrow
-
question_answer43) Chloro compound of Vanadium has only spin magnetic moment of 1.73 BM. This Vanadium chloride has the formula:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[VC{{l}_{2}}\]
done
clear
B)
\[VC{{l}_{4}}\]
done
clear
C)
\[VC{{l}_{3}}\]
done
clear
D)
\[VC{{l}_{5}}\]
done
clear
View Answer play_arrow
-
question_answer44) An octahedral complex of \[C{{o}^{3+}}\]is diamagnetic. The hybridisation involved in the formation of the complex is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[s{{p}^{3}}{{d}^{2}}\]
done
clear
B)
\[ds{{p}^{2}}\]
done
clear
C)
\[{{d}^{2}}s{{p}^{3}}\]
done
clear
D)
\[s{{p}^{3}}d\]
done
clear
View Answer play_arrow
-
question_answer45) Which of the following is not formed when \[{{H}_{2}}S\] reacts with acidic \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]solution?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[CrS{{O}_{4}}\]
done
clear
B)
\[C{{r}_{2}}{{(S{{O}_{4}})}_{3}}\]
done
clear
C)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
D)
\[S\]
done
clear
View Answer play_arrow
-
question_answer46) Which of the following has unpaired electron(s)?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[{{N}_{2}}\]
done
clear
B)
\[O_{2}^{-}\]
done
clear
C)
\[N_{2}^{2+}\]
done
clear
D)
\[N_{2}^{2-}\]
done
clear
View Answer play_arrow
-
question_answer47)
In the following sets of reactants which two sets best exhibit the amphoteric characters of \[A{{l}_{2}}{{O}_{3}}.x{{H}_{2}}O\]?
|
Set \[1:A{{l}_{2}}{{O}_{3}}.x{{H}_{2}}O(s)\]and \[O{{H}^{-}}(aq)\]
|
|
Set \[2:A{{l}_{2}}{{O}_{3}}.x{{H}_{2}}O(s)\]and \[{{H}_{2}}O(l)\]
|
|
Set \[3:A{{l}_{2}}{{O}_{3}}.x{{H}_{2}}O(s)\]and \[{{H}^{+}}(aq)\]
|
|
Set \[4:A{{l}_{2}}{{O}_{3}}.x{{H}_{2}}O(s)\]and \[N{{H}_{3}}(aq)\]
|
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[1 and 2\]
done
clear
B)
\[1 and 3\]
done
clear
C)
\[2 and 4\]
done
clear
D)
\[3 and 4\]
done
clear
View Answer play_arrow
-
question_answer48) The number and type of bonds in \[C_{2}^{2-}\]ion in \[Ca{{C}_{2}}\] are:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
One \[\sigma \] bond and one \[\text{ }\!\!\pi\!\!\text{ -}\]bond
done
clear
B)
One \[\sigma \] bond and two \[\text{ }\!\!\pi\!\!\text{ -}\]bond
done
clear
C)
Two \[\sigma \] bond and two \[\text{ }\!\!\pi\!\!\text{ -}\]bond
done
clear
D)
Two \[\sigma \] bond and one \[\text{ }\!\!\pi\!\!\text{ -}\]bond
done
clear
View Answer play_arrow
-
question_answer49) The form of iron obtained from blast furnace is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
Steel
done
clear
B)
Cast Iron
done
clear
C)
Pig Iron
done
clear
D)
Wrought Iron
done
clear
View Answer play_arrow
-
question_answer50)
View Answer play_arrow
-
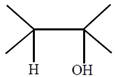
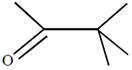
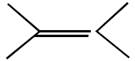
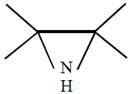
question_answer51) Which one of the following reactions will not result in the formation of carbon-carbon bond?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
Reimer-Tie man reaction
done
clear
B)
Friedel Craft's acylation
done
clear
C)
Wurtz reaction
done
clear
D)
Cannizzaro reaction
done
clear
View Answer play_arrow
-
question_answer52) In the hydrocarboration - oxidation reaction of propene with diborane, \[{{H}_{2}}{{O}_{2}}\]and NaOH, the organic compound formed is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{3}}CHOHC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}COH\]
done
clear
View Answer play_arrow
-
question_answer53)
The major product of the reaction 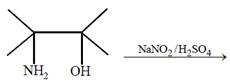 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer54) For the compounds\[C{{H}_{3}}Cl,C{{H}_{3}}Br,C{{H}_{3}}I\]and \[C{{H}_{3}}F,\]the correct order of increasing C-halogen bond length is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[C{{H}_{3}}F<C{{H}_{3}}Cl<C{{H}_{3}}Br<C{{H}_{3}}I\]
done
clear
B)
\[C{{H}_{3}}F<C{{H}_{3}}Br<C{{H}_{3}}CI<C{{H}_{3}}I\]
done
clear
C)
\[C{{H}_{3}}F<C{{H}_{3}}I<C{{H}_{3}}Br<C{{H}_{3}}Cl\]
done
clear
D)
\[C{{H}_{3}}Cl<C{{H}_{3}}Br<C{{H}_{3}}F<C{{H}_{3}}I\]
done
clear
View Answer play_arrow
-
question_answer55) Allyl phenyl ether can be prepared by heating:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[{{C}_{6}}{{H}_{5}}Br+C{{H}_{2}}=CH-C{{H}_{2}}-ONa\]
done
clear
B)
\[C{{H}_{2}}=CH-C{{H}_{2}}-Br-{{C}_{6}}{{H}_{5}}ONa\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}-CH=CH-Br-C{{H}_{3}}-ONa\]
done
clear
D)
\[C{{H}_{2}}=CH-Br-{{C}_{6}}{{H}_{5}}-C{{H}_{2}}-ONa\]
done
clear
View Answer play_arrow
-
question_answer56) In a nucleophilic substitution reaction: \[R-Br+C{{l}^{-}}\xrightarrow[{}]{DMF}R-Cl+B{{r}^{-}},\]which one of the following undergoes complete inversion of configuration?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[{{C}_{6}}{{H}_{5}}CH{{C}_{6}}{{H}_{5}}Br\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}Br\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}CHC{{H}_{3}}Br\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}CC{{H}_{3}}{{C}_{6}}{{H}_{5}}Br\]
done
clear
View Answer play_arrow
-
question_answer57) In which of the following pairs A is more stable than B?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
done
clear
B)
done
clear
C)
done
clear
D)
\[P{{h}_{3}}{{C}^{\bullet }},{{(C{{H}_{3}})}_{3}}{{C}^{\bullet }}\]
done
clear
View Answer play_arrow
-
question_answer58) Structure of some important polymers are given. Which one represents Buna-S?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[(-C{{H}_{2}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,=CH-C{{H}_{2}}-)n\]
done
clear
B)
\[(-C{{H}_{2}}-CH=CH-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ {{C}_{6}}{{H}_{5}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}\overline{n})\]
done
clear
C)
\[(-C{{H}_{2}}-CH=CH-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ CN \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-)n\]
done
clear
D)
\[{{(-C{{H}_{2}}-\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}\,=CH-C{{H}_{2}}-)}_{n}}\]
done
clear
View Answer play_arrow
-
question_answer59) Which is major product formed when acetone is heated with iodine and potassium hydroxide?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
Iodoacetone
done
clear
B)
Acetic acid
done
clear
C)
Iodoform
done
clear
D)
Acetophenone
done
clear
View Answer play_arrow
-
question_answer60) Which one of the following class of compounds is obtained by polymerization of acetylene?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
Poly-yne
done
clear
B)
Poly-ene
done
clear
C)
Poly-ester
done
clear
D)
Poly-amine
done
clear
View Answer play_arrow
-
question_answer61) Let P be the relation defined on the set of all real numbers such that \[P=\{(a,b):se{{c}^{2}}a-ta{{n}^{2}}b=1\}.\]Then P is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
reflexive and symmetric but not transitive.
done
clear
B)
reflexive and transitive but not symmetric.
done
clear
C)
symmetric and transitive but not reflexive.
done
clear
D)
an equivalence relation.
done
clear
View Answer play_arrow
-
question_answer62) Let \[w(Imw\ne 0)\]be a complex number. Then the set of all complex number z satisfying the equation\[w-\overline{w}z=k(1-z),\]for some real number k, is
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[\left\{ z:\left| z \right|=1 \right\}\]
done
clear
B)
\[\left\{ z:z=\overline{z} \right\}\]
done
clear
C)
\[\left\{ z:z\ne 1 \right\}\]
done
clear
D)
\[\left\{ z:\left| z \right|=1,z\ne 1 \right\}\]
done
clear
View Answer play_arrow
-
question_answer63) If equations \[a{{x}^{2}}+bx+c=0(a,b,c\in R,a\ne 0)\]and \[2{{x}^{2}}+3x+4=0\]have a common root, then a : b : c equals:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
: 2 : 3
done
clear
B)
2 : 3 : 4
done
clear
C)
4 : 3 : 2
done
clear
D)
3 : 2 : 1
done
clear
View Answer play_arrow
-
question_answer64) If\[\frac{1}{\sqrt{\alpha }}\]and\[\frac{1}{\sqrt{\beta }}\] are the roots of the equation,\[a{{x}^{2}}+bx+1=0\]\[(a\ne 0,a,b,\in R),\]then the equation,\[x\left( x+{{b}^{3}} \right)+\left( {{a}^{3}}-3abx \right)=0\]has roots:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[{{\alpha }^{{}^{3}/{}_{2}}}\]and\[{{\beta }^{{}^{3}/{}_{2}}}\]
done
clear
B)
\[\alpha {{\beta }^{{}^{1}/{}_{2}}}\]and\[{{\alpha }^{{}^{1}/{}_{2}}}\beta \]
done
clear
C)
\[\sqrt{\alpha \beta }\]and\[\alpha \beta \]
done
clear
D)
\[{{\alpha }^{-\frac{3}{2}}}\]and\[{{\beta }^{-\frac{3}{2}}}\]
done
clear
View Answer play_arrow
-
question_answer65)
If a, b, c are non-zero real numbers and if the system of equations
|
(a - 1) x = y + z
|
|
(b - 1) y = z + x
|
|
(c - 1) z = x + y
|
has a non-trivial solution, then ab + bc + ca equals:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
a + b + c
done
clear
B)
abc
done
clear
C)
1
done
clear
D)
- 1
done
clear
View Answer play_arrow
-
question_answer66) If B is a \[3\times 3\]matrix such that \[{{B}^{2}}=0,\]then det. \[[{{(I+B)}^{50}}-50B]\]is equal to:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
50
done
clear
View Answer play_arrow
-
question_answer67) The number of terms in the expansion of\[{{(1+x)}^{101}}{{(1+{{x}^{2}}-x)}^{100}}\]in powers of x is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
302
done
clear
B)
301
done
clear
C)
202
done
clear
D)
101
done
clear
View Answer play_arrow
-
question_answer68) The sum of the digits in the unit's place of all the 4-digitnumbers formed by using the numbers 3, 4, 5 and 6, without repetition, is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
432
done
clear
B)
108
done
clear
C)
36
done
clear
D)
18
done
clear
View Answer play_arrow
-
question_answer69) Given an A.P. whose terms are all positive integers. The sum of its first nine terms is greater than 200 and less than 220. If the second term in it is 12, then its 4th term is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
8
done
clear
B)
16
done
clear
C)
20
done
clear
D)
24
done
clear
View Answer play_arrow
-
question_answer70) If the sum\[\frac{3}{{{1}^{2}}}+\frac{5}{{{1}^{2}}+{{2}^{2}}}+\frac{7}{{{1}^{2}}+{{2}^{2}}+{{3}^{2}}}+.......+\]up to 20 terms is equalto\[\frac{k}{21},\]then k is equal to:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
120
done
clear
B)
180
done
clear
C)
240
done
clear
D)
60
done
clear
View Answer play_arrow
-
question_answer71) If f(x) is continuous and\[f\left( \frac{9}{2} \right)=\frac{2}{9},\]then \[\underset{x\to 0}{\mathop{\lim }}\,f\left( \frac{1-\cos 3x}{{{x}^{2}}} \right)\]is equal to:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[\frac{9}{2}\]
done
clear
B)
\[\frac{2}{9}\]
done
clear
C)
0
done
clear
D)
\[\frac{8}{9}\]
done
clear
View Answer play_arrow
-
question_answer72) If\[y={{e}^{nx}},\]then\[\left( \frac{{{d}^{2}}y}{d{{x}^{2}}} \right)\left( \frac{{{d}^{2}}x}{d{{y}^{2}}} \right)\]is equal to:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[n{{e}^{nx}}\]
done
clear
B)
\[n{{e}^{-nx}}\]
done
clear
C)
1
done
clear
D)
\[-n{{e}^{-nx}}\]
done
clear
View Answer play_arrow
-
question_answer73) If the Rolle's theorem holds for the function\[f(x)=2{{x}^{3}}+a{{x}^{2}}+bx\] in the interval [-1, 1] for the point\[c=\frac{1}{2},\]then the value of 2a + b is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[1\]
done
clear
B)
\[-1\]
done
clear
C)
\[2\]
done
clear
D)
\[- 2\]
done
clear
View Answer play_arrow
-
question_answer74) If \[f(x)={{\left( \frac{3}{5} \right)}^{x}}+{{\left( \frac{4}{5} \right)}^{x}}=-1,x\in R,\]then the equation f(x) =0 has :
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
no solution
done
clear
B)
one solution
done
clear
C)
two solutions
done
clear
D)
more than two solutions
done
clear
View Answer play_arrow
-
question_answer75) \[\int_{{}}^{{}}{\frac{{{\sin }^{8}}x-{{\cos }^{8}}x}{\left( 1-2\sin x{{\cos }^{2}}x \right)}}dx\]is equal to:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[\frac{1}{2}\sin 2x+c\]
done
clear
B)
\[-\frac{1}{2}\sin 2x+c\]
done
clear
C)
\[-\frac{1}{2}\sin x+c\]
done
clear
D)
\[-{{\sin }^{2}}x+c\]
done
clear
View Answer play_arrow
-
question_answer76) The integral \[\int\limits_{0}^{\frac{1}{2}}{\frac{\ln \left( 1+2x \right)}{1+4{{x}^{2}}}}dx,\]equals:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[\frac{\pi }{4}\ln 2\]
done
clear
B)
\[\frac{\pi }{8}\ln 2\]
done
clear
C)
\[\frac{\pi }{16}\ln 2\]
done
clear
D)
\[\frac{\pi }{32}\ln 2\]
done
clear
View Answer play_arrow
-
question_answer77) Let \[A=\{(x,y):{{y}^{2}}\le 4x,y-2x\ge -4\}.\]The area (in square units) of the region A is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
8
done
clear
B)
9
done
clear
C)
10
done
clear
D)
11
done
clear
View Answer play_arrow
-
question_answer78) If the differential equation representing the family of all circles touching x-axis at the origin is \[\left( {{x}^{2}}-{{y}^{2}} \right)\frac{dy}{dx}=g\left( x \right)y,\]then g(x) equals:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[\frac{1}{2}x\]
done
clear
B)
\[2{{x}^{2}}\]
done
clear
C)
\[2x\]
done
clear
D)
\[\frac{1}{2}{{x}^{2}}\]
done
clear
View Answer play_arrow
-
question_answer79) Let a and b be any two numbers satisfying\[\frac{1}{{{a}^{2}}}+\frac{1}{{{b}^{2}}}=\frac{1}{4}.\] Then, the foot of perpendicular from the origin on the variable line,\[\frac{x}{a}+\frac{y}{b}=1,\]lies on:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
a hyperbola with each semi-axis \[=\sqrt{2}\]
done
clear
B)
a hyperbola with each semi-axis = 2
done
clear
C)
a circle of radius = 2
done
clear
D)
a circle of radius \[=\sqrt{2}\]
done
clear
View Answer play_arrow
-
question_answer80) Given three points \[P, Q, R\] with \[P(5, 3)\] and R lies on the x-axis. If equation of RQ is \[x - 2y = 2\] and PQ is parallel to the x-axis, then the centroid of \[DPQR\] lies on the line:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[2x+y-9=0\text{ }~\]
done
clear
B)
\[x-2y+1=0\]
done
clear
C)
\[5x - 2y = 0\]
done
clear
D)
\[2x - 5y = 0\]
done
clear
View Answer play_arrow
-
question_answer81) If the point (1, 4) lies inside the circle \[{{x}^{2}}+{{y}^{2}}-6x-10y+p=0\] and the circle does not touch or intersect the coordinate axes, then the set of all possible values of p is the interval:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[(0, 25)\]
done
clear
B)
\[(25, 39)\]
done
clear
C)
\[(9, 25)\]
done
clear
D)
\[(25, 29)\]
done
clear
View Answer play_arrow
-
question_answer82) If OB is the semi-minor axis of an ellipse, \[{{F}_{1}}\] and \[{{F}_{2}}\] are its foci and the angle between \[{{F}_{1}}B\]and \[{{F}_{2}}B\] is a right angle, then the square of the eccentricity of the ellipse is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
(a)\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{\sqrt{2}}\]
done
clear
C)
\[\frac{1}{2\sqrt{2}}\]
done
clear
D)
\[\frac{1}{4}\]
done
clear
View Answer play_arrow
-
question_answer83) Equation of the plane which passes through the point of inter section of lines \[\frac{x-1}{3}=\frac{y-2}{1}=\frac{z-3}{2}\]and\[\frac{x-3}{1}=\frac{y-1}{2}=\frac{z-2}{3}\]and has the largest distance from the origin is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[7x\text{ }+\text{ }2y\text{ }+\text{ }4z\text{ }=\text{ }54~~\]
done
clear
B)
\[3x\text{ }+\text{ }4y\text{ }+\text{ }5z\text{ }=\text{ }49\]
done
clear
C)
\[4x\text{ }+\text{ }3y\text{ }+\text{ }5z\text{ }=\text{ }50\]
done
clear
D)
\[5x\text{ }+\text{ }4y\text{ }+\text{ }3z\text{ }=\text{ }57\]
done
clear
View Answer play_arrow
-
question_answer84) A line in the 3-dimensional space makes an angle\[\theta \left( 0<\theta \le \frac{\pi }{2} \right)\]with both the x and y axes. Then the set of all values of \[\theta \] is the interval:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[\left( 0,\frac{\pi }{4} \right]\]
done
clear
B)
\[\left[ \frac{\pi }{6},\frac{\pi }{3} \right]\]
done
clear
C)
\[\left[ \frac{\pi }{4},\frac{\pi }{2} \right]\]
done
clear
D)
\[\left( \frac{\pi }{3},\frac{\pi }{2} \right]\]
done
clear
View Answer play_arrow
-
question_answer85) If\[\left| \overset{\to }{\mathop{a}}\, \right|=2,\left| \overset{\to }{\mathop{b}}\, \right|=3\]and\[\left| 2\overset{\to }{\mathop{a}}\,-\overset{\to }{\mathop{b}}\, \right|=5,\]then\[\left| 2\overset{\to }{\mathop{a}}\,+\overset{\to }{\mathop{b}}\, \right|\] equals:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
17
done
clear
B)
7
done
clear
C)
5
done
clear
D)
1
done
clear
View Answer play_arrow
-
question_answer86) In a set of 2n distinct observations, each of the observations below the median of all the observations is increased by 5and each of the remaining observations is decreased by 3. Then the mean of the new set of observations:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
increases by 1
done
clear
B)
decreases by 1
done
clear
C)
decreases by 2
done
clear
D)
increases by 2
done
clear
View Answer play_arrow
-
question_answer87) If A and B are two events such that \[P\left( A\cup B \right)=P\left( A\cap B \right),\]then the incorrect statement amongst the following statements is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
A and B are equally likely
done
clear
B)
\[P\left( A\cap B' \right)=0\]
done
clear
C)
\[P\left( A'\cap B \right)=0\]
done
clear
D)
\[P(A)+P(B)=1\]
done
clear
View Answer play_arrow
-
question_answer88) The number of values of \[\alpha \] in \[[0,2\pi ]\]for which \[2{{\sin }^{3}}\alpha -7{{\sin }^{2}}\alpha +7\sin \alpha =2,\]is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
6
done
clear
B)
4
done
clear
C)
3
done
clear
D)
1
done
clear
View Answer play_arrow
-
question_answer89) If\[\cos ec\theta =\frac{p+q}{p-w}(p\ne q\ne 0),\]then\[\left| \cot \left( \frac{\pi }{4}+\frac{\theta }{2} \right) \right|\]then cotto:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[\sqrt{\frac{p}{q}}\]
done
clear
B)
\[\sqrt{\frac{q}{p}}\]
done
clear
C)
\[\sqrt{pq}\]
done
clear
D)
\[pq\]
done
clear
View Answer play_arrow
-
question_answer90) The contrapositive of the statement "I go to school if it does not rain" is
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
If it rains, I do not go to school.
done
clear
B)
If I do not go to school, it rains.
done
clear
C)
If it rains, I go to school.
done
clear
D)
If I go to school, it rains.
done
clear
View Answer play_arrow
 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
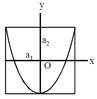
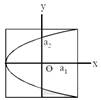
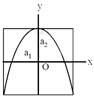
 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
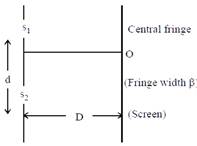
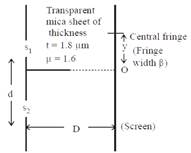
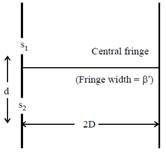 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )