question_answer 1) Threshold wavelength depends upon:
A)
Frequency of radiation
done
clear
B)
velocity of electrons
done
clear
C)
work function
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 2) A galvanometer of 25 ohms and having full scale deflection for a current of 10 mA is changed into voltmeter of range 100 volts by connecting a resistance R in series with the galvanometer. The resistance R in ohms is:
A)
10000
done
clear
B)
975
done
clear
C)
10025
done
clear
D)
9975
done
clear
View Answer play_arrow
question_answer 3) A far-sighted man who has lost his spectacles, reads a book by looking through a small hole in a sheet of paper:
A)
the hole produces an image of the letters at a longer distance
done
clear
B)
in doing so, the focal length of the eye lens is effectively decreased in doing so, the focal length of the eye
done
clear
C)
lens is effectively decreased
done
clear
D)
because in doing so, equivalent focal length remains the same as with spectacles
done
clear
View Answer play_arrow
question_answer 4) Four identical copper cubes are painted with different types of paint. Which one would you expect to lose heat rapidly, if all cubes are heated to the same temperature and are left in a vacuum?
A)
Rough white
done
clear
B)
Shinning white
done
clear
C)
Rough black
done
clear
D)
Shinning black
done
clear
View Answer play_arrow
question_answer 5) In a photometer two sources of light when placed at distances 20 cm and 30 cm respectively produce shadows of equal intensities. Their luminous intensities are in the ratio of;
A)
\[{{\left( \frac{2}{3} \right)}^{1/2}}\]
done
clear
B)
\[2/3\]
done
clear
C)
\[4/9\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 6) A moving cycle can be easily balanced. This can be explain by the law of conservation of:
A)
linear momentum
done
clear
B)
translational kinetic energy
done
clear
C)
rotational kinetic energy
done
clear
D)
angular momentum
done
clear
View Answer play_arrow
question_answer 7) The ferromagnetic substance is converted into paramagnetic substances:
A)
below the curie temperature
done
clear
B)
at the curie temperature
done
clear
C)
above the curie temperature
done
clear
D)
at the absolute zero
done
clear
View Answer play_arrow
question_answer 8) Water is supplied to different localities from a tank. The pressure of water is:
A)
less in the localities near the tank
done
clear
B)
same in different localities
done
clear
C)
more in the localities near the tank
done
clear
D)
more at higher altitudes
done
clear
View Answer play_arrow
question_answer 9) The tension in piano wire is 10 kg. What should be the tension in the wire to produce a note of double the frequency?
A)
5 kg
done
clear
B)
20 kg
done
clear
C)
40 kg
done
clear
D)
80 kg
done
clear
View Answer play_arrow
question_answer 10) There is no hole current in good conductors because:
A)
they do not have valence band
done
clear
B)
they do not have conduction band
done
clear
C)
have neither valence nor conduction bands
done
clear
D)
their valence and conduction bands overlap
done
clear
View Answer play_arrow
question_answer 11) A proton is fired through a magnetic field in a direction opposite to that of the field. The particle will:
A)
be deflected with no change in speed
done
clear
B)
be deflected with a change in speed
done
clear
C)
not be deflected but have a change in speed
done
clear
D)
neither be deflected nor have a change in speed
done
clear
View Answer play_arrow
question_answer 12) Two lenses of power +1.5 diopter and +1.0 diopter are placed in contact, the effective power of the combination is:
A)
2.5 diopter
done
clear
B)
\[\frac{3}{5}\]diopter
done
clear
C)
0.5 diopter
done
clear
D)
\[\frac{5}{3}\]diopter
done
clear
View Answer play_arrow
question_answer 13) The bulk modulus of a fluid is inversely proportional to:
A)
change in pressure
done
clear
B)
volume of fluid
done
clear
C)
mass of fluid
done
clear
D)
compressibility
done
clear
View Answer play_arrow
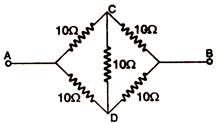
question_answer 14) What is the resistance between A and B in the figure:
A)
10\[\Omega \]
done
clear
B)
20\[\Omega \]
done
clear
C)
40\[\Omega \]
done
clear
D)
50\[\Omega \]
done
clear
View Answer play_arrow
question_answer 15) Which of the following types of wheels of same mass and radius will have largest moment of inertia?
A)
Ring
done
clear
B)
Annular disc
done
clear
C)
Solid disc
done
clear
D)
Cylindrical
done
clear
View Answer play_arrow
question_answer 16) A certain radioactive element has a half-life of 20 years. If you had a block with 10 g of the element in it, after how many years will there be just 2.5 g of the element in the block?
A)
40 years
done
clear
B)
60 years
done
clear
C)
80 years
done
clear
D)
100 years
done
clear
View Answer play_arrow
question_answer 17) If \[\] is the density of the material of the body falling through a medium of density \[{{}_{m,}}\] then the terminal velocity of the body is proportional to:
A)
\[\]
done
clear
B)
\[{{}_{m}}\]
done
clear
C)
\[\text{+}{{}_{m}}\]
done
clear
D)
\[\text{-}{{}_{m}}\]
done
clear
View Answer play_arrow
question_answer 18) Doubling of which of the following will double the frequency of oscillation of a simple pendulum?
A)
Mass of the bob
done
clear
B)
Length of the pendulum
done
clear
C)
Amplitude of oscillation
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 19) Hubbles Law is based on:
A)
Dopplers effect
done
clear
B)
Weins Law
done
clear
C)
Law of Gravitation
done
clear
D)
Stefens Law
done
clear
View Answer play_arrow
question_answer 20) A capacitor is charged by using a battery which is then disconnected. A dielectric slab is then slipped between the plates which results in:
A)
reduction of charges on the plates and increase of potential difference across the plates
done
clear
B)
increase in the potential difference across the plates reduction in stored energy but no change in the charge on the plates
done
clear
C)
decrease in the potential difference across the plates reduction in stored energy but no change in the charge on the plates
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 21) What causes the Fraunhoffers lines in the solar spectrum?
A)
Absorption of radiation
done
clear
B)
Emission of radiation
done
clear
C)
Both absorption and emission of radiation
done
clear
D)
Neither absorption nor emission of radiation
done
clear
View Answer play_arrow
question_answer 22) Two air bubbles of unequal size are connected to each other so that the air can flow from one to the other. Which of the following is likely to occur?
A)
Smaller bubble expands and larger one contracts
done
clear
B)
Smaller bubble contracts and larger one expands
done
clear
C)
No change in the size of the bubbles
done
clear
D)
Size oscillate, that is first smaller bubble expands, and then contracts and so on
done
clear
View Answer play_arrow
question_answer 23) What is the dimensional formula for the gravitational constant?
A)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{\text{-1}}}{{\text{L}}^{\text{3}}}{{\text{T}}^{\text{-2}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
B)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{\text{-1}}}{{\text{L}}^{\text{-1}}}{{\text{T}}^{\text{-3}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
C)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{\text{-1}}}{{\text{L}}^{\text{-3}}}{{\text{T}}^{\text{-1}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
D)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{\text{-2}}}{{\text{L}}^{\text{-3}}}{{\text{T}}^{\text{-2}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 24) For a stationary satellite, which of the following statement is not correct?
A)
Its height is fixed
done
clear
B)
Time period of rotation is same as that of earth
done
clear
C)
Its orbital plane is inclined at a small angle to the axis of rotation of the earth
done
clear
D)
Direction of rotation is same as that of earth
done
clear
View Answer play_arrow
question_answer 25) A transformer is used to light 140 W, 24 V lamp from 240 V AC mains. The currents in the mains is 0.7 A. The efficiency of the transformer is nearest to:
A)
60%
done
clear
B)
70%
done
clear
C)
80%
done
clear
D)
90%
done
clear
View Answer play_arrow
question_answer 26) When the liquid flows through an orifice, the rate of volume of liquid escaping out does not depend upon :
A)
height of liquid level
done
clear
B)
density of liquid
done
clear
C)
acceleration due to gravity
done
clear
D)
radius of orifice
done
clear
View Answer play_arrow
question_answer 27) In the atmosphere of the earth which of the following continuously decreases with height?
A)
Pressure
done
clear
B)
Temperature
done
clear
C)
Humidity
done
clear
D)
Wind velocity
done
clear
View Answer play_arrow
question_answer 28) If x and x are the co-ordinates of a particle in two frames of reference S and S moving with respect to each other with a velocity \[\upsilon \] along the v-axis and having the co-ordinate axes parallel to each other, then which of the following is correct relation?
A)
\[x=x\]
done
clear
B)
\[\frac{dx}{dt}=\frac{dx}{dt}\]
done
clear
C)
\[\frac{{{d}^{2}}x}{d{{t}^{2}}}=\frac{{{d}^{2}}x}{dt{{}^{2}}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 29) The resolving power of a telescope increases when:
A)
wavelength of light increases
done
clear
B)
wavelength of light decreases
done
clear
C)
focal length of the eye piece increases
done
clear
D)
focal length of eye piece decreases
done
clear
View Answer play_arrow
question_answer 30) We heat a gas sample from \[27{}^\circ C\] to \[327{}^\circ C\]. The initial average kinetic energy of the molecules was E. What will be the average kinetic energy after hearing?
A)
327E
done
clear
B)
300E
done
clear
C)
2E
done
clear
D)
\[\sqrt{2}E\]
done
clear
View Answer play_arrow
question_answer 31) The distance between virtual sources in a biprism of an angle a and refractive index \[\mu .\] of the source is placed at \[a\] distance a from it is:
A)
\[(\mu -1)\alpha \]
done
clear
B)
\[2(\mu -1)\alpha \,a\]
done
clear
C)
\[2(\mu -1)\alpha \,\]
done
clear
D)
\[(\mu -1)\alpha \,a\]
done
clear
View Answer play_arrow
question_answer 32) A flask containing air at \[27{}^\circ \] at atmosphere pressure is corked up. A pressure of 2.5 at m would force the cork out, the temperature at which it happen is :
A)
620 K
done
clear
B)
750 K
done
clear
C)
\[67.5{}^\circ C\]
done
clear
D)
\[577{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 33) The equation of a certain gas can be written as \[{{\left( \frac{{{T}^{7}}}{{{P}^{2}}} \right)}^{1/5}}=\]constant. The specific heat at constant volume of this gas is (in J/mol K)
A)
0.5R
done
clear
B)
1.5R
done
clear
C)
2R
done
clear
D)
2.5R
done
clear
View Answer play_arrow
question_answer 34) A thin glass prism of n = 1.5 is immersed in water of n = 1.33. The ratio of deviation of the ray water to that in air for the same prism is:
A)
1 : 2
done
clear
B)
1 : 4
done
clear
C)
1 : 3
done
clear
D)
1 : 8
done
clear
View Answer play_arrow
question_answer 35) The power of water pump is 4 kW. The volume of water it can raise in one minute to a height of: (Assume \[g=10\,m/{{s}^{2}}\])
A)
\[4\,{{m}^{3}}\]
done
clear
B)
\[0.1\,{{m}^{3}}\]
done
clear
C)
\[2.0\,{{m}^{3}}\]
done
clear
D)
\[2.4\,{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 36) When temperature goes down what happens to the coefficient of viscosity of liquids and gases:
A)
increases for both
done
clear
B)
decreases for both
done
clear
C)
increases for liquids and decreases for gases
done
clear
D)
decreases for liquids and increases for gases
done
clear
View Answer play_arrow
question_answer 37) Which of the following is dimensionless quantity?
A)
Strain
done
clear
B)
Stress
done
clear
C)
Quantity of heat
done
clear
D)
Specific heat
done
clear
View Answer play_arrow
question_answer 38) A bucket containing water is rotated in a vertical circle. The water does not fall out of the bucket even when it is at the top, because:
A)
weight of water is zero
done
clear
B)
sufficient time is not available for the all
done
clear
C)
inertia prevents it from falling
done
clear
D)
cohesive forces do not allow it to fall
done
clear
View Answer play_arrow
question_answer 39) The Einsteins photoelectric equation may be written as \[hv={{E}_{k+h{{v}_{0.}}}}\] Here \[{{E}_{k}}\] represents:
A)
kinetic energy of photoelectrons
done
clear
B)
mean kinetic energy of the photo- electrons
done
clear
C)
minimum kinetic energy of photo- electrons
done
clear
D)
maximum kinetic energy of the photo- electrons
done
clear
View Answer play_arrow
question_answer 40) A thin rectangular magnet suspended freely has a period of oscillation 4 s. If it is broken into two halves each having half their initial length, then when suspended similarly, the time period of oscillation of each part will be:
A)
4 s
done
clear
B)
2 s
done
clear
C)
1 s
done
clear
D)
0.25 s
done
clear
View Answer play_arrow
question_answer 41) A artificial satellite is going round the earth assumed to be uniform sphere. Its period t will depend on the density \[\rho \] by the relation (k is numerical constant)
A)
\[t=k{{\rho }^{1/2}}\]
done
clear
B)
\[t=k\rho \]
done
clear
C)
\[t=k{{\rho }^{-1}}\]
done
clear
D)
\[t=k{{\rho }^{-1/2}}\]
done
clear
View Answer play_arrow
question_answer 42) The minimum acceleration of a fireman to climb down a rope of breaking tension 0.8 of his weight is
A)
0.8 g
done
clear
B)
0.4 g
done
clear
C)
0.2 g
done
clear
D)
g
done
clear
View Answer play_arrow
question_answer 43) \[\text{M}{{\text{L}}^{\text{2}}}{{\text{T}}^{\text{3}}}{{\text{I}}^{\text{-2}}}\] is the dimension of:
A)
resistivity
done
clear
B)
resistance
done
clear
C)
potential difference
done
clear
D)
electric field
done
clear
View Answer play_arrow
question_answer 44) The period of a simple pendulum at rest is 2 second. When its period will be measured in a lift accelerating upward with \[\,2\,\,m/{{s}^{2}}\] then its period will be:
A)
\[\sqrt{\frac{40}{8}}\]
done
clear
B)
\[\sqrt{\frac{72}{40}}\]
done
clear
C)
\[\sqrt{\frac{40}{12}}\]
done
clear
D)
\[\frac{8}{4}\]
done
clear
View Answer play_arrow
question_answer 45) A body is dropped from a height rebounds to 0.81 of original height after impact, what is value of e?
A)
2
done
clear
B)
0.5
done
clear
C)
1
done
clear
D)
0.9
done
clear
View Answer play_arrow
question_answer 46) The ratio of the intensities of two simple harmonic waves is 1 : 9. The ratio of their amplitudes is:
A)
1 : 9
done
clear
B)
1 : 3
done
clear
C)
9 : 1
done
clear
D)
3 : 1
done
clear
View Answer play_arrow
question_answer 47) The displacement of a particle executing S.H.M. is \[y=a\,\sin \,(\omega t-\phi ).\] The velocity of the particle at time \[t=\frac{\phi }{\omega }\] is:
A)
\[\omega \,\cos \,\phi \]
done
clear
B)
\[a\omega \]
done
clear
C)
\[\omega \,\cos \,2\phi \]
done
clear
D)
\[-a\omega \,\cos \,2\phi \]
done
clear
View Answer play_arrow
question_answer 48) What will happen to the weight of the body at the North Pole, if the earth stops rotating about its axis?
A)
No change
done
clear
B)
Reduces to zero
done
clear
C)
Decreases but does not become zero
done
clear
D)
Increases
done
clear
View Answer play_arrow
question_answer 49) How, should the force act on a body to exert maximum torque on it?
A)
In any direction and at maximum
done
clear
B)
Distance from the axis of rotation Perpendicular to the axis of rotation with the position vector of the point of action of force also perpendicular to the axis of rotation
done
clear
C)
At maximum distance perpendicular to the axis of rotation
done
clear
D)
In any direction with the line action of the force passing through the axis of rotation
done
clear
View Answer play_arrow
question_answer 50) An ideal gas is heated from \[27{}^\circ C\] to\[627{}^\circ C\]. It initial volume was\[4{{m}^{3}}\]. Its final volume will be:
A)
\[\sqrt{2}\times 4{{m}^{3}}\]
done
clear
B)
\[8{{m}^{3}}\]
done
clear
C)
\[\sqrt{3}\times 4{{m}^{3}}\]
done
clear
D)
\[12{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 51) The force acting on a body of mass 10 kg is \[(2\mathbf{\hat{i}}+\mathbf{\hat{j}}\,-\mathbf{\hat{k}})\] N. If the body is initially at rest. The velocity at the end of the 20 sec is:
A)
\[2\sqrt{3}\]
done
clear
B)
\[3\sqrt{2}\]
done
clear
C)
\[6\sqrt{2}\]
done
clear
D)
\[2\sqrt{6}\]
done
clear
View Answer play_arrow
question_answer 52) A mass of 12 kg at rest explodes into two pieces of masses 4 kg and 8 kg. Which move in opposite directions. If the velocity of 8 kg mass is 6 m/s. Then kinetic energy of the 4 kg mass is:
A)
288 J
done
clear
B)
144 J
done
clear
C)
128 J
done
clear
D)
64 J
done
clear
View Answer play_arrow
question_answer 53) The mass of the planet is \[\frac{1}{9}\]th of the mass of the earth and its radius is half that of the earth. If a body weighs 450 N on the earth, then it is weight on the planet would be:
A)
100 N
done
clear
B)
200 N
done
clear
C)
450 N
done
clear
D)
900 N
done
clear
View Answer play_arrow
question_answer 54) An open and closed pipe have same lengths. The ratio of frequencies in their \[p\] the mode of vibration, will be :
A)
\[\frac{2p}{2p-1}\]
done
clear
B)
\[\frac{p}{2p-1}\]
done
clear
C)
\[\frac{2p}{2p+1}\]
done
clear
D)
\[\frac{p}{2p+1}\]
done
clear
View Answer play_arrow
question_answer 55) Two soap bubbles are blown. In the first soap bubble the pressure is 2 times that of the other soap bubble. The ratio of their radii will be:
A)
1 : 4
done
clear
B)
4 : 1
done
clear
C)
1 : 2
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 56) A series LCR circuit is tuned to resonance. The impedance of the circuit now is:
A)
\[{{\left[ {{R}^{2}}+{{\left( \omega L-\frac{1}{\omega C} \right)}^{2}} \right]}^{1/2}}\]
done
clear
B)
\[{{\left[ {{R}^{2}}+{{(\omega L)}^{2}}+{{\left( \frac{1}{\omega C} \right)}^{2}} \right]}^{1/2}}\]
done
clear
C)
\[{{\left[ {{R}^{2}}+{{\left( \frac{1}{\omega C}-\omega L \right)}^{2}} \right]}^{1/2}}\]
done
clear
D)
\[R\]
done
clear
View Answer play_arrow
question_answer 57) In the nuclear reaction \[_{\text{2}}\text{H}{{\text{e}}^{\text{4}}}{{\text{+}}_{\text{7}}}{{\text{N}}^{\text{14}}}\xrightarrow{{}}\text{a}{{\text{X}}^{\text{b}}}{{\text{+}}_{\text{1}}}{{\text{H}}^{\text{1}}}\] What is the value of a and b?
A)
\[a=8,b=16\]
done
clear
B)
\[a=8,b=17\]
done
clear
C)
\[a=7,b=16\]
done
clear
D)
\[a=7,b=17\]
done
clear
View Answer play_arrow
question_answer 58) A toaster and a light bulb connected in parallel. The toaster produces more heat than bulb, then the resistance of toaster is:
A)
greater than that of the bulb
done
clear
B)
equal to that of the bulb
done
clear
C)
less than that of the bulb
done
clear
D)
not comparable to that of bulb
done
clear
View Answer play_arrow
question_answer 59) Two insulated charged spheres of radii \[{{R}_{1}}\] and \[{{R}_{2}}\] having charges \[{{Q}_{1}}\] and \[{{Q}_{2}}\] respectively connected to each other. There is:
A)
an increase in the energy of the system
done
clear
B)
no change in the energy of the system
done
clear
C)
always decrease in energy
done
clear
D)
a decrease in energy of the system unless \[{{Q}_{1}}{{R}_{2}}={{Q}_{2}}{{R}_{1}}\]
done
clear
View Answer play_arrow
question_answer 60) A converging lens is used to form an image on a screen. When the upper half of the lens is covered by an opaque screen:
A)
half the image will disappear
done
clear
B)
complete image will be formed
done
clear
C)
intensity of image will increase
done
clear
D)
intensity of the image will decrease
done
clear
View Answer play_arrow
question_answer 61) Lucas reagent is a mixture of:
A)
cone. \[HCl\] + anhyd. \[ZnC{{l}_{2}}\]
done
clear
B)
cone. \[HCl\] + hydrated \[ZnC{{l}_{2}}\]
done
clear
C)
cone. \[HN{{O}_{3}}\]+ hydrated \[ZnC{{l}_{2}}\]
done
clear
D)
cone. \[HN{{O}_{3}}\]+ anhyd. \[ZnC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 62) The most abundant transition element is:
A)
Cr
done
clear
B)
Zn
done
clear
C)
Fe
done
clear
D)
W
done
clear
View Answer play_arrow
question_answer 63) Which of the following carbonates, will not decompose on heating?
A)
\[CaC{{O}_{3}}\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
C)
\[BaC{{O}_{3}}\]
done
clear
D)
\[CdC{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 64) Be exhibits the diagonal relationship with:
A)
Mg
done
clear
B)
Li
done
clear
C)
Si
done
clear
D)
Al
done
clear
View Answer play_arrow
question_answer 65) Which of the following hydroxides, is a strongest base?
A)
\[LiOH\]
done
clear
B)
\[NaOH\]
done
clear
C)
\[KOH\]
done
clear
D)
\[Ca{{(OH)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 66) Which of the following molecules, has the largest bond angle around the central atom?
A)
\[{{H}_{2}}O\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}S\]
done
clear
D)
\[{{H}_{2}}Se\]
done
clear
View Answer play_arrow
question_answer 67) A 3d-electron having spin quantum number, \[s=+\frac{1}{2},\] can have a magnetic quantum number:
A)
+ 3
done
clear
B)
- 2
done
clear
C)
+ 4
done
clear
D)
- 3
done
clear
View Answer play_arrow
question_answer 68) The kinetic energy of electron in \[H{{e}^{+}}\]is maximum in:
A)
IInd orbit
done
clear
B)
IIIrd orbit
done
clear
C)
Ist orbit
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 69) The hybrid state of sulphur in \[SO_{4}^{2-}\] is:
A)
\[s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
\[sp\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 70) Which one is the electron deficient corn pound?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[PC{{l}_{3}}\]
done
clear
C)
\[BC{{l}_{3}}\]
done
clear
D)
\[Cl{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 71) Which of the following compound would exhibit co-ordination isomerism?
A)
\[[Cr{{(en)}_{2}}]\,N{{O}_{2}}\]
done
clear
B)
\[[Cr{{({{H}_{2}}O)}_{6}}]\,C{{l}_{3}}\]
done
clear
C)
\[[Cr{{(N{{H}_{3}})}_{6}}]\,\,[Co{{(CN)}_{6}}]\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 72) The blue complex formed on addition of cone. \[N{{H}_{4}}OH\] solution to a \[C{{u}^{2+}}\] salt solution has the structure:
A)
\[{{[Cu\,{{(N{{H}_{3}})}_{2}}]}^{2+}}\]
done
clear
B)
\[{{[Cu\,{{(N{{H}_{3}})}_{4}}]}^{2+}}\]
done
clear
C)
\[{{[Cu\,{{(N{{H}_{4}})}_{4}}]}^{2+}}\]
done
clear
D)
\[{{[Cu\,{{(N{{H}_{4}})}_{2}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 73) Which of the substance, Na, Hg, S, Pt and graphite, can be used as electrodes in electrolytic cells having aqueous solution?
A)
Hg, Pt and graphite
done
clear
B)
Na, S and Hg
done
clear
C)
Pt and graphite only
done
clear
D)
Na and S only
done
clear
View Answer play_arrow
question_answer 74) The most stable oxidation state of Fe is:
A)
+ 2
done
clear
B)
+ 3
done
clear
C)
- 2
done
clear
D)
- 3
done
clear
View Answer play_arrow
question_answer 75) Which of the following ion in aqueous medium has orange colour?
A)
\[C{{r}^{+3}}\]
done
clear
B)
\[C{{r}_{2}}O_{7}^{2-}\]
done
clear
C)
\[MnO_{4}^{-}\]
done
clear
D)
\[MnO_{4}^{2-}\]
done
clear
View Answer play_arrow
question_answer 76) Which of the following is used for galvanizing iron?
A)
Cr
done
clear
B)
Zn
done
clear
C)
Ni
done
clear
D)
Sn
done
clear
View Answer play_arrow
question_answer 77) Which of the following does not have S?S bond?
A)
\[{{S}_{2}}O_{3}^{2-}\]
done
clear
B)
\[{{S}_{2}}O_{6}^{2-}\]
done
clear
C)
\[{{S}_{2}}O_{4}^{2-}\]
done
clear
D)
\[{{S}_{2}}O_{7}^{2-}\]
done
clear
View Answer play_arrow
question_answer 78) Which of the following cannot be hydrolyzed?
A)
\[GeC{{l}_{4}}\]
done
clear
B)
\[SnC{{l}_{4}}\]
done
clear
C)
\[SiC{{l}_{4}}\]
done
clear
D)
\[CC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 79) What is the oxidation number of chromium in chromyl chloride?
A)
+ 3
done
clear
B)
+ 6
done
clear
C)
+ 4
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 80) The second most electronegative element is:
A)
F
done
clear
B)
Cl
done
clear
C)
O
done
clear
D)
N
done
clear
View Answer play_arrow
question_answer 81) In which of the following state of matter, the average distance between the molecules lies between \[{{10}^{-5}}\]cm to \[{{10}^{-7}}\]cm?
A)
Liquid
done
clear
B)
Gas
done
clear
C)
Solid
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 82) The volume of the resulting solution obtained by mixing 25 ml of acetone and 25ml of \[CHC{{l}_{3}}\]will be:
A)
50 ml
done
clear
B)
< 50 ml
done
clear
C)
> 50 ml
done
clear
D)
unpredictable
done
clear
View Answer play_arrow
question_answer 83) The \[\beta -\]decay of \[{}_{11}{{N}^{24}}\]will produce an isotope of:
A)
Mg
done
clear
B)
Ne
done
clear
C)
Na
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 84) Which of the following statement is true for degree of dissociation of weak electrolytes?
A)
it is inversely proportional to the initial concentration of the electrolyte
done
clear
B)
it is directly proportional to the initial concentration of the electrolyte
done
clear
C)
it is independent of the initial concentration of the electrolyte
done
clear
D)
it depends on the equilibrium concentration of electrolyte
done
clear
View Answer play_arrow
question_answer 85) Which of the following quantities are equal at equilibrium under the particular set of conditions?
A)
\[\Delta H\] and \[\Delta G\]
done
clear
B)
\[\Delta H\] and \[\Delta S\]
done
clear
C)
\[\Delta G\] and \[\Delta S\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 86) A chemical reaction will not occur at all if the conditions are chosen so that:
A)
enthalpy change and entropy change, are both negative
done
clear
B)
enthalpy change and entropy change, are both positive
done
clear
C)
enthalpy change is negative and entropy change is positive
done
clear
D)
enthalpy change is positive and entropy change is negative
done
clear
View Answer play_arrow
question_answer 87) For a hypothetical reaction, \[aA+bB\xrightarrow{{}}\text{Product,}\] the rate law is \[r=k\,{{[A]}^{x}}{{[B]}^{y}},\]then:
A)
\[(a+b)\,>(x+y)\]
done
clear
B)
\[(a+b)\,>(x+y)\]
done
clear
C)
\[(a+b)=(x+y)\]
done
clear
D)
\[(a+b)\] may or may not be equal to \[(x+y)\]
done
clear
View Answer play_arrow
question_answer 88) For an endothermic reaction, carried out in a closed vessel:
A)
\[\Delta H>0\]
done
clear
B)
\[\Delta H<0\]
done
clear
C)
\[\Delta H=\Delta E\]
done
clear
D)
\[\Delta H=0\]
done
clear
View Answer play_arrow
question_answer 89) The order of a reaction, with respect to one of the reacting component, \[\Upsilon \] is zero. It implies that:
A)
the reaction is going on at a constant rate
done
clear
B)
the rate of reaction does not vary with temperature
done
clear
C)
the reaction rate is independent of the concentration of \[\Upsilon \]
done
clear
D)
the rate of formation of activated complex is zero
done
clear
View Answer play_arrow
question_answer 90) The existence of line spectrum of hydrogen provided an evidence that:
A)
there is absorption of energy
done
clear
B)
the electrons do not lose energy while coming back in stationary states
done
clear
C)
the energy of electron is quantized
done
clear
D)
there is an emission of energy when hydrogen is heated
done
clear
View Answer play_arrow
question_answer 91) The basic principle involved in hydrogen bomb is:
A)
nuclear fission
done
clear
B)
nuclear fusion
done
clear
C)
nuclear disintegration
done
clear
D)
nuclear spallation
done
clear
View Answer play_arrow
question_answer 92) For the transformation, \[{}_{7}{{N}^{14}}+...\xrightarrow{{}}{}_{6}{{C}^{14}}+{}_{1}{{H}^{1}}\] the bombarding particle is:
A)
proton
done
clear
B)
neutron
done
clear
C)
deuteron
done
clear
D)
electron
done
clear
View Answer play_arrow
question_answer 93) Emulsifier is an agent which:
A)
homogenesis the emulsion
done
clear
B)
stabilise the emulsion
done
clear
C)
accelerates the dispersion
done
clear
D)
breaks up the emulsion
done
clear
View Answer play_arrow
question_answer 94) A solution of \[5\times {{10}^{-8}}M\]HCl has pH value:
A)
7
done
clear
B)
7.3010
done
clear
C)
6.9
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 95) Two electrodes \[{{A}^{2+}}\text{/}A\] and \[{{B}^{+}}\text{/}B\] have \[E_{red}^{\text{o}},\]\[0.62\,V\]and \[-\,1.25\,V\]respectively. The e.m.f. of cell will be:
A)
0.63 V
done
clear
B)
- 0.63 V
done
clear
C)
1.87 V
done
clear
D)
data are insufficient
done
clear
View Answer play_arrow
question_answer 96) The molar conductivity of an aqueous solution of strong electrolyte:
A)
bears no relationship with concentration
done
clear
B)
decreases with increase in dilution
done
clear
C)
remains constant at all concentration
done
clear
D)
increases with dilution
done
clear
View Answer play_arrow
question_answer 97) Out of molality (m), molarity (M), formality (F), normality (N) and mole-fraction (x), the two quantities, independent of temperature, are:
A)
N, m
done
clear
B)
m, x
done
clear
C)
m, N
done
clear
D)
F, N
done
clear
View Answer play_arrow
question_answer 98) The isotonic solutions have equal:
A)
osmotic pressure
done
clear
B)
vapour pressure
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 99) When formaldehyde is heated with ammonia, the compound formed is:
A)
methyl amine
done
clear
B)
amino formaldehyde
done
clear
C)
hexamethylene tetramine
done
clear
D)
formaline
done
clear
View Answer play_arrow
question_answer 100) Which of the following is not found in alkenes?
A)
Chain isomerism
done
clear
B)
Geometrical isomerism
done
clear
C)
Tautomerism
done
clear
D)
Position isomerism
done
clear
View Answer play_arrow
question_answer 101) Which of the following is the weakest base?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
D)
all are equally basic
done
clear
View Answer play_arrow
question_answer 102) Which of the following alcohol, cannot be dehydrated to give alkene?
A)
t-butyl alcohol
done
clear
B)
Neopentyl alcohol
done
clear
C)
Isopropyl alcohol
done
clear
D)
all of these can be dehydrated
done
clear
View Answer play_arrow
question_answer 103) A paste of bleaching powder on heating with ethanol gives:
A)
chloroform
done
clear
B)
ethylene
done
clear
C)
carbon tetrachloride
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 104) A mixture of equal parts of enantiomers, is called:
A)
configuration
done
clear
B)
resolution
done
clear
C)
racemic mixture
done
clear
D)
meso mixture
done
clear
View Answer play_arrow
question_answer 105) Which of the following acids, will be able to give silver mirror test?
A)
Acetic acid
done
clear
B)
Butyric acid
done
clear
C)
Formic acid
done
clear
D)
Carboxylic acids do not give this test
done
clear
View Answer play_arrow
question_answer 106) The hybrid state of positively charged carbon in vinyl carbocation is \[(C{{H}_{2}}=C{{H}^{+}}--)\]:
A)
\[s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
\[sp\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 107) The conjugate acid of \[C{{H}_{3}}N{{H}_{2}}\]is:
A)
\[C{{H}_{3}}NH_{3}^{+}\]
done
clear
B)
\[C{{H}_{3}}N{{H}^{-}}\]
done
clear
C)
\[C{{H}_{3}}OH\]
done
clear
D)
\[NH_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 108) In alkenes, the negative part of addendum adds on to the carbon atom joined to the least number of hydrogen atoms. This statement is called:
A)
Peroxide effect
done
clear
B)
Baeyers strain theory
done
clear
C)
Wallachs rule
done
clear
D)
Markownikoffs rule
done
clear
View Answer play_arrow
question_answer 109) The reaction, \[{{C}_{6}}{{H}_{5}}{{N}_{2}}Cl\xrightarrow{C{{u}_{2}}C{{l}_{2}}/HCl}{{C}_{2}}{{H}_{5}}Cl+{{N}_{2}}\uparrow \] is called:
A)
Sandmeyers reaction
done
clear
B)
Gattermann reaction
done
clear
C)
Gattermann-Koch reaction
done
clear
D)
Lederrer Mannase reaction
done
clear
View Answer play_arrow
question_answer 110) The Kolbes synthesis of ethane involves :
A)
electrophilic substitution
done
clear
B)
nucelophilic addition
done
clear
C)
free-radical addition
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 111) Isopropyl alcohol, on mild oxidation, gives:
A)
acetone
done
clear
B)
propanoic acid
done
clear
C)
propene
done
clear
D)
propane
done
clear
View Answer play_arrow
question_answer 112) Ethyl amine reacts with \[HN{{O}_{2}}\] to give:
A)
methanol
done
clear
B)
ethanol
done
clear
C)
nitrosoethyl amine
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 113) The number of possible optical isomers of lactic acid are:
A)
2
done
clear
B)
4
done
clear
C)
many
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 114)
The reaction,
A)
Claisen condensation
done
clear
B)
Claisen rearrangement
done
clear
C)
Perkin reaction
done
clear
D)
Benzoin condensation
done
clear
View Answer play_arrow
question_answer 115) Which of the following is not an explosive?
A)
nitroglycerine
done
clear
B)
o-aminotoluene
done
clear
C)
dyanamite
done
clear
D)
trinitrotoluene
done
clear
View Answer play_arrow
question_answer 116) Riboflavin is:
A)
enzyme
done
clear
B)
hormone
done
clear
C)
co-enzyme
done
clear
D)
vitamin
done
clear
View Answer play_arrow
question_answer 117) The law of electrolysis were given by:
A)
Coulomb
done
clear
B)
Faraday
done
clear
C)
Kohlrausch
done
clear
D)
Soddy
done
clear
View Answer play_arrow
question_answer 118) The entropy of a solid is zero at absolute zero temperature. This statement is given by:
A)
zeroth law of thermodynamics
done
clear
B)
Ist law of thermodynamics
done
clear
C)
IInd law of thermodynamics
done
clear
D)
IIIrd law of thermodynamics
done
clear
View Answer play_arrow
question_answer 119) Carborundum is the commerical name of:
A)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
B)
\[HBr\]
done
clear
C)
\[BN\]
done
clear
D)
\[SiC\]
done
clear
View Answer play_arrow
question_answer 120) Gun metal is an alloy of copper and tin with:
A)
iron
done
clear
B)
magnesium
done
clear
C)
zinc
done
clear
D)
nickel
done
clear
View Answer play_arrow
question_answer 121) What causes the liquid part of blood filter out form glomerulus into the renal tubule?
A)
Diapedesis
done
clear
B)
Pelvis
done
clear
C)
Hydrostatic pressure
done
clear
D)
Dialysis
done
clear
View Answer play_arrow
question_answer 122) Which is not a connecting link in evolution?
A)
Pheretima
done
clear
B)
Ecdina
done
clear
C)
Sphenodon
done
clear
D)
Peripatus
done
clear
View Answer play_arrow
question_answer 123) Which of the following sets represents all endodermal structures?
A)
Liver, brain, spinal cord
done
clear
B)
Kidney, liver, pancreas
done
clear
C)
Lung, kidney, brain
done
clear
D)
Liver, lung, pancreas
done
clear
View Answer play_arrow
question_answer 124) Which one is replacing bone?
A)
Exoccipital
done
clear
B)
Pterygoid
done
clear
C)
Squamosal
done
clear
D)
Vomer
done
clear
View Answer play_arrow
question_answer 125) The adult male Ascaris can be identified by:
A)
five pair of post-anal papillae
done
clear
B)
pineal setae
done
clear
C)
fifty pairs of pre-anal papillae
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 126) Which one of the following organs are homologous?
A)
Tail of scorpion, tail of bird, tail of monkey
done
clear
B)
Wing of butterfly, wing of flying fish, wing of bird
done
clear
C)
Paddles of whale, front legs of horse and arms of man
done
clear
D)
Sting of honeybee, sting of scorpion and poison fangs of snakes
done
clear
View Answer play_arrow
question_answer 127) Which of the following pairs of specific names belongs to same common genus?
A)
histolytica and rocktertil
done
clear
B)
histolytica and falciparum
done
clear
C)
histolytica and coli
done
clear
D)
histolytica and protons
done
clear
View Answer play_arrow
question_answer 128) An experiment to prove that organic compounds were the basis of life was performed by:
A)
Miller
done
clear
B)
Fox
done
clear
C)
Oparin
done
clear
D)
Malvin
done
clear
View Answer play_arrow
question_answer 129) 80S ribosomes have two sub units which are:
A)
20S and 60S
done
clear
B)
30S and 50S
done
clear
C)
40S and 60S
done
clear
D)
40S and 40S
done
clear
View Answer play_arrow
question_answer 130) Ureotelic animals excrete out:
A)
urea
done
clear
B)
uric add
done
clear
C)
amino acids
done
clear
D)
ammonia
done
clear
View Answer play_arrow
question_answer 131) Which of the following stages is the longest in animals?
A)
Leptotcne
done
clear
B)
Prophase
done
clear
C)
Pachytene
done
clear
D)
Telophase
done
clear
View Answer play_arrow
question_answer 132) Which is a true fish?
A)
Cat fish
done
clear
B)
Silver fish
done
clear
C)
Cuttle fish
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 133) When a man starves, he first of all consumes to the stored:
A)
protein
done
clear
B)
carbohydrate
done
clear
C)
glycogen
done
clear
D)
fat
done
clear
View Answer play_arrow
question_answer 134) Gigantism and acromegaly are due to improper functioning of:
A)
kidney
done
clear
B)
pituitary
done
clear
C)
thyroid
done
clear
D)
adrenal
done
clear
View Answer play_arrow
question_answer 135) Gonadotrophins are secreted from:
A)
posterior pituitary
done
clear
B)
gonads
done
clear
C)
adrenal
done
clear
D)
anterior pituitary
done
clear
View Answer play_arrow
question_answer 136) Amphibians arose in:
A)
Devonian
done
clear
B)
Carboniferous
done
clear
C)
Permian
done
clear
D)
Jurrasic
done
clear
View Answer play_arrow
question_answer 137) Which of the following are exclusively marine?
A)
Porifera
done
clear
B)
Amphibians
done
clear
C)
Echinodermates
done
clear
D)
Mollusca
done
clear
View Answer play_arrow
question_answer 138) No special respiratory organs are found in:
A)
mosquito
done
clear
B)
cockroach
done
clear
C)
earthworm
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 139) Thiamine is a/an:
A)
enzyme
done
clear
B)
sugar
done
clear
C)
vitamin of B complex
done
clear
D)
nitrogen base
done
clear
View Answer play_arrow
question_answer 140) Chromosomes are associated with proteins called:
A)
histones
done
clear
B)
non- histones
done
clear
C)
heteroproteins
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 141) Cisternae are a part of:
A)
golgi apparatus
done
clear
B)
mitochondria
done
clear
C)
chloroplasts
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 142) In a plant cell, starch is stored by:
A)
amyloplasts
done
clear
B)
eleoplasts
done
clear
C)
chloroplasts
done
clear
D)
aleuronplasts
done
clear
View Answer play_arrow
question_answer 143) The joint between numerous and ulna is:
A)
pivot
done
clear
B)
hinge
done
clear
C)
ball and socket
done
clear
D)
gliding
done
clear
View Answer play_arrow
question_answer 144) Fishes, amphibians and reptiles have in common:
A)
eggs
done
clear
B)
gills
done
clear
C)
scales
done
clear
D)
both (a) and (c)
done
clear
View Answer play_arrow
question_answer 145) Leyding cells secrete:
A)
glycogen
done
clear
B)
oxytocin
done
clear
C)
\[HCl\]
done
clear
D)
testosterone
done
clear
View Answer play_arrow
question_answer 146) Natural parthenogenesis is seen in:
A)
hen
done
clear
B)
honeybee
done
clear
C)
frog
done
clear
D)
cockroach
done
clear
View Answer play_arrow
question_answer 147) Branch of zoology dealing with study of fossils is:
A)
eugenics
done
clear
B)
palaeozoology
done
clear
C)
anatomy
done
clear
D)
ecology
done
clear
View Answer play_arrow
question_answer 148) Chloragogen cells of Pheretima are equivalent to:
A)
spleen
done
clear
B)
kidney
done
clear
C)
liver
done
clear
D)
sweat gland
done
clear
View Answer play_arrow
question_answer 149) Purkinje fibres are:
A)
elastinfibres
done
clear
B)
collagen fibres
done
clear
C)
musclefibres
done
clear
D)
nerve fibres
done
clear
View Answer play_arrow
question_answer 150) Linkage in Drosophila was first discovered by:
A)
Strutevant
done
clear
B)
Bridges
done
clear
C)
Bateson and Punnet
done
clear
D)
Morgan
done
clear
View Answer play_arrow
question_answer 151) The structure formed by bacterial genomeis called:
A)
nucleoside
done
clear
B)
nucleolus
done
clear
C)
nucleoid
done
clear
D)
nucleus
done
clear
View Answer play_arrow
question_answer 152) In Rhizopuszygospore germinates to produce:
A)
mycelium
done
clear
B)
heterothallus
done
clear
C)
promycelium
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 153) Pyrenoids are found in one of the following organs in Spirogyra:
A)
cell wall
done
clear
B)
chloroplast
done
clear
C)
cytoplasm
done
clear
D)
vacuole
done
clear
View Answer play_arrow
question_answer 154) The leaves of Mimosa pudica drop down on touch because:
A)
the leaves tissues are injured
done
clear
B)
the plant has nervous system
done
clear
C)
the leaves are very tender
done
clear
D)
the turgor pressure of leaf base changes
done
clear
View Answer play_arrow
question_answer 155) Mineral, salts are absorbed in the form of:
A)
compounds
done
clear
B)
ions
done
clear
C)
mixtures
done
clear
D)
molecules
done
clear
View Answer play_arrow
question_answer 156) Transpiration pull and cohesion tension theory is responsible for:
A)
osmotic absorption
done
clear
B)
passive absorption
done
clear
C)
active absorption
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 157) When a cell is fully turgid which of the following will be zero:
A)
W.P.
done
clear
B)
O.P.
done
clear
C)
T.P.
done
clear
D)
DPD
done
clear
View Answer play_arrow
question_answer 158) Photoperiodism is associated with the formation of:
A)
gibberellins
done
clear
B)
florigen
done
clear
C)
chlorophyll
done
clear
D)
auxins
done
clear
View Answer play_arrow
question_answer 159) Substance which originate at the tip of stem and control growth elsewhere are:
A)
vitamins
done
clear
B)
enzymes
done
clear
C)
hormones
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 160) The simple mechanical tissue of living cells is:
A)
sclerenchyma
done
clear
B)
parenchyma
done
clear
C)
collenchyma
done
clear
D)
chlorenchyma
done
clear
View Answer play_arrow
question_answer 161) The function of cork cambium is to produce:
A)
cork and secondary cortex
done
clear
B)
secondary xylem and secondary phloem
done
clear
C)
cork
done
clear
D)
secondary cortex and phelIoderm
done
clear
View Answer play_arrow
question_answer 162) Conjoint, collateral, endarch and open vascular bundles are found in:
A)
dicot root
done
clear
B)
monocot root
done
clear
C)
monocot stem
done
clear
D)
dicot stem
done
clear
View Answer play_arrow
question_answer 163) In funaria spore germinates to produce:
A)
main plant
done
clear
B)
protonema
done
clear
C)
sporangium
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 164) Prothallus of Dryoptcris bears:
A)
archegonia
done
clear
B)
antheridia
done
clear
C)
sporangia
done
clear
D)
both (a) and (b)
done
clear
View Answer play_arrow
question_answer 165) Seeds of Pinus have:
A)
two cotyledons
done
clear
B)
three cotyledons
done
clear
C)
many cotyledons
done
clear
D)
one cotyledons
done
clear
View Answer play_arrow
question_answer 166) Seeds of Brassica campestris provide:
A)
oil and oil cake
done
clear
B)
medicines
done
clear
C)
oil
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 167) Renewable natural resources are:
A)
air and gases
done
clear
B)
metallic, ores, fossil, fuels
done
clear
C)
water, wood, natural vegetation
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 168) Soil formation occurs by:
A)
weathering
done
clear
B)
pedogenesis
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 169) One of the basis of variation is:
A)
equational separation of chromosomes
done
clear
B)
pairing and segregation of chromosomes
done
clear
C)
disjuntional separation of chromosomes
done
clear
D)
crossing over during meiosis
done
clear
View Answer play_arrow
question_answer 170) Diploid cell which undergo meiosis, is:
A)
zygote
done
clear
B)
antheridia
done
clear
C)
somatic cell
done
clear
D)
gamete
done
clear
View Answer play_arrow
question_answer 171) An individual with the genotype Aa Bb will produce:
A)
all gametes of same type
done
clear
B)
four types of gametes
done
clear
C)
two gametes of one type and remaining gametes of second type
done
clear
D)
two types of gametes
done
clear
View Answer play_arrow
question_answer 172) Trisomy of 21st chromosome leads to:
A)
Downs syndrome
done
clear
B)
Turners syndrome
done
clear
C)
Edwards syndrome
done
clear
D)
none of above
done
clear
View Answer play_arrow
question_answer 173) Almost all bones provide support and protection to soft body parts but which of the following is concerned with another function?
A)
Atlas
done
clear
B)
Rib
done
clear
C)
Radius
done
clear
D)
Malleus
done
clear
View Answer play_arrow
question_answer 174) Blastopore in frogs development occurs in:
A)
blastula and opens in blastocoel
done
clear
B)
gastrula and opens in archentron
done
clear
C)
blastula and opens in archenteron
done
clear
D)
gastrula and opens in blastocoel
done
clear
View Answer play_arrow
question_answer 175) Which of the following is responsible for emotional state such as fear, anger, pain and causes rise in blood pressure and rate of heart beat?
A)
Thyroxin
done
clear
B)
Insulin
done
clear
C)
Adrenalin
done
clear
D)
Progesterone
done
clear
View Answer play_arrow
question_answer 176) A person having blood group B can take blood from personal having blood groups:
A)
B only
done
clear
B)
AB and B
done
clear
C)
B and O
done
clear
D)
AB and A
done
clear
View Answer play_arrow
question_answer 177) The infective stage of Plasmodium vivax in man is:
A)
Sporozoite
done
clear
B)
Cryptozoite
done
clear
C)
Merozoite
done
clear
D)
Trophozoite
done
clear
View Answer play_arrow
question_answer 178) Portuguese man of war is:
A)
portuguese soldier
done
clear
B)
a sponge
done
clear
C)
a cocelentrate
done
clear
D)
soldiers of first world war
done
clear
View Answer play_arrow
question_answer 179) Elementary particles occur:
A)
in mitrochondria
done
clear
B)
in the cytoplasm
done
clear
C)
in chloroplast
done
clear
D)
in all of these
done
clear
View Answer play_arrow
question_answer 180) Which of the following shows heterotrichous habit?
A)
Ectocarpus
done
clear
B)
Euglina
done
clear
C)
Ulothrix
done
clear
D)
Spirogyra
done
clear
View Answer play_arrow
question_answer 181) Directions: Pick one of the choices which complete each of the following sentences correctly. She asked him when ...... ready:
A)
will he be
done
clear
B)
would he be
done
clear
C)
make not
done
clear
D)
not make
done
clear
View Answer play_arrow
question_answer 182) Directions: Pick one of the choices which complete each of the following sentences correctly. You are asked to write ...... ink:
A)
with
done
clear
B)
by
done
clear
C)
in
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 183) Directions: Pick one of the choices which complete each of the following sentences correctly. He will arrive ...... a few days:
A)
on
done
clear
B)
after
done
clear
C)
in
done
clear
D)
by
done
clear
View Answer play_arrow
question_answer 184) Directions: Pick one of the choices which complete each of the following sentences correctly. The child ...... the mother a lot:
A)
resembles with
done
clear
B)
resembles by
done
clear
C)
resembles at
done
clear
D)
resembles
done
clear
View Answer play_arrow
question_answer 185) Directions: Pick one of the choices which complete each of the following sentences correctly. I ...... experiencing trouble with my machine
A)
have been experiencing
done
clear
B)
have experienced
done
clear
C)
had experienced
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 186) What is meant by temper in the passage?
A)
Trend
done
clear
B)
Spirit
done
clear
C)
Tone
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 187) What is immediate problem?
A)
Technological advancement
done
clear
B)
Removal of poverty
done
clear
C)
Changes in social customs
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 188) The title of the passage should be:
A)
Social economic, technological advancement for removing poverty
done
clear
B)
Removal of poverty
done
clear
C)
Poverty
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 189) The main idea of the passage is ......
A)
Removal of poverty needs social, economic and technological advancement
done
clear
B)
We must remove poverty
done
clear
C)
Technological advancement is necessary
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 190) What is meant by combat poverty:
A)
Recognizing poverty
done
clear
B)
Removing poverty
done
clear
C)
Fighting poverty
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 191) Directions: Each word in the capital letters is followed by four synonyms. Pick up the correct synonyms. BRUTAL:
A)
ascent
done
clear
B)
merciless
done
clear
C)
peaceful
done
clear
D)
cheat
done
clear
View Answer play_arrow
question_answer 192) Directions: Each word in the capital letters is followed by four synonyms. Pick up the correct synonyms. FICKLE:
A)
fiction
done
clear
B)
dominating
done
clear
C)
changing
done
clear
D)
transient
done
clear
View Answer play_arrow
question_answer 193) Directions: Pick up correct antonyms from the choices for each word given in capital letters. ENTIRE:
A)
incomplete
done
clear
B)
partial
done
clear
C)
whole
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 194) Directions: Pick up correct antonyms from the choices for each word given in capital letters. EARN:
A)
divide
done
clear
B)
rob
done
clear
C)
win
done
clear
D)
lose
done
clear
View Answer play_arrow
question_answer 195) Directions: In each of the following questions four words are given. One of these is incorrectly spelled. Pick up that word.
A)
liutenant
done
clear
B)
exercise
done
clear
C)
humane
done
clear
D)
complacent
done
clear
View Answer play_arrow
question_answer 196) Directions: In each of the following questions four words are given. One of these is incorrectly spelled. Pick up that word.
A)
conceive
done
clear
B)
deceive
done
clear
C)
receive
done
clear
D)
perceive
done
clear
View Answer play_arrow
question_answer 197) Directions: Four parts of each of the following sentences are underlined. Find which of the underlined parts (a or b or c or d) is not acceptable in the standard written English. If none of the parts is incorrect mark e.
A)
The Himalayas is
done
clear
B)
in
done
clear
C)
the north
done
clear
D)
of India.
done
clear
E)
No error:
done
clear
View Answer play_arrow
question_answer 198) Directions: Four parts of each of the following sentences are underlined. Find which of the underlined parts (a or b or c or d) is not acceptable in the standard written English. If none of the parts is incorrect mark e.
A)
Both his friends as well as
done
clear
B)
his uncle are
done
clear
C)
to
done
clear
D)
leave. Bombay today.
done
clear
E)
No Error:
done
clear
View Answer play_arrow
question_answer 199) Directions: Four parts of each of the following sentences are underlined. Find which of the underlined parts (a or b or c or d) is not acceptable in the standard written English. If none of the parts is incorrect mark e.
A)
Did he travel
done
clear
B)
by air
done
clear
C)
or by
done
clear
D)
the sea.
done
clear
E)
No Error:
done
clear
View Answer play_arrow
question_answer 200) Directions: Four parts of each of the following sentences are underlined. Find which of the underlined parts (a or b or c or d) is not acceptable in the standard written English. If none of the parts is incorrect mark e.
A)
The
done
clear
B)
stitch
done
clear
C)
in time
done
clear
D)
saves life.
done
clear
E)
No Error:
done
clear
View Answer play_arrow
 10\[\Omega \]
10\[\Omega \] 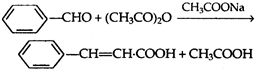 is called:
is called:
