question_answer 1) A body of mass 10 kg is lying on a rough plane inclined at an angle of \[30{}^\circ \] to the horizontal and the coefficient of friction is 0.5. The minimum force required to pull the body up the plane is:
A)
914 N
done
clear
B)
91.4 N
done
clear
C)
9.14 N
done
clear
D)
0.914 N
done
clear
View Answer play_arrow
question_answer 2) Two spheres of radius \[{{R}_{1}}\] and \[{{R}_{2}}\] respectively are charged and joined by a wire. The ratio of electric field of the spheres is:
A)
\[\frac{R\,_{2}^{2}}{R\,_{1}^{2}}\]
done
clear
B)
\[\frac{R\,_{1}^{2}}{R\,_{2}^{2}}\]
done
clear
C)
\[\frac{R{{\,}_{2}}}{R{{\,}_{1}}}\]
done
clear
D)
\[\frac{R{{\,}_{1}}}{R{{\,}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 3) Monochromatic light is refracted from air into the glass of refractive index\[\mu \]. The ratio of the wavelength of incident and refracted waves is:
A)
1:1
done
clear
B)
\[\mu :1\]
done
clear
C)
1:\[{{\mu }^{2}}\]
done
clear
D)
\[1 :\mu \]
done
clear
View Answer play_arrow
question_answer 4) A person cannot see objects clearly beyond 2.0 m. The power of lens required to correct his vision will be :
A)
\[-\,0.5\text{ }D\]
done
clear
B)
\[+\,1.0\text{ }D\]
done
clear
C)
\[-\,1.0\,D\]
done
clear
D)
\[+\,2.0\,D\]
done
clear
View Answer play_arrow
question_answer 5) The dominant mechanisms for motion of charge carriers in forward and reverse biased silicon\[\text{p-n}\]junctions are:
A)
drift in both forward and reverse bias
done
clear
B)
diffusion in both forward and reverse bias
done
clear
C)
diffusion in forward bias, drift in reverse bias
done
clear
D)
drift in forward bias diffusion in reverse bias
done
clear
View Answer play_arrow
question_answer 6) If half-life of a radioactive atom is 2.3 days, then its decay constant would be:
A)
0.2
done
clear
B)
0.1
done
clear
C)
2.3
done
clear
D)
0.3
done
clear
View Answer play_arrow
question_answer 7) In Youngs double slit experiment, a mica slit of thickness t and refractive index \[\mu \] is introduced in the ray from the first source\[{{S}_{1.}}\]. By how much distance the fringes pattern will be displaced?
A)
\[\frac{D}{d}(\mu -1)\]
done
clear
B)
\[\frac{d}{(\mu -1)D}\]
done
clear
C)
\[\frac{D}{d}(\mu -1)t\]
done
clear
D)
\[\frac{d}{D}(\mu -1)t\]
done
clear
View Answer play_arrow
question_answer 8) A uniform chain of length L and mass M is lying on a smooth table and one-third of its length is hanging vertically down over the edge of the table. If g is the acceleration due to gravity, the work required to pull the hanging part on to the table is:
A)
\[\frac{MgL}{18}\]
done
clear
B)
\[\frac{MgL}{9}\]
done
clear
C)
\[\frac{MgL}{3}\]
done
clear
D)
\[MgL\]
done
clear
View Answer play_arrow
question_answer 9) A (100 W, 200V) bulb is connected to a to V power supply. The power consumption would be:
A)
80 W
done
clear
B)
125 W
done
clear
C)
64 W
done
clear
D)
100 W
done
clear
View Answer play_arrow
question_answer 10) A body is moved along a straight line by a machine delivering constant power. The distance moved by the body in time t is proportional to:
A)
\[{{t}^{1/2}}\]
done
clear
B)
\[{{t}^{3/2}}\]
done
clear
C)
\[{{t}^{2}}\]
done
clear
D)
\[{{t}^{3/4}}\]
done
clear
View Answer play_arrow
question_answer 11) The wavelength of the energy emitted when electron come from fourth orbit to second orbit in hydrogen is \[20,397\text{ }c{{m}^{-1}}\]. The wavelength of energy for the same transition in He+ is :
A)
\[81,988c{{m}^{-1}}\]
done
clear
B)
\[40,994c{{m}^{-1}}\]
done
clear
C)
\[20,497c{{m}^{-1}}\]
done
clear
D)
\[5,099\text{ }c{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 12) 12. If the binding energy per nucleon in \[L{{i}^{7}}\] and \[H{{e}^{4}}\] nuclei are respectively 5.60 MeV and 7.06 MeV, then energy of reaction \[L{{i}^{7}}+p\xrightarrow{{}}2\,{{\,}_{2}}H{{e}^{4}}\] is:
A)
17.3 MeV
done
clear
B)
8.4 MeV
done
clear
C)
2.4 MeV
done
clear
D)
19.6 MeV
done
clear
View Answer play_arrow
question_answer 13) A convex lens of focal length 0.5 m and concave lens of focal length 1 m are combined. The power of the resulting lens will be:
A)
- 1 D
done
clear
B)
0.5 D
done
clear
C)
1D
done
clear
D)
- 0.5 D
done
clear
View Answer play_arrow
question_answer 14) Half lives of two radioactive substances A and B are respectively 20 minute and 40 minute. Initially the sample of A and B have equal number of nuclei. After 80 minutes, the ratio remains in number of A and B nuclei is.
A)
4 : 1
done
clear
B)
1 : 16
done
clear
C)
1 : 1
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 15) The plates of a parallel plate capacitor of capacity 50\[\mu F\] are charged to a potential of 100 volts and then separated from each other so that the distance between them is doubled. How much is the energy spent in doing so?
A)
\[\text{12}\text{.5 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-2}}}\text{J}\]
done
clear
B)
\[\text{-25 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-2}}}\text{J}\]
done
clear
C)
\[\text{-12}\text{.5 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-2}}}\text{J}\]
done
clear
D)
\[\text{25 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-2}}}\text{J}\]
done
clear
View Answer play_arrow
question_answer 16) Two point charges + 3 \[\mu C\] and + 8\[\mu C\] repel each other with a force of 40 N. If a charge of - 5 \[\mu C\] is added to each of them, then the force between them will become:
A)
+10 N
done
clear
B)
+20 N
done
clear
C)
-20 N
done
clear
D)
-10 N
done
clear
View Answer play_arrow
question_answer 17) Two waves \[y=0.25\text{ }sin\,316t\] and \[y=0.25\,\sin 310\,t\] are travelling in same direction. The number of beats produced per second will be :
A)
3
done
clear
B)
6
done
clear
C)
\[3\pi \]
done
clear
D)
\[3/\pi \]
done
clear
View Answer play_arrow
question_answer 18) The intensity of radiation emitted by the sun has its maximum value at a wavelength of 510 nm and that emitted by the North Star has the maximum value at 350 nm. If these stars behave like black bodies, then the ratio of the surface temperature of the sun and north star is:
A)
0.83
done
clear
B)
1.21
done
clear
C)
0.69
done
clear
D)
1.46
done
clear
View Answer play_arrow
question_answer 19) The unit of luminous efficiency of electric bulb is:
A)
lumen
done
clear
B)
watt
done
clear
C)
lux
done
clear
D)
lumen/watt
done
clear
View Answer play_arrow
question_answer 20) An electron having charge \[1.6\times {{10}^{-19}}\] and mass \[9\times {{10}^{-31}}kg\] is moving with \[4\times {{10}^{6}}m{{s}^{-1}}\] speed in a magnetic field \[2\times {{10}^{-1}}\] tesla in a circular orbit. The force acting on electron and the radius of the circular orbit will be:
A)
\[1.28\times {{10}^{-13}}N,\text{ }1.1\times {{10}^{-4}}m\]
done
clear
B)
\[1.28\times {{10}^{-13~~}}N,\text{ }1.1\text{ }{{10}^{-3}}m\]
done
clear
C)
\[1.28\times {{10}^{-14~}}N,\text{ }1.1\times {{10}^{-3}}m\]
done
clear
D)
\[12.8\times {{10}^{-13~}}N,\text{ }1.1\times {{10}^{-4}}m\]
done
clear
View Answer play_arrow
question_answer 21) An astronomical telescope has an angular magnification of magnitude 5 for distant objects. The separation between the objective and the eyepiece is 36 cm and final image is formed at infinity. The focal lengths of the objective and eyepiece are respectively:
A)
20 cm, 16 cm
done
clear
B)
50 cm, 10 cm
done
clear
C)
30 cm, 6 cm
done
clear
D)
45 cm, - 9 cm
done
clear
View Answer play_arrow
question_answer 22) The nucleus \[_{\text{48}}^{\text{115}}\text{Cd}\] after two successive\[\text{-}\]decays will give:
A)
\[_{\text{50}}^{\text{115}}\text{Sn}\]
done
clear
B)
\[_{\text{50}}^{\text{113}}\text{Sn}\]
done
clear
C)
\[_{49}^{\text{114}}\text{ln}\]
done
clear
D)
\[_{46}^{\text{115}}\text{Pa}\]
done
clear
View Answer play_arrow
question_answer 23) The refractive index of water with respect to air is 4/3 and the refractive index of glass with respect to air is 3/2. The refractive index of water with respect to glass is:
A)
8/9
done
clear
B)
9/8
done
clear
C)
2
done
clear
D)
1/2
done
clear
View Answer play_arrow
question_answer 24) For the structural analysis of crystals, X-rays are used because:
A)
X-rays are highly penetrating radiations
done
clear
B)
wavelength of X-rays is of the order of nuclear size
done
clear
C)
X-rays have wavelength of the order of interatomic spacing
done
clear
D)
X-rays are coherent radiations
done
clear
View Answer play_arrow
question_answer 25) 110 J of heat are added to a gaseous system whose internal energy is 40 J, then the amount of external work done is:
A)
40 J
done
clear
B)
110 J
done
clear
C)
70 J
done
clear
D)
150 J
done
clear
View Answer play_arrow
question_answer 26) Two trains, each 50 m long are travelling in opposite direction with velocity 10 m/s and 15 m/s. The time of crossing is:
A)
\[\frac{10}{3}s\]
done
clear
B)
\[2\,s\]
done
clear
C)
\[2\sqrt{3}\,s\]
done
clear
D)
\[2\sqrt{3}\,s\]
done
clear
View Answer play_arrow
question_answer 27) If mass of body is M on the earths surface, then the mass of the same body on the moons surface is:
A)
zero
done
clear
B)
M/6
done
clear
C)
M
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 28) A spherical source of power 4 W and frequency 800 Hz is emitting sound waves. The intensity of waves at a distance 200 m is:
A)
\[\text{2 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-4}}}\text{W/}{{\text{m}}^{\text{2}}}\]
done
clear
B)
\[\text{8 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-6}}}\text{W/}{{\text{m}}^{\text{2}}}\]
done
clear
C)
\[\text{4W/}{{\text{m}}^{\text{2}}}\]
done
clear
D)
\[\text{1 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-4}}}\text{W/}{{\text{m}}^{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 29) A body of mass 2 kg is moving with velocity 10 m/s towards east. Another body of same mass and same velocity moving towards north collides with former and coalesces and moves towards North-East. Its velocity is :
A)
5 m/s
done
clear
B)
2.5 m/s
done
clear
C)
10 m/s
done
clear
D)
5\[\sqrt{2}\] m/s
done
clear
View Answer play_arrow
question_answer 30) A man fires a bullet of mass 200 g at a speed of 5 m/s. The gun is of one kg mass. By what velocity the gun rebounds backwards:
A)
0.01 m/s
done
clear
B)
1 m/s
done
clear
C)
10 m/s
done
clear
D)
0.1 m/s
done
clear
View Answer play_arrow
question_answer 31) Which of the following formula is wrong?
A)
\[{{\text{C}}_{\text{P}}}\text{-}\,{{\text{C}}_{}}\text{=}\,\text{2}\,\text{R}\]
done
clear
B)
\[\frac{{{\text{C}}_{\text{P}}}}{{{\text{C}}_{}}}\text{=}\,\]
done
clear
C)
\[{{C}_{\upsilon }}=\frac{R}{-1}\]
done
clear
D)
\[{{C}_{\upsilon }}=\frac{R}{-1}\]
done
clear
View Answer play_arrow
question_answer 32) The mass of the earth is 81 times that of the moon and the radius of the earth is 3.5 times that of the moon. The ratio of the escape velocity on the surface of earth to that on the surface of moon will be :
A)
0.39
done
clear
B)
4.81
done
clear
C)
2.57
done
clear
D)
0.2
done
clear
View Answer play_arrow
question_answer 33) When the displacement is half the amplitude the ratio of potential energy to the total energy is:
A)
1/4
done
clear
B)
1/2
done
clear
C)
1/8
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 34) The number of turns in the primary coil of a transformer is 200 and the number of turns in the secondary coil is 10. If 240 volt AC is applied to the primary, the output from the secondary will be:
A)
6V
done
clear
B)
12V
done
clear
C)
24V
done
clear
D)
48V
done
clear
View Answer play_arrow
question_answer 35) If the pressure amplitude in a sound wave is tripled, then the intensity of sound is increased by a factor of:
A)
6
done
clear
B)
3
done
clear
C)
9
done
clear
D)
\[\sqrt{3}\]
done
clear
View Answer play_arrow
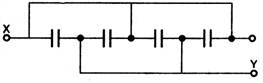
question_answer 36) Four condensers are connected as shown in the given figure, the capacity of each is 8\[\mu F\]. The capacity between points X and Y will be:
A)
\[16\mu F\]
done
clear
B)
\[64\mu F\]
done
clear
C)
\[2\mu F\]
done
clear
D)
\[32\mu F\]
done
clear
View Answer play_arrow
question_answer 37) The bob of a simple pendulum of mass and total energy \[{{\text{E}}_{\text{k}}}\] will have maximum linear momentum equal to:
A)
\[\text{ME}_{k}^{2}\]
done
clear
B)
\[\text{2mE}_{k}^{{}}\]
done
clear
C)
\[\sqrt{\frac{\text{2E}_{k}^{{}}}{m}}\]
done
clear
D)
\[\sqrt{\text{2mE}_{k}^{{}}}\]
done
clear
View Answer play_arrow
question_answer 38) Light of wavelength of \[6000\overset{\text{o}}{\mathop{\text{A}}}\,\] has a frequency of:
A)
\[5\times {{10}^{14}}Hz\]
done
clear
B)
\[5\times {{10}^{12}}Hz\]
done
clear
C)
\[5\times {{10}^{15}}Hz\]
done
clear
D)
\[5\times {{10}^{6}}Hz\]
done
clear
View Answer play_arrow
question_answer 39) You are provided three bulbs of 15, 40 and 60 watt. Which of them has lowest resistance:
A)
40 watt bulb
done
clear
B)
60 watt bulb
done
clear
C)
25 watt bulb
done
clear
D)
Information is insufficient
done
clear
View Answer play_arrow
question_answer 40) Heat produced in a wire of resistance R due to current flowing at constant potential difference, is proportional to :
A)
\[\frac{1}{{{R}^{2}}}\]
done
clear
B)
\[\frac{1}{R}\]
done
clear
C)
\[R\]
done
clear
D)
\[{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 41) Standing waves arc educed in a 10 m long stretched string. If the string vibrates in 5 segments and the wave velocity is 20 m/s, the frequency is:
A)
10 Hz
done
clear
B)
5 Hz
done
clear
C)
4 Hz
done
clear
D)
2 Hz
done
clear
View Answer play_arrow
question_answer 42) A sample of gas expands from volume \[{{V}_{1}}\] to \[{{V}_{2}}\] The amount of work done by the gas is greatest when the expansion is:
A)
isobaric
done
clear
B)
isothermal
done
clear
C)
adiabatic
done
clear
D)
equal in all cases
done
clear
View Answer play_arrow
question_answer 43) A soap bubble in vacuum has a radius of 3 cm and another soap bubble in vaccum has a radius of 4 cm. If the two bubbles coalesce under isothermal condition, then the radius of the new bubble is :
A)
4.5cm,
done
clear
B)
2.3cm
done
clear
C)
7 cm
done
clear
D)
5 cm
done
clear
View Answer play_arrow
question_answer 44) A body is whirled in a horizontal circle of radius 20 cm. It has angular velocity of 10 rad/s. What is its linear velocity at any point on circular path?
A)
2 m/s
done
clear
B)
10 m/s
done
clear
C)
20 m/s
done
clear
D)
\[\sqrt{2}\]m/s
done
clear
View Answer play_arrow
question_answer 45) A car of mass m is driven with acceleration a along a straight level road against a constant external resistive force R. When the velocity of the car is v, the rate at which the engine of the car is doing work, will be:
A)
\[(ma-R)\upsilon \]
done
clear
B)
\[(R+ma)\upsilon \]
done
clear
C)
\[ma\upsilon \]
done
clear
D)
\[R\upsilon \]
done
clear
View Answer play_arrow
question_answer 46) When light of wavelength 300 nm falls on a photoelectric emitter, photoelectrons are liberated. For another emitter, however light of 600 nm wavelength is sufficient for creating photoemission. What is the ratio of the work functions of the two emitters:
A)
1 : 4
done
clear
B)
4 : 1
done
clear
C)
2 : 1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 47) A spring 40 mm long is stretched by the application of a force. If 10 N force is required to stretch the spring through 1 mm, then work done in stretching the spring through 40 mm is:
A)
8 J
done
clear
B)
23 J
done
clear
C)
68 J
done
clear
D)
84 J
done
clear
View Answer play_arrow
question_answer 48) A spring of force constant k is cut into three equal parts and then they are suspended from the same point. The force constant of the system is:
A)
k
done
clear
B)
3k
done
clear
C)
9k
done
clear
D)
\[\frac{k}{9}\]
done
clear
View Answer play_arrow
question_answer 49) The accelerating voltage of an electron is increased to two times, its de Broglie wavelength will be:
A)
increase 2 times
done
clear
B)
decrease 0.5 times
done
clear
C)
increase 1.4 times
done
clear
D)
decrease 0.7 times
done
clear
View Answer play_arrow
question_answer 50) The capacitance of a metallic sphere will be \[1\mu F,\] if it radius is nearly:
A)
9 km
done
clear
B)
10 m
done
clear
C)
1.11 m
done
clear
D)
1.11 cm
done
clear
View Answer play_arrow
question_answer 51) The interference phenomenon can take place:
A)
in longitudinal waves
done
clear
B)
in standing waves only
done
clear
C)
interference waves
done
clear
D)
in all waves
done
clear
View Answer play_arrow
question_answer 52) The energy of a photon of light of wavelength 450 nm is:
A)
\[2.5\times {{10}^{-17}}J\]
done
clear
B)
\[1.25\,\times {{10}^{-17}}J\]
done
clear
C)
\[2.5\times {{10}^{-19}}J\]
done
clear
D)
\[4.4\times {{10}^{-19}}J\]
done
clear
View Answer play_arrow
question_answer 53) Moment of inertia of a ring of mass m = 3 gm and radius r = 1 cm about an axis passing through its edge and parallel to its natural axis is:
A)
\[1\text{ }gm\text{ }c{{m}^{2}}\]
done
clear
B)
\[6\text{ }gm\text{ }c{{m}^{2}}\]
done
clear
C)
\[100\text{ }gm\text{ }c{{m}^{2}}\]
done
clear
D)
\[10\text{ }gm\text{ }c{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 54) A bullet of mass 0.1 kg is fired with a speed of 100 m/sec, the mass of gun is 50 kg. The velocity of recoil is :
A)
0.05 m/sec
done
clear
B)
0.5 m/sec
done
clear
C)
0.1 m/sec
done
clear
D)
0.2 m/sec
done
clear
View Answer play_arrow
question_answer 55) At constant volume, temperature is increased. Then:
A)
collisions will be in straight lines
done
clear
B)
collisions on walls will be less
done
clear
C)
number of collisions per unit time will increase
done
clear
D)
collisions will not change
done
clear
View Answer play_arrow
question_answer 56) Which one of the following can detect infrared radiation :
A)
ordinary photographic plate
done
clear
B)
fluorescence screen
done
clear
C)
LCR circuit
done
clear
D)
thermopile
done
clear
View Answer play_arrow
question_answer 57) The minimum thickness of air film which appears dark when viewed under 500 nm light normally is:
A)
1000 nm
done
clear
B)
500 nm
done
clear
C)
750 nm
done
clear
D)
250 nm
done
clear
View Answer play_arrow
question_answer 58) The number of lines of force coming out but from + 1 coulomb is :
A)
1
done
clear
B)
\[1.11\times {{10}^{-10}}\]
done
clear
C)
\[1.12\times {{10}^{4}}\]
done
clear
D)
\[1.1\times {{10}^{19}}\]
done
clear
View Answer play_arrow
question_answer 59) For a transistor \[\,\text{=}\,\text{62,}\]\[{{R}_{L}}\,\text{=}\,5000\,\Omega \text{,}\]\[{{R}_{i}}\,\text{=}\,500\,\Omega .\] Voltage amplification is:
A)
260
done
clear
B)
620
done
clear
C)
2.6
done
clear
D)
6.2
done
clear
View Answer play_arrow
question_answer 60) A refrigerator has a coefficient of performance is 9. If the surrounding temperature is \[27{}^\circ C\]. The minimum temperature it can cool a body inside is (in\[{}^\circ C\]):
A)
3
done
clear
B)
zero
done
clear
C)
10
done
clear
D)
-3
done
clear
View Answer play_arrow
question_answer 61) Although CO is neutral gas, yet it shows acidic nature or reaction with: (at high pressure and temperature)
A)
\[LiOH\]
done
clear
B)
\[Ca{{(OH)}_{2}}\]
done
clear
C)
\[NaOH\]
done
clear
D)
\[Zn{{(OH)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 62) Which of the following has maximum hydration energy?
A)
\[N{{a}^{+}}\]
done
clear
B)
\[L{{i}^{+}}\]
done
clear
C)
\[M{{g}^{2+}}\]
done
clear
D)
\[C{{a}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 63) Which species does not exist?
A)
\[{{(SiC{{l}_{6}})}^{2-}}\]
done
clear
B)
\[CCl_{6}^{2-}\]
done
clear
C)
\[GeCl_{6}^{2-}\]
done
clear
D)
\[AlCl_{4}^{-}\]
done
clear
View Answer play_arrow
question_answer 64) What is the weight of 1 mole atoms of Ag?
A)
10.79
done
clear
B)
107.9
done
clear
C)
108.9
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 65) When \[{{H}_{2}}S\] reacts with halogens, the halogens:
A)
form sulphur halides
done
clear
B)
are oxidised
done
clear
C)
are reduced
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 66) \[Pb{{O}_{2}}\] is:
A)
basic
done
clear
B)
acidic
done
clear
C)
neutral
done
clear
D)
amphoteric
done
clear
View Answer play_arrow
question_answer 67) Nitrogen combines with hot magnesium wire to form:
A)
magnesium nitrate
done
clear
B)
magnesium nitride
done
clear
C)
magnesium oxide
done
clear
D)
nitrosyl chloride
done
clear
View Answer play_arrow
question_answer 68) Lead pipes are not suitable for drinking water because:
A)
lead reacts with air to form litharge
done
clear
B)
lead reacts with water containing air to form \[Pb{{(OH)}_{2}}\]
done
clear
C)
a layer of lead dioxide is deposited over pipes
done
clear
D)
lead forms basic lead carbonate
done
clear
View Answer play_arrow
question_answer 69) Which of the following is not oxidised by\[KMnO_{4}^{-}\]?
A)
\[C{{l}^{-}}\]
done
clear
B)
\[{{I}^{-}}\]
done
clear
C)
\[B{{r}^{-}}\]
done
clear
D)
\[{{F}^{-}}\]
done
clear
View Answer play_arrow
question_answer 70) The chief component of cement is:
A)
\[Si{{O}_{2}}\]
done
clear
B)
\[CaO\]
done
clear
C)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 71) Which metal displace hydrogen from acids?
A)
\[Hg\]
done
clear
B)
\[Ca\]
done
clear
C)
\[Zn\]
done
clear
D)
\[Al\]
done
clear
View Answer play_arrow
question_answer 72) The ionic carbide is:
A)
\[ZnC\]
done
clear
B)
\[SiC\]
done
clear
C)
\[Ca{{C}_{2}}\]
done
clear
D)
\[TiC\]
done
clear
View Answer play_arrow
question_answer 73) The most dangerous reaction of an acid is with the metal:
A)
\[Al\]
done
clear
B)
\[K\]
done
clear
C)
\[Fe\]
done
clear
D)
\[Zn\]
done
clear
View Answer play_arrow
question_answer 74) In which of the following compounds, the central atom has not completed octet?
A)
\[BeC{{l}_{2}}\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 75) Which set has the same number of unpaired electrons in their ground state?
A)
\[C{{l}^{-}},F{{e}^{3+}},C{{r}^{3+}}\]
done
clear
B)
\[N{{a}^{+}},M{{g}^{2+}},Al\]
done
clear
C)
\[Na,P,Cl\]
done
clear
D)
\[N,P,V\]
done
clear
View Answer play_arrow
question_answer 76) Uranium \[{}_{92}{{U}^{238}}\]decays into a stable isotope of:
A)
\[Np\]
done
clear
B)
\[Pb\]
done
clear
C)
\[Rn\]
done
clear
D)
\[Ra\]
done
clear
View Answer play_arrow
question_answer 77) Artificial radioactivity was discovered by:
A)
Madame Curie
done
clear
B)
Wilson
done
clear
C)
Rutherford
done
clear
D)
Soddy
done
clear
View Answer play_arrow
question_answer 78) The de-Broglie wavelength associated with a material particle is:
A)
directly proportional to its energy
done
clear
B)
directly proportional to momentum
done
clear
C)
inversely proportional to its energy
done
clear
D)
inversely proportional to momentum
done
clear
View Answer play_arrow
question_answer 79) V versus T curves, at constant pressure \[{{P}_{1}}\] and \[{{P}_{2}}\] for an ideal gas are shown in figure. Which statement is correct?
A)
\[{{P}_{1}}<{{P}_{2}}\]
done
clear
B)
\[{{P}_{1}}>{{P}_{2}}\]
done
clear
C)
\[{{P}_{1}}={{P}_{2}}\]
done
clear
D)
all of the above are correct
done
clear
View Answer play_arrow
question_answer 80) As a result of osmosis, the volume of concentrate solution:
A)
increases
done
clear
B)
decreases
done
clear
C)
remains constant
done
clear
D)
first increase and then decrease
done
clear
View Answer play_arrow
question_answer 81) The pH of a 0.01 M solution of a weak acid having degree of dissociation 12.5% is:
A)
5.623
done
clear
B)
2.903
done
clear
C)
4.509
done
clear
D)
3.723
done
clear
View Answer play_arrow
question_answer 82) Which of the following is a Buffer solution?
A)
\[NaOH+NaCl\]
done
clear
B)
\[KOH+KN{{O}_{3}}\]
done
clear
C)
\[C{{H}_{3}}COOH+C{{H}_{3}}COONa\]
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 83) Equilibrium concentration of \[HI,\,\,{{I}_{2}}\] and \[{{H}_{2}}\]are 0.7, 0.1 and 0.1 M respectively. The value of equilibrium constant for the reaction, \[{{H}_{2}}+{{I}_{2}}\rightleftharpoons 2HI\] is:
A)
36
done
clear
B)
49
done
clear
C)
0.49
done
clear
D)
0.36
done
clear
View Answer play_arrow
question_answer 84) The activation energy for a reaction is 9.0 kcal/mol. The increase in the rate constant when its temperature is increased from 298 K to 308 K is:
A)
63%
done
clear
B)
50%
done
clear
C)
100%
done
clear
D)
10%
done
clear
View Answer play_arrow
question_answer 85) The ratio of energy of photon of \[3000\,\overset{\text{o}}{\mathop{\text{A}}}\,\] and \[6000\,\overset{\text{o}}{\mathop{\text{A}}}\,\] wavelength radiation is:
A)
2
done
clear
B)
1/2
done
clear
C)
4
done
clear
D)
1/4
done
clear
View Answer play_arrow
question_answer 86) According to Le Chateliers principle, an increasing temperature of an endothermic reaction at equilibrium, what will happen?
A)
Heat will evolve
done
clear
B)
Reaction will proceed backward
done
clear
C)
Reaction will proceed forward
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 87) Heat of neutralisation of the acid-base reaction is 57.32 kJ for:
A)
\[HN{{O}_{3}}+LiOH\]
done
clear
B)
\[HCOOH+KOH\]
done
clear
C)
\[HCl+N{{H}_{4}}OH\]
done
clear
D)
\[C{{H}_{3}}COOH+NaOH\]
done
clear
View Answer play_arrow
question_answer 88) Joule Thomson expansion is:
A)
isobaric
done
clear
B)
isothermal
done
clear
C)
iso-enthalpic
done
clear
D)
iso-entropic
done
clear
View Answer play_arrow
question_answer 89) The co-ordination number of bcc crystal is:
A)
8
done
clear
B)
6
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 90) What is the pH for a neutral solution at the body temperature\[\left( 38{}^\circ C \right)\]?
A)
7.2
done
clear
B)
14.0
done
clear
C)
6.8
done
clear
D)
6.0
done
clear
View Answer play_arrow
question_answer 91) For a reversible reaction, \[{{N}_{2}}+3{{H}_{2}}\rightleftharpoons 2N{{H}_{3}},\] \[{{K}_{c}}\]is \[2.37\times {{10}^{-3}}.\] If at equilibrium, \[[{{N}_{2}}]=2M,\,\,[{{H}_{2}}]=3M,\] the concentration of\[N{{H}_{3}}\]is:
A)
0.00358 M
done
clear
B)
0.0358 M
done
clear
C)
3.58 M
done
clear
D)
0.358 M
done
clear
View Answer play_arrow
question_answer 92) The volume of a gas is 100 ml at \[100{}^\circ C\]. If the pressure is kept constant, at what temperature will the gas have a volume of 200ml?
A)
\[50{}^\circ C\]
done
clear
B)
\[473{}^\circ C\]
done
clear
C)
\[446{}^\circ C\]
done
clear
D)
\[200{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 93) Which solution has the maximum pH?
A)
\[1\,M\text{-}KOH\]
done
clear
B)
\[10\,M\,{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
Chlorine-water
done
clear
D)
Water containing carbon-dioxide
done
clear
View Answer play_arrow
question_answer 94) A reaction of first-order completed 90% in 90 minutes, hence it is completed 50% in approximately:
A)
50 minutes
done
clear
B)
54 minutes
done
clear
C)
27 minutes
done
clear
D)
62 minutes
done
clear
View Answer play_arrow
question_answer 95) Chlorobenzene is prepared commercially by:
A)
Raschig process
done
clear
B)
Wurtz-Fittig reaction
done
clear
C)
Friedel-Crafts reaction
done
clear
D)
Grignard reaction
done
clear
View Answer play_arrow
question_answer 96) A mixture of camphor and benzoic acid can be easily separated by:
A)
fractional crystallization
done
clear
B)
chemical method
done
clear
C)
extraction with solvent
done
clear
D)
sublimation
done
clear
View Answer play_arrow
question_answer 97) Butyne-1 and butyne-2 can be distinguished by:
A)
Benedicts reagent
done
clear
B)
alkaline \[KMn{{O}_{4}}\]
done
clear
C)
ammonical cuprous chloride
done
clear
D)
bromine water
done
clear
View Answer play_arrow
question_answer 98) Isopropyl alcohol on passing over heated copper at \[300{}^\circ C\] gives:
A)
acetone
done
clear
B)
acetaldehyde
done
clear
C)
propylene
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 99) Which of the following reacts with \[COC{{l}_{2}}\] to give phenyl isocyanate?
A)
Chlorobenzene
done
clear
B)
Nitrobenzene
done
clear
C)
Aminophenol
done
clear
D)
Aniline
done
clear
View Answer play_arrow
question_answer 100) \[{{o}^{-}},\,{{m}^{-}}\] and p-xylenes, on oxidation with acidic \[KMn{{O}_{4}}\] gives:
A)
terphthalic acid
done
clear
B)
phthalic acid
done
clear
C)
isophthalic acid
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 101) In the case of homologous series of alkanes, which one of the following statements is incorrect?
A)
The members of the series are isomers of each other
done
clear
B)
The members of the series have similar chemical properties
done
clear
C)
The members can be prepared by some common methods
done
clear
D)
The members have the general formula \[{{C}_{n}}{{H}_{2n+2}}\]
done
clear
View Answer play_arrow
question_answer 102) Carbanions initiates:
A)
substitution reactions
done
clear
B)
addition reactions
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 103) 1, 3-butadiene has:
A)
\[sp\] and \[s{{p}^{2}}\text{-}\]hybridised C-atoms
done
clear
B)
\[sp\] \[s{{p}^{2}}\] and \[s{{p}^{3}}\text{-}\]hybridised C-atoms
done
clear
C)
only \[s{{p}^{2}}\text{-}\] hybridised C-atoms
done
clear
D)
only \[sp\]- hybridised C-atoms
done
clear
View Answer play_arrow
question_answer 104) When HCHO is treated with \[{{C}_{6}}{{H}_{5}}CHO\] in presence of KOH, the product are:
A)
\[HCOONa\] and \[{{C}_{6}}{{H}_{5}}C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{3}}OH\]and \[{{C}_{6}}{{H}_{5}}COONa\]
done
clear
C)
\[C{{H}_{3}}OH\]and \[{{C}_{6}}{{H}_{5}}COONa\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}OH\]and \[{{C}_{6}}{{H}_{5}}COONa\]
done
clear
View Answer play_arrow
question_answer 105) Vinyl chloride reacts with \[HCl\] to form:
A)
1, 1-dichloro ethane
done
clear
B)
1, 2-dichloro ethane
done
clear
C)
tetrachloro ethylene
done
clear
D)
mixtures of 1, 2 and 1, 1-dichloro ethane
done
clear
View Answer play_arrow
question_answer 106) What is the end product in the following reaction? \[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\xrightarrow{HN{{O}_{2}}}A\xrightarrow{PC{{l}_{5}}}B\xrightarrow{N{{H}_{3}}}C\]
A)
Methyl amine
done
clear
B)
Acetamide
done
clear
C)
Ethyl cyanide
done
clear
D)
Ethyl amine
done
clear
View Answer play_arrow
question_answer 107) What is obtained, when aniline is sulphonated?
A)
Anthranilic acid
done
clear
B)
o-amino benzene sulphonic acid
done
clear
C)
Sulphanilic acid
done
clear
D)
m-amino benzene sulphonic acid
done
clear
View Answer play_arrow
question_answer 108) The product obtained, when benzene reacts with an alkyl chloride \[(RCl)\] in presence of anhydrous \[AlC{{l}_{3}}\]:
A)
\[{{C}_{6}}{{H}_{5}}AlC{{l}_{3}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}Cl\]
done
clear
C)
no reaction takes place
done
clear
D)
\[{{C}_{6}}{{H}_{5}}R\]
done
clear
View Answer play_arrow
question_answer 109) \[C{{H}_{3}}C{{H}_{2}}COOH\xrightarrow[Fe]{C{{l}_{2}}}X\xrightarrow{alc.\,KOH}Y,\]the compound \[Y\] is:
A)
\[C{{H}_{2}}==CHCOOH\]
done
clear
B)
\[C{{H}_{2}}CHClCOOH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CN\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 110) Which of the following reactions, follows free-radical mechanism?
A)
Addition of HBr to alkene
done
clear
B)
Friedel-Craffs reaction
done
clear
C)
Sandmeyer reaction
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 111) The correct IUPAC name of \[H--\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,--C{{H}_{2}}C{{H}_{2}}COOH\]is:
A)
2-carboxy ethanol
done
clear
B)
4-formyl butanoic acid
done
clear
C)
3-formyl propanoic acid
done
clear
D)
3-keto propanoic acid
done
clear
View Answer play_arrow
question_answer 112) An element X, with the electronic configuration \[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}\]would be expected to form the chloride with the formula:
A)
\[XC{{l}_{3}}\]
done
clear
B)
\[XC{{l}_{2}}\]
done
clear
C)
\[XCl\]
done
clear
D)
\[{{X}_{2}}Cl\]
done
clear
View Answer play_arrow
question_answer 113) On heating \[NaCl+{{K}_{2}}C{{r}_{2}}{{O}_{7}}+\] cone. \[{{H}_{2}}S{{O}_{4}},\] the gas comes out is:
A)
\[{{O}_{2}}\]
done
clear
B)
\[C{{l}_{2}}\]
done
clear
C)
\[CrOC{{l}_{2}}\]
done
clear
D)
\[Cr{{O}_{2}}C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 114) The change in optical rotation with time, of freshly prepared solution of sugar is known as:
A)
mutor rotation
done
clear
B)
rotatory motion
done
clear
C)
inversion
done
clear
D)
specific rotation
done
clear
View Answer play_arrow
question_answer 115) Night blindness is caused by the deficiency of:
A)
ascorbic acid
done
clear
B)
Calciferol
done
clear
C)
retionol
done
clear
D)
tocopherol
done
clear
View Answer play_arrow
question_answer 116) RNA does not contain:
A)
ribose sugar
done
clear
B)
phosphate group
done
clear
C)
pyridine base
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 117) The pH of blood is:
A)
\[\approx 2\]
done
clear
B)
\[<7\]but \[>6\]
done
clear
C)
\[>7\] but \[<8\]
done
clear
D)
equal to 7
done
clear
View Answer play_arrow
question_answer 118) The noble metal is:
A)
\[Na\]
done
clear
B)
\[Ag\]
done
clear
C)
\[Hf\]
done
clear
D)
\[Pt\]
done
clear
View Answer play_arrow
question_answer 119) The compound that is not a Lewis acid, is
A)
\[B{{F}_{3}}\]
done
clear
B)
\[BaC{{l}_{2}}\]
done
clear
C)
\[AlC{{l}_{3}}\]
done
clear
D)
\[SnC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 120) The laws of electrolysis, were given by:
A)
Nernst
done
clear
B)
M. Berthelot
done
clear
C)
M. Faraday
done
clear
D)
Aristotle
done
clear
View Answer play_arrow
question_answer 121) Hormones of hypothalamus are called:
A)
angiotensis
done
clear
B)
trophic hormones
done
clear
C)
growth hormones
done
clear
D)
regulatory hormones
done
clear
View Answer play_arrow
question_answer 122) The phenomenon of fertilization was first perceived by:
A)
Hertwig
done
clear
B)
Robert Hooke
done
clear
C)
Leeuwenhoek
done
clear
D)
Weismann
done
clear
View Answer play_arrow
question_answer 123) The mammalian follicle in ovary was first described by:
A)
Fabricius
done
clear
B)
Robert Hooke
done
clear
C)
Von Baer
done
clear
D)
de Graaf
done
clear
View Answer play_arrow
question_answer 124) Which one of the following phenomena of hereditary characters was not known to Mendel?
A)
Segregation of alleles
done
clear
B)
Linkage
done
clear
C)
Dominant and recessive traits
done
clear
D)
Independent assortment
done
clear
View Answer play_arrow
question_answer 125) In alkaptonuria:
A)
the urine turns black
done
clear
B)
the pathway of phenylalanine metaboIism is blocked
done
clear
C)
two genes for the trait are inherited
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 126) Enzyme necessary for transcription is:
A)
Endonuclease
done
clear
B)
RNA polymerase
done
clear
C)
DNA polymerase
done
clear
D)
RNA ase
done
clear
View Answer play_arrow
question_answer 127) Dubois in 1891 found the fossil of Java ape man. It is:
A)
Homo sapiens
done
clear
B)
Homo rhodesiensis
done
clear
C)
Pithecanthropus erectus
done
clear
D)
Sinanthropuspe kinensis
done
clear
View Answer play_arrow
question_answer 128) Sesame oil is obtained from the seeds of:
A)
Ricinuscommunis
done
clear
B)
Sesamumindicum
done
clear
C)
Carthamustinctorius
done
clear
D)
Brassica campestris
done
clear
View Answer play_arrow
question_answer 129) Seshania rostrata is a bio-fertilizer crop. It contains:
A)
root nodules
done
clear
B)
stem nodules
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 130) Cell in the neck of archegonia of Dryopteris are in how many longitudinal orvertical rows?
A)
2
done
clear
B)
8
done
clear
C)
4
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 131) Which one of the following is a fresh water sponge?
A)
Euplectella
done
clear
B)
Spongilla
done
clear
C)
Euspongia
done
clear
D)
Sycon
done
clear
View Answer play_arrow
question_answer 132) Hepatic caecae in cockroach are derived from:
A)
crop
done
clear
B)
ileum
done
clear
C)
midgut
done
clear
D)
gizzard
done
clear
View Answer play_arrow
question_answer 133) In 1980, F. Sanger was awarded Nobel prize second time to be shared by Gilbert for their work on:
A)
genetic mapping
done
clear
B)
determining the base sequences of DNA of a virus
done
clear
C)
determining the structure of DNA
done
clear
D)
determing amino acid sequence of insulin
done
clear
View Answer play_arrow
question_answer 134) Some of the species of Selaginella perennate by means of:
A)
bulbils
done
clear
B)
stem tubers
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 135) A sponge differs from all other matazoans in having:
A)
nerve cells
done
clear
B)
absence of blood
done
clear
C)
numerous mouthlets and one exit
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 136) What is the name of class to which pearl oyster belongs?
A)
Cephalopoda
done
clear
B)
Pelecypoda
done
clear
C)
Gastropoda
done
clear
D)
Scapoda
done
clear
View Answer play_arrow
question_answer 137) Male cockroach can be identified from the female by the presence of
A)
anal styles
done
clear
B)
wingless body
done
clear
C)
elongated abdomen
done
clear
D)
long antennae
done
clear
View Answer play_arrow
question_answer 138) The distinguishing feature of all chordates is:
A)
a water vascular system
done
clear
B)
an elastic rod (notochord)
done
clear
C)
achitinous exoskeleton
done
clear
D)
a ventrally placed nerve cord
done
clear
View Answer play_arrow
question_answer 139) Which of the birds cannot fly?
A)
Strok
done
clear
B)
Emu
done
clear
C)
Duck
done
clear
D)
Peacock
done
clear
View Answer play_arrow
question_answer 140) An obligate parasite is one which:
A)
always requires a living host
done
clear
B)
is usually a saprophyte but can become a parasite
done
clear
C)
always requires dead organic matter to survive
done
clear
D)
is usually a parasite but can become a Saprophyte
done
clear
View Answer play_arrow
question_answer 141) Fibrous roots develop in maize from:
A)
upper internodes
done
clear
B)
upper nodes
done
clear
C)
lower nodes
done
clear
D)
lower internodes
done
clear
View Answer play_arrow
question_answer 142) A tissue whose living cells form the mechanical tissue of activity growing organs and whose cell walls show cellulosic unlignified thickenings often at the corners of its cells is called:
A)
parenchyma
done
clear
B)
chlorenchyman
done
clear
C)
collenchyma
done
clear
D)
sclerenchyma
done
clear
View Answer play_arrow
question_answer 143) Eustele is present in:
A)
algae
done
clear
B)
dicots
done
clear
C)
bryophytes
done
clear
D)
pteridophytes
done
clear
View Answer play_arrow
question_answer 144) Which of the following plants keep its stomata open during the night and closed during the day?
A)
Hibiscus
done
clear
B)
Cactus
done
clear
C)
Ivy
done
clear
D)
Water lily
done
clear
View Answer play_arrow
question_answer 145) Which of the following is a component of middle lamella in plant cells?
A)
Fe
done
clear
B)
K
done
clear
C)
\[Ca\]
done
clear
D)
Mg
done
clear
View Answer play_arrow
question_answer 146) The source of \[{{O}_{2}}\] liberated in photo-synthesis is:
A)
carbon dioxide
done
clear
B)
water
done
clear
C)
alcohol
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 147) Vegetables are rich in:
A)
carbohydrates and fats
done
clear
B)
fats and vitamins
done
clear
C)
proteins and vitamins
done
clear
D)
minerals and vitamins
done
clear
View Answer play_arrow
question_answer 148) Bacillus Calmette Guerine (BCG) vaccineis anti:
A)
tuberculosis
done
clear
B)
polio
done
clear
C)
pneumonia
done
clear
D)
emphysema
done
clear
View Answer play_arrow
question_answer 149) A branch of botany concerned with the classification, nomenclature and identification of plants is:
A)
Physiology
done
clear
B)
Morphology
done
clear
C)
Ecology
done
clear
D)
Systematic Botany
done
clear
View Answer play_arrow
question_answer 150) Reducing sugars possesses:
A)
ketonic group
done
clear
B)
aldehyde group
done
clear
C)
carboxylic group
done
clear
D)
both a and b
done
clear
View Answer play_arrow
question_answer 151) FAD or FMN is a coenzyme, which vitamin is incorporated in its structure:
A)
vitamin C
done
clear
B)
vitamin \[{{B}_{6}}\]
done
clear
C)
vitamin \[{{B}_{2}}\]
done
clear
D)
vitamin \[{{B}_{1}}\]
done
clear
View Answer play_arrow
question_answer 152) Who among the following scientists developed eytochemical technique for the presence of DNA?
A)
Johannsen
done
clear
B)
Hammerling
done
clear
C)
McClintock
done
clear
D)
Feulgen and Rossenbeck
done
clear
View Answer play_arrow
question_answer 153) Ribosomes of bacteria, mitochondria and chloroplasts are of:
A)
30S type
done
clear
B)
70S type
done
clear
C)
80S type
done
clear
D)
50S type
done
clear
View Answer play_arrow
question_answer 154) When yeast ferments glucose the products are:
A)
ethanol and carbondioxide
done
clear
B)
methanol and \[C{{O}_{2}}\]
done
clear
C)
water and carbondioxide
done
clear
D)
ethanol and water
done
clear
View Answer play_arrow
question_answer 155) Anastral mitosis is the characteristic of:
A)
lower animals
done
clear
B)
higher animals
done
clear
C)
higher plants
done
clear
D)
all living organisms
done
clear
View Answer play_arrow
question_answer 156) The genetic material of bacteria is present as:
A)
spherosome
done
clear
B)
genophore
done
clear
C)
lomasomes
done
clear
D)
mesosome
done
clear
View Answer play_arrow
question_answer 157) Which of the following is an edible fungus?
A)
Neurospora
done
clear
B)
Penicillium
done
clear
C)
Agaricus
done
clear
D)
Rhizopus
done
clear
View Answer play_arrow
question_answer 158) Heterotrichous habit is shown by:
A)
Ulothrix
done
clear
B)
Oedogonium
done
clear
C)
Chalmydomonas
done
clear
D)
Stigeoclonium
done
clear
View Answer play_arrow
question_answer 159) Avena curvature test is used for biosynthesis of:
A)
auxin
done
clear
B)
gibberellin
done
clear
C)
ethylene
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 160) Largest ecosystem of the world are:
A)
forests
done
clear
B)
oceans
done
clear
C)
great lakes
done
clear
D)
grasslands
done
clear
View Answer play_arrow
question_answer 161) In a pond if there is too much wastage, then the BOD of pond will:
A)
remain same
done
clear
B)
decrease
done
clear
C)
increase
done
clear
D)
both (b) and (c)
done
clear
View Answer play_arrow
question_answer 162) Narcotic and soothing properties of tobacco is due to or harmful alkaloid contained in the leaves of tobacco is:
A)
codeine
done
clear
B)
nicotine
done
clear
C)
aconite
done
clear
D)
caffeine
done
clear
View Answer play_arrow
question_answer 163) Richest source of vitamins C is:
A)
lemon
done
clear
B)
orange
done
clear
C)
Emblica officinalis (Amla)
done
clear
D)
Capsicum fruit escence
done
clear
View Answer play_arrow
question_answer 164) Trypanosomiasis is transmitted by:
A)
tse-tse fly
done
clear
B)
sand fly
done
clear
C)
louse
done
clear
D)
may fly
done
clear
View Answer play_arrow
question_answer 165) Monocytes and neutrophils are important cells participating in:
A)
antibody production
done
clear
B)
passive immunity
done
clear
C)
phagocytosis
done
clear
D)
perforin production
done
clear
View Answer play_arrow
question_answer 166) X-rays were discovered in the year:
A)
1929
done
clear
B)
1906
done
clear
C)
1895
done
clear
D)
1870
done
clear
View Answer play_arrow
question_answer 167) The term antibiotic was coined by:
A)
Louis Pasteur
done
clear
B)
Alexander Flemming
done
clear
C)
Edward Jenner
done
clear
D)
Selman Waskman
done
clear
View Answer play_arrow
question_answer 168) Which of the following protein is present in Cartilage?
A)
Actine
done
clear
B)
Chondrin
done
clear
C)
Casein
done
clear
D)
Ostein
done
clear
View Answer play_arrow
question_answer 169) In a spadix inflorescence the spa the encloses a:
A)
umbel
done
clear
B)
biparous cyme
done
clear
C)
catkin
done
clear
D)
spike
done
clear
View Answer play_arrow
question_answer 170) Gynobasic style is found in:
A)
Centella
done
clear
B)
Colocasia
done
clear
C)
Ranunculus
done
clear
D)
Ocimum
done
clear
View Answer play_arrow
question_answer 171) A microspore mother cell forms:
A)
an ovule
done
clear
B)
pollen grains
done
clear
C)
embryosac
done
clear
D)
a pollen sac
done
clear
View Answer play_arrow
question_answer 172) The movement of auxin is largely:
A)
acropetal
done
clear
B)
centripetal
done
clear
C)
basipetal
done
clear
D)
both (a) and (c)
done
clear
View Answer play_arrow
question_answer 173) Cartilage is produced by:
A)
chondroblasts
done
clear
B)
fibroblasts
done
clear
C)
epithelium
done
clear
D)
osteoblasts
done
clear
View Answer play_arrow
question_answer 174) When coenzyme is combined with apo-enzyme it is called:
A)
vitamin A
done
clear
B)
holoenzyme
done
clear
C)
subtrate enzyme complex
done
clear
D)
cofactor
done
clear
View Answer play_arrow
question_answer 175) Which of the following events takes place during inspiration in rabbit?
A)
Due to contraction of external inter costal muscles and flattening of diaphragm the volume of thoracic cavity decreases
done
clear
B)
The abdominal muscles contract
done
clear
C)
Due to contraction of external inter costal muscles and flattening of diaphragm the volume of thoracic cavity increases
done
clear
D)
The internal intercostal muscles relax
done
clear
View Answer play_arrow
question_answer 176) Haematocrit is related with:
A)
counting number of RBC
done
clear
B)
oxygen carrying capacity of haemoglobin When a sample of blood is rendered non coagulable by adding sodium oxalate and then centrifuged in haematocrit tube. RBCs sediment to the bottom of the tube and the relative volume of RBCs can be read as a percentage of blood volume. This is called Haematocrit value.
done
clear
C)
cell volume when packed together
done
clear
D)
amount of Hb/100 ml of blood
done
clear
View Answer play_arrow
question_answer 177) About how many nephrons are there in each kidney of a human:
A)
1, 00, 000
done
clear
B)
1, 000
done
clear
C)
200
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 178) Glenoid cavity is present in:
A)
skull of rabbit
done
clear
B)
xiphisternum
done
clear
C)
pelvic girdle
done
clear
D)
pectoral girdle
done
clear
View Answer play_arrow
question_answer 179) Outermost covering of brain is called as:
A)
gray mater
done
clear
B)
pericardium
done
clear
C)
pia mater
done
clear
D)
duramater
done
clear
View Answer play_arrow
question_answer 180) The recapitulation theory was proposed by:
A)
E. Haeckel
done
clear
B)
Harvey
done
clear
C)
Lamarck
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 181) Directions: Find out the miss-spelt word...
A)
trauma
done
clear
B)
tinker
done
clear
C)
takitum
done
clear
D)
turpitude
done
clear
View Answer play_arrow
question_answer 182) Directions: Find out the miss-spelt word...
A)
synchronize
done
clear
B)
simulate
done
clear
C)
stimulate
done
clear
D)
scoul
done
clear
View Answer play_arrow
question_answer 183) Directions: Find out the miss-spelt word...
A)
groupie
done
clear
B)
gurantee
done
clear
C)
gubernatorial
done
clear
D)
guard-rail
done
clear
View Answer play_arrow
question_answer 184) Directions: Find out the miss-spelt word...
A)
loubrow
done
clear
B)
loutish
done
clear
C)
luminosity
done
clear
D)
lumpen
done
clear
View Answer play_arrow
question_answer 185) Directions: Find out the miss-spelt word...
A)
indefatigable
done
clear
B)
indentured
done
clear
C)
indicative
done
clear
D)
intelligentsia
done
clear
View Answer play_arrow
question_answer 186) (A) tree (B) the (C) fell (D) The (E) from (F) boy:
A)
DFCEBA
done
clear
B)
BACEDF
done
clear
C)
BFCDAE
done
clear
D)
EDACBF
done
clear
View Answer play_arrow
question_answer 187) (A) grinded (B) to (C) the (D) a (E) train (F) halt:
A)
DEABCF
done
clear
B)
DFABCE
done
clear
C)
CEABDF
done
clear
D)
FEACBD
done
clear
View Answer play_arrow
question_answer 188) (A) dejected (B) students (C) lot (D) the (E) a (F) were:
A)
ABFECD
done
clear
B)
EACFDB
done
clear
C)
DBFEAC
done
clear
D)
ECFDAB
done
clear
View Answer play_arrow
question_answer 189) (A) convicted (B) court (C) of (D) murder (E) the (F) him:
A)
EDAFCB
done
clear
B)
CEDBAF
done
clear
C)
FAEBCD
done
clear
D)
EBAFCD
done
clear
View Answer play_arrow
question_answer 190) (A) vehicle (B) accosted (C) the (D) speeding (E) sergeant (F) the:
A)
FEBCDA
done
clear
B)
FBACDE
done
clear
C)
CDABFE
done
clear
D)
BFEDAC
done
clear
View Answer play_arrow
question_answer 191) It is now well known that stress 191 up a 192 of body chemicals that eventually 193 your health. Now scientists 194 further findings, It 195 that every time one goes through 196 stress, the bodys stress thermostat gets 197 to a greater 198 Researchers compare this to an allergy once the body is 199 to a substance. It reacts strongly even at a mere 200 of the stuff.
A)
generates
done
clear
B)
brings
done
clear
C)
develops
done
clear
D)
stirs
done
clear
View Answer play_arrow
question_answer 192) It is now well known that stress 191 up a 192 of body chemicals that eventually 193 your health. Now scientists 194 further findings, It 195 that every time one goes through 196 stress, the bodys stress thermostat gets 197 to a greater 198 Researchers compare this to an allergy once the body is 199 to a substance. It reacts strongly even at a mere 200 of the stuff.
A)
flow
done
clear
B)
storm
done
clear
C)
plethora
done
clear
D)
array
done
clear
View Answer play_arrow
question_answer 193) It is now well known that stress 191 up a 192 of body chemicals that eventually 193 your health. Now scientists 194 further findings, It 195 that every time one goes through 196 stress, the bodys stress thermostat gets 197 to a greater 198 Researchers compare this to an allergy once the body is 199 to a substance. It reacts strongly even at a mere 200 of the stuff.
A)
effect
done
clear
B)
destroy
done
clear
C)
ruins
done
clear
D)
affect
done
clear
View Answer play_arrow
question_answer 194) It is now well known that stress 191 up a 192 of body chemicals that eventually 193 your health. Now scientists 194 further findings, It 195 that every time one goes through 196 stress, the bodys stress thermostat gets 197 to a greater 198 Researchers compare this to an allergy once the body is 199 to a substance. It reacts strongly even at a mere 200 of the stuff.
A)
develop
done
clear
B)
report
done
clear
C)
hypothesize
done
clear
D)
relate
done
clear
View Answer play_arrow
question_answer 195) It is now well known that stress 191 up a 192 of body chemicals that eventually 193 your health. Now scientists 194 further findings, It 195 that every time one goes through 196 stress, the bodys stress thermostat gets 197 to a greater 198 Researchers compare this to an allergy once the body is 199 to a substance. It reacts strongly even at a mere 200 of the stuff.
A)
appears
done
clear
B)
signifies
done
clear
C)
propagates
done
clear
D)
shows
done
clear
View Answer play_arrow
question_answer 196) It is now well known that stress 191 up a 192 of body chemicals that eventually 193 your health. Now scientists 194 further findings, It 195 that every time one goes through 196 stress, the bodys stress thermostat gets 197 to a greater 198 Researchers compare this to an allergy once the body is 199 to a substance. It reacts strongly even at a mere 200 of the stuff.
A)
prolonged
done
clear
B)
serious
done
clear
C)
any
done
clear
D)
proper
done
clear
View Answer play_arrow
question_answer 197) It is now well known that stress 191 up a 192 of body chemicals that eventually 193 your health. Now scientists 194 further findings, It 195 that every time one goes through 196 stress, the bodys stress thermostat gets 197 to a greater 198 Researchers compare this to an allergy once the body is 199 to a substance. It reacts strongly even at a mere 200 of the stuff.
A)
alarmed
done
clear
B)
activated
done
clear
C)
reset
done
clear
D)
ready
done
clear
View Answer play_arrow
question_answer 198) It is now well known that stress 191 up a 192 of body chemicals that eventually 193 your health. Now scientists 194 further findings, It 195 that every time one goes through 196 stress, the bodys stress thermostat gets 197 to a greater 198 Researchers compare this to an allergy once the body is 199 to a substance. It reacts strongly even at a mere 200 of the stuff.
A)
level
done
clear
B)
program
done
clear
C)
energy
done
clear
D)
sensitivity
done
clear
View Answer play_arrow
question_answer 199) It is now well known that stress 191 up a 192 of body chemicals that eventually 193 your health. Now scientists 194 further findings, It 195 that every time one goes through 196 stress, the bodys stress thermostat gets 197 to a greater 198 Researchers compare this to an allergy once the body is 199 to a substance. It reacts strongly even at a mere 200 of the stuff.
A)
alert
done
clear
B)
allergetic
done
clear
C)
sensitized
done
clear
D)
immune
done
clear
View Answer play_arrow
question_answer 200) It is now well known that stress 191 up a 192 of body chemicals that eventually 193 your health. Now scientists 194 further findings, It 195 that every time one goes through 196 stress, the bodys stress thermostat gets 197 to a greater 198 Researchers compare this to an allergy once the body is 199 to a substance. It reacts strongly even at a mere 200 of the stuff.
A)
disturbance
done
clear
B)
whiff
done
clear
C)
addition
done
clear
D)
smell
done
clear
View Answer play_arrow
 \[16\mu F\]
\[16\mu F\] 