A)
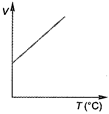
B)
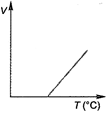
C)
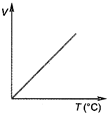
D)
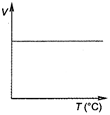
Correct Answer: C
Solution :
At constant pressure, the volume of a given mass of a gas is directly proportional to its absolute temperature\[(T)\]. \[ie,\] \[\frac{V}{T}=\]constant.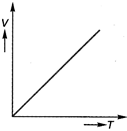 This is another form of Charles law. Hence, variation of volume with temperature is as follows. Hence, correct graph will be (c).
This is another form of Charles law. Hence, variation of volume with temperature is as follows. Hence, correct graph will be (c).
You need to login to perform this action.
You will be redirected in
3 sec
