A) \[s{{p}^{3}},\text{ }ds{{p}^{2}},ds{{p}^{2}}\]
B) \[s{{p}^{3}},\text{ }ds{{p}^{2}},\text{ }s{{p}^{3}}\]
C) \[s{{p}^{3}},\text{ }s{{p}^{3}},\text{ }ds{{p}^{2}}\]
D) \[ds{{p}^{2}},\text{ }s{{p}^{3}},\text{ }sp\]
Correct Answer: B
Solution :
(I) In\[Ni{{(CO)}_{4}},\]nickel is\[s{{p}^{3}}-\]hybrid because in it oxidation state of\[Ni\] is zero. So configuration of \[_{28}Ni=1{{s}^{2}}2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{8}},4{{s}^{2}}\]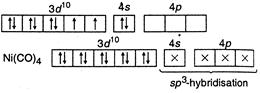 (II) In\[{{[ni{{(CN)}_{4}}]}^{2-}},\]nickel is present as\[N{{i}^{2+}},\] so its configuration \[=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{8}}\]
(II) In\[{{[ni{{(CN)}_{4}}]}^{2-}},\]nickel is present as\[N{{i}^{2+}},\] so its configuration \[=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{8}}\] 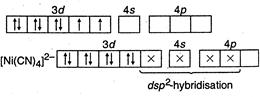 \[C{{N}^{-}}\]is strong field ligand, hence it makes\[N{{I}^{2+}}\] electrons to be paired up. (Ill) In\[{{[NiC{{l}_{4}}]}^{2-}}\]species, nickel is present as \[N{{i}^{2+}},\]so its configuration \[=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}},3{{d}^{8}}.\]
\[C{{N}^{-}}\]is strong field ligand, hence it makes\[N{{I}^{2+}}\] electrons to be paired up. (Ill) In\[{{[NiC{{l}_{4}}]}^{2-}}\]species, nickel is present as \[N{{i}^{2+}},\]so its configuration \[=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}},3{{d}^{8}}.\] 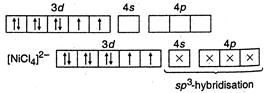 \[C{{l}^{-}}\]is weak field ligand, hence\[N{{i}^{2+}}\]electrons are not paired.
\[C{{l}^{-}}\]is weak field ligand, hence\[N{{i}^{2+}}\]electrons are not paired.
You need to login to perform this action.
You will be redirected in
3 sec
