A) four\[P-P\]single bonds
B) four lone pair of electrons
C) PPP angle of\[60{}^\circ \]
D) light\[P-P\]single bonds
Correct Answer: C
Solution :
\[{{P}_{4}}\] molecule, Bond angle\[=60{}^\circ \] Six \[P-P=\]single bonds Lone pair =4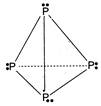
You need to login to perform this action.
You will be redirected in
3 sec
