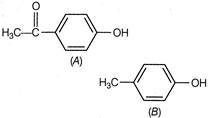
A) The larger size of ketone group helps to stabilize the conjugate base
B) The ketone group exert a large inductive effect in conjugate base of A
C) The ketone group allows for resonance delocalisation of the charge in conjugate base
D) The OH oxygen in A is more electronegative than the OH oxygen in B
Correct Answer: C
Solution :
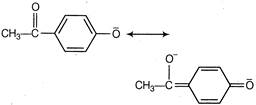 Compound A is more acidic than B because of the delocalisation of the charge in its conjugate base due to resonance.
Compound A is more acidic than B because of the delocalisation of the charge in its conjugate base due to resonance.
You need to login to perform this action.
You will be redirected in
3 sec
