question_answer 1) Two open organ pipes of length 25 cm and; 25.5 cm produce 0.1 beat/sec. The velocity of sound will be:
A)
255m/s
done
clear
B)
250 m/s
done
clear
C)
350 m/s
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 2) The velocity of photon is proportional to (where v is frequency):
A)
\[\frac{{{v}^{2}}}{2}\]
done
clear
B)
\[\frac{1}{\sqrt{v}}\]
done
clear
C)
\[\sqrt{v}\]
done
clear
D)
\[v\]
done
clear
View Answer play_arrow
question_answer 3) Which one of the following is different from others?
A)
Amplitude
done
clear
B)
Frequency
done
clear
C)
Velocity
done
clear
D)
Wavelength
done
clear
View Answer play_arrow
question_answer 4) Intensity of an electric field E due to a dipole, depends on distance r as:
A)
\[E\propto \frac{1}{{{r}^{4}}}\]
done
clear
B)
\[E\propto \frac{1}{{{r}^{3}}}\]
done
clear
C)
\[E\propto \frac{1}{{{r}^{2}}}\]
done
clear
D)
\[E\propto \frac{1}{r}\]
done
clear
View Answer play_arrow
question_answer 5) A simple pendulum is vibrating in an evacuated chamber, it will oscillate with:
A)
increasing amplitude
done
clear
B)
constant amplitude
done
clear
C)
decreasing amplitude
done
clear
D)
first [c] then [a]
done
clear
View Answer play_arrow
question_answer 6) The effective acceleration of a body, when thrown upwards with acceleration a will be:
A)
\[\sqrt{a-{{g}^{2}}}\]
done
clear
B)
\[\sqrt{{{a}^{2}}+{{g}^{2}}}\]
done
clear
C)
\[(a-g)\]
done
clear
D)
\[(a+g)\]
done
clear
View Answer play_arrow
question_answer 7) A diver in a swimming pool bends his head before diving, because it:
A)
decreases his moment of inertia
done
clear
B)
decreases his angular velocity
done
clear
C)
increases his moment of inertia
done
clear
D)
increases his linear velocity
done
clear
View Answer play_arrow
question_answer 8) If\[|\overrightarrow{A}\times \overrightarrow{B}|=|\overrightarrow{A}.\overrightarrow{B}|\]then the angle between\[\overrightarrow{A}\]and \[\overrightarrow{B}\]will be:
A)
\[90{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 9) The units of Plancks constant are:
A)
\[J\,{{s}^{-2}}\]
done
clear
B)
\[J\,{{s}^{2}}\]
done
clear
C)
\[J\,s\]
done
clear
D)
\[J/s\]
done
clear
View Answer play_arrow
question_answer 10) If a force on a rocket moving with a velocity of 300 m/s is 210 N. Then the rate of combustion of the fuel, will be:
A)
1.7 kg/sec
done
clear
B)
0.7 kg/sec
done
clear
C)
2.7 kg/sec
done
clear
D)
1.5 kg/sec
done
clear
View Answer play_arrow
question_answer 11) At what depth below the surface of the earth, acceleration due to gravity g will be half its value 1600 km above the surface of the earth?
A)
\[4.2\times {{10}^{6}}m\]
done
clear
B)
\[3.19\times {{10}^{6}}m\]
done
clear
C)
\[1.59\times {{10}^{6}}m\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 12) A man of weight 75 kg is standing m an elevator which is moving with an acceleration of\[5\text{ }m/{{s}^{2}}\]in upward direction the apparent weight of the man will be:\[(g=10m/{{s}^{2}})\]
A)
1425 N
done
clear
B)
1375 N
done
clear
C)
1250 N
done
clear
D)
1125 N
done
clear
View Answer play_arrow
question_answer 13) A cylinder of 10 kg is rolling in a plane with an initial velocity of 10 m/s. If the coefficient of friction between the surface and cylinder is 0.5 kg then before slipping, it will cover:\[(g=10m/{{s}^{2}})\]
A)
2.5m
done
clear
B)
5m
done
clear
C)
7.5m
done
clear
D)
10m
done
clear
View Answer play_arrow
question_answer 14) The new resistance of wire of\[R\text{ }\Omega ,\]whose radius is reduced half, is:
A)
16R
done
clear
B)
3R
done
clear
C)
2R
done
clear
D)
R
done
clear
View Answer play_arrow
question_answer 15) When a dielectric material is introduced between the plates of a charged condenser, then electric field between the plates:
A)
rem ain constant
done
clear
B)
decreases
done
clear
C)
increases
done
clear
D)
first increases then decreases
done
clear
View Answer play_arrow
question_answer 16) The pitch of a note depends upon its:
A)
wavelength
done
clear
B)
amplitude
done
clear
C)
frequency
done
clear
D)
speed
done
clear
View Answer play_arrow
question_answer 17) A perfectly black body is one where:
A)
absorptive power in infinity
done
clear
B)
absorption point is 0
done
clear
C)
emmissive power is 1
done
clear
D)
absorptive power is 1
done
clear
View Answer play_arrow
question_answer 18) A potential energy of satellite, having mass m and rotating at height of\[6.4\times {{10}^{6}}m\]from the earth surface will be:
A)
\[-0.5\,mg{{R}_{e}}\]
done
clear
B)
\[-1.5mg{{R}_{e}}\]
done
clear
C)
\[-0.67mg{{R}_{e}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 19) If a freely falling body travels in the last second a distance equal to the distance travelled by it in the first three second, the time of the travel is:
A)
6 sec
done
clear
B)
5 sec
done
clear
C)
4 sec
done
clear
D)
3 sec
done
clear
View Answer play_arrow
question_answer 20) The escape velocity from the surface of the earth\[{{\upsilon }_{es}}\]. The escape velocity from the surface of a planet whose mass arid radius are three times those of earth; will be:
A)
\[1/3{{\upsilon }_{e}}\]
done
clear
B)
\[9{{\upsilon }_{e}}\]
done
clear
C)
\[3{{\upsilon }_{e}}\]
done
clear
D)
\[{{\upsilon }_{e}}\]
done
clear
View Answer play_arrow
question_answer 21) A packet is dropped from a balloon which is going upwards with the velocity 12 m/s, the velocity of the packet after 2 seconds will be:
A)
\[-12\text{ }m/s\]
done
clear
B)
12 m/s
done
clear
C)
\[-7.6\text{ }m/s\]
done
clear
D)
7.6 m/s
done
clear
View Answer play_arrow
question_answer 22) On a planet a freely falling body takes 2 sec when it is dropped from a height of 8 m, the time period of simple pendulum of length 1 m on that planet is:
A)
3.14 sec
done
clear
B)
16.28 sec
done
clear
C)
1.57 sec
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 23) The experiments of Frank and Hertz showed\ that:
A)
an atom has energy states having a continuous distribution
done
clear
B)
nothing can be send a bond energy states bf atom
done
clear
C)
at atom has energy states, having discreat values
done
clear
D)
atom is an indivisible particle
done
clear
View Answer play_arrow
question_answer 24) If a copper ring is moved quickly towards south pole of a powerful stationary bar magnet, then:
A)
current flows through the copper ring
done
clear
B)
voltage in the magnet increases
done
clear
C)
current flows in the magnet
done
clear
D)
copper ring will get magnetized
done
clear
View Answer play_arrow
question_answer 25) Velocity time curve for a body, projected vertically upwards, will be:
A)
straight line
done
clear
B)
hyperbola
done
clear
C)
parabola
done
clear
D)
ellipse
done
clear
View Answer play_arrow
question_answer 26) The ratio of charge to the potential of body:
A)
resistance
done
clear
B)
inductance
done
clear
C)
conductance
done
clear
D)
capacitance
done
clear
View Answer play_arrow
question_answer 27) Taking Rydbergs constant\[{{R}_{H}}=1.097\]\[\times {{10}^{7}}m\] first and second wavelength of Balmer series m hydrogen spectrm, is:
A)
\[2000\overset{o}{\mathop{\text{A}}}\,,3000\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[1575\overset{o}{\mathop{\text{A}}}\,,2960\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[6529\overset{o}{\mathop{\text{A}}}\,,4280\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[6552\overset{o}{\mathop{\text{A}}}\,,4863\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
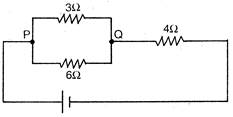
question_answer 28)
If the current through\[3\,\Omega \] resistor is 0.8 A, then potential drop through\[4\,\Omega \] reisistor is:
A)
1.5 V
done
clear
B)
2.6 V
done
clear
C)
4.8 V
done
clear
D)
9.8 V
done
clear
View Answer play_arrow
question_answer 29) A stepup transformer has transformation ratio\[3:2\]. The voltage in the secondary coil, if the voltage m the primary is 30 volt, will be:
A)
300 V
done
clear
B)
90 V
done
clear
C)
45 V
done
clear
D)
23 V
done
clear
View Answer play_arrow
question_answer 30) If three resistors of resistance\[2\Omega ,4\Omega \]and\[5\Omega \] are connected in parallel then the total resistance of the combination will be:
A)
\[\frac{20}{19}\Omega \]
done
clear
B)
\[\frac{19}{20}\Omega \]
done
clear
C)
\[\frac{19}{10}\Omega \]
done
clear
D)
\[\frac{10}{19}\Omega \]
done
clear
View Answer play_arrow
question_answer 31) When a lamp is connected in series with capacitor, then:
A)
lamp will not glow
done
clear
B)
lamp will brust out
done
clear
C)
lamp will glow normally
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 32) The current is flowing in south direction along a power line. The direction of magnetic field above the power line (neglecting earths field) is:
A)
south
done
clear
B)
east
done
clear
C)
north
done
clear
D)
west
done
clear
View Answer play_arrow
question_answer 33) In a junction diode the holes due to:
A)
missing electron
done
clear
B)
extra neutron
done
clear
C)
protons
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 34) What is the respective number of\[\alpha \]and\[\beta -\]particles emitted in the following radioactive decay? \[_{90}{{X}^{200}}{{\xrightarrow[{}]{{}}}_{80}}{{X}^{168}}\]
A)
\[8\alpha ,8\beta \]
done
clear
B)
\[8\alpha ,6\beta \]
done
clear
C)
\[6\alpha ,6\beta \]
done
clear
D)
\[6\alpha ,8\beta \]
done
clear
View Answer play_arrow
question_answer 35) Hygens wave theory of light could not explain:
A)
photoelectric effect
done
clear
B)
polarization
done
clear
C)
interference
done
clear
D)
diffraction
done
clear
View Answer play_arrow
question_answer 36) If the refractive index of material of equilateral prism is\[\sqrt{3}\]then angle of minimum deviation of the prism will be:
A)
\[25{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 37) In Bohrs model the atomic radius of the first orbit is\[{{r}_{0}},\]the radius of the third orbit will be:
A)
\[9{{r}_{0}}\]
done
clear
B)
\[3{{r}_{0}}\]
done
clear
C)
\[{{r}_{0}}\]
done
clear
D)
\[\frac{{{r}_{0}}}{3}\]
done
clear
View Answer play_arrow
question_answer 38) A process in which temperature T of the system remains constant though other variable P and V may change, is called:
A)
isochoric process
done
clear
B)
isothermal process
done
clear
C)
isobaric process
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 39) In achromatic combination of lenses is formed by joining:
A)
1 convex lens and 1 plane mirror
done
clear
B)
1 convex, 1 concave lens
done
clear
C)
2 convex lenses
done
clear
D)
2 concave lenses
done
clear
View Answer play_arrow
question_answer 40) Which of me unipolar transistor-
A)
point contact transistor
done
clear
B)
field effect transistor
done
clear
C)
p-n-p transistor
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 41) In a meter bridge, the balancing length from the left end (standard resistance of 1 ohm m the gap) is found to be 20 cm. The value of the unknown resistance will be.
A)
\[0.25\,\Omega \]
done
clear
B)
\[0.4\,\Omega \]
done
clear
C)
\[0.5\,\Omega \]
done
clear
D)
\[0.8\,\Omega \]
done
clear
View Answer play_arrow
question_answer 42) The magnetic flux linked with coil, in web is given by the equation, \[\phi =5{{t}^{2}}+3t+16.\] The induced emf in the coil m the fourth second is:
A)
10 V
done
clear
B)
30 V
done
clear
C)
45 V
done
clear
D)
90 V
done
clear
View Answer play_arrow
question_answer 43) Serious draw back of semiconductor device is:
A)
they cannot be used with high voltage
done
clear
B)
hey pollute the environment
done
clear
C)
they are costly
done
clear
D)
they do not last for long time
done
clear
View Answer play_arrow
question_answer 44) The potential difference between the cathode and the target in a Coolidge tube is 100 kV. The minimum wavelength of the X-ray emitted by the tube is:
A)
\[0.66\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[938\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[0.246\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[0.123\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 45) For an isotropic medium\[B,\mu ,H\]and\[M\]are related as (where\[B,{{\mu }_{0}},H\]and M have their usual meaning in the context of magnetic material:
A)
\[(B-M)={{\mu }_{0}}H\]
done
clear
B)
\[M={{\mu }_{0}}(H+M)\]
done
clear
C)
\[H={{\mu }_{0}}(H+M)\]
done
clear
D)
\[B={{\mu }_{0}}(H+M)\]
done
clear
View Answer play_arrow
question_answer 46) A cricket ball is hit at\[45{}^\circ \]to the horizontal with kinetic energy\[K,\]The kinetic energy at the highest point is:
A)
\[K\]
done
clear
B)
\[K/\sqrt{2}\]
done
clear
C)
\[K/2\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 47) The root mean square (rms) speed of oxygen molecules\[{{O}_{2}}\]at a temperature T (degree absolute) is\[\upsilon \]. If the temperature is doubled and oxygen gas dissociates into atomic oxygen. The rms speed:
A)
becomes\[\upsilon /\sqrt{2}\]
done
clear
B)
remains\[\upsilon \]
done
clear
C)
becomes\[\sqrt{2}\upsilon \]
done
clear
D)
becomes\[2\upsilon \]
done
clear
View Answer play_arrow
question_answer 48) Minimum excitation potential of Bohrs orbit in hydrogen atom is.
A)
3.6 V
done
clear
B)
10.2 V
done
clear
C)
3.4 V
done
clear
D)
13.6 V
done
clear
View Answer play_arrow
question_answer 49) A X-ray has a wavelength\[0.01\,\overset{o}{\mathop{\text{A}}}\,\]Its momentum is:
A)
\[6.626\times {{10}^{-22}}kg\,m/s\]
done
clear
B)
\[3.45\times {{10}^{-25}}kg\,m/s\]
done
clear
C)
\[3.313\times {{10}^{-22}}kg\,m/s\]
done
clear
D)
\[2.126\times {{10}^{-23}}kg\text{ }m/s\]
done
clear
View Answer play_arrow
question_answer 50) \[Rn\]decay info\[{{P}_{o}}\]by emitting\[\alpha -\]particles with life of 4 days a sample contains\[6.4\times {{10}^{10}}\]atom of Rn. After 12 days, the number of atoms of\[Rn\] left in the sample will be:
A)
\[0.8\times {{10}^{10}}\]
done
clear
B)
\[2.1\times {{10}^{10}}\]
done
clear
C)
\[3.2\times {{10}^{10}}\]
done
clear
D)
\[0.3\times {{10}^{10}}\]
done
clear
View Answer play_arrow
question_answer 51) The trivalent ion having largest size in larithanide series is:
A)
\[Ti\]
done
clear
B)
\[Zr\]
done
clear
C)
\[HF\]
done
clear
D)
\[La\]
done
clear
View Answer play_arrow
question_answer 52) The aldehyde which react with\[NaOH\]to produce an alcohol and sodium salt is:
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CHO\]
done
clear
View Answer play_arrow
question_answer 53) The IUPAC name of \[{{(C{{H}_{3}})}_{2}}-CHC{{H}_{2}}-C{{H}_{2}}Br\]is:
A)
1-bromo pentane
done
clear
B)
2-methyl-4-bromo butane
done
clear
C)
2-methyl-3-bromo pentane
done
clear
D)
1-bromo-3-methyl butane
done
clear
View Answer play_arrow
question_answer 54) A system absorb 600 J of heat and work equivalent to 300 J on its surroundings. The change in internal energy is:
A)
300J
done
clear
B)
400J
done
clear
C)
500J
done
clear
D)
600J
done
clear
View Answer play_arrow
question_answer 55) Glucose and bromine water reacts to produce:
A)
glucarric acid
done
clear
B)
gluconic acid
done
clear
C)
sorbitol
done
clear
D)
laevulinic acid
done
clear
View Answer play_arrow
question_answer 56) The number of atoms present in unit cell of a monoatomic substance of simple cubic lattice is:
A)
6
done
clear
B)
3
done
clear
C)
2
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 57) The ore carnalite is represented by structure:
A)
\[N{{a}_{2}}A{{l}_{2}}{{O}_{3}}\]
done
clear
B)
\[N{{a}_{3}}Al{{F}_{6}}\]
done
clear
C)
\[KClMgC{{l}_{2}}6{{H}_{2}}O\]
done
clear
D)
\[F{{e}_{3}}{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 58) Quartz is a crystalline variety of:
A)
silicon carbide
done
clear
B)
sodium silicate
done
clear
C)
silica
done
clear
D)
silicon
done
clear
View Answer play_arrow
question_answer 59) The conjugate acid of\[N{{H}_{3}}\]ion is:
A)
\[N{{H}_{2}}OH\]
done
clear
B)
\[NH_{4}^{+}\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[NH_{2}^{+}\]
done
clear
View Answer play_arrow
question_answer 60) The emf of a galvanic cell, with electrode potential of zinc\[=+0.76V\]and that of copper = 0.34 V, is:
A)
\[0.76V\]
done
clear
B)
\[1.1V\]
done
clear
C)
\[-1,1V\]
done
clear
D)
\[1.6V\]
done
clear
View Answer play_arrow
question_answer 61) The maximum number of electrons present in an orbit\[l=3,\]is:
A)
6
done
clear
B)
8
done
clear
C)
10
done
clear
D)
14
done
clear
View Answer play_arrow
question_answer 62) One mole of water at\[100{}^\circ C\]is converted into steam at\[100{}^\circ C\]at a constant pressure of, 1 atm. The change in entropy is [heat of vaporisation of water at\[100{}^\circ C=540\]cal/gm]:
A)
8.74
done
clear
B)
18.76
done
clear
C)
24.06
done
clear
D)
26.06
done
clear
View Answer play_arrow
question_answer 63) The charge on gamma rays is:
A)
zero
done
clear
B)
\[+1\]
done
clear
C)
\[-1\]
done
clear
D)
\[+2\]
done
clear
View Answer play_arrow
question_answer 64) The equilibrium concentration of the species in the reaction\[A+B=C+D\]are 3,5,10 and 15 mole\[{{L}^{-1}}\]respectively at 300 K the\[\Delta G\]for the reaction is:
A)
13.81
done
clear
B)
\[-1381.8\]
done
clear
C)
\[-138.18\]
done
clear
D)
1391.6
done
clear
View Answer play_arrow
question_answer 65) Acetaldehyde gives orange coloured precipitate on treatment with:
A)
2,4-DNP
done
clear
B)
\[N{{H}_{2}}OH\]
done
clear
C)
\[NaHS{{O}_{3}}\]
done
clear
D)
\[NaOH\]
done
clear
View Answer play_arrow
question_answer 66) Oxygen is not evolved on reaction of ozone with:
A)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
B)
\[S{{O}_{3}}\]
done
clear
C)
\[Hg\]
done
clear
D)
\[KI\]
done
clear
View Answer play_arrow
question_answer 67) The volume occupied by one mole of water is [ density of water\[=1\text{ }gm/c{{m}^{3}}\]:
A)
\[22400\text{ }c{{m}^{3}}\]
done
clear
B)
\[6.02\times {{10}^{-23}}c{{m}^{3}}\]
done
clear
C)
\[3.0\times {{10}^{-23}}c{{m}^{3}}\]
done
clear
D)
\[11200\text{ }c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 68) Alcoholic beverages contains:
A)
\[C{{H}_{3}}OH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}CHOH\]
done
clear
View Answer play_arrow
question_answer 69) Calcium cyanamide on treatment with steam produce:
A)
\[CaC{{O}_{3}}+N{{H}_{3}}\]
done
clear
B)
\[CaHC{{O}_{3}}+N{{H}_{3}}\]
done
clear
C)
\[CaO+N{{H}_{3}}\]
done
clear
D)
\[Ca{{(OH)}_{2}}+N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 70) The laboratory method for the preparation of\[{{H}_{2}}{{O}_{2}}\]is:
A)
by adding\[Pb{{O}_{2}}\]into\[KMn{{O}_{4}}\]solution
done
clear
B)
by adding\[N{{a}_{2}}O\]to cold water
done
clear
C)
by adding\[Mn{{O}_{2}}\]to dil \[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
by passing\[C{{O}_{2}}\]into\[Ba{{O}_{2}}\]solution
done
clear
View Answer play_arrow
question_answer 71) The rate of reaction depends upon:
A)
concentration of reactants
done
clear
B)
pressure
done
clear
C)
volume
done
clear
D)
force
done
clear
View Answer play_arrow
question_answer 72) Among the following the weakest one is:
A)
metallic bond
done
clear
B)
ionic bond
done
clear
C)
van der Waals force
done
clear
D)
covalent bond
done
clear
View Answer play_arrow
question_answer 73) The hybridisation in\[B{{F}_{3}}\]molecule is:
A)
\[sp\]
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{3}}\]
done
clear
D)
\[s{{p}^{3}}d\]
done
clear
View Answer play_arrow
question_answer 74) The molality of 90%\[{{H}_{2}}S{{O}_{4}}\]solution is [density\[=1.8\text{ }g\text{ }ml\]]:
A)
1.8
done
clear
B)
48.4
done
clear
C)
91.8
done
clear
D)
94.6
done
clear
View Answer play_arrow
question_answer 75) The volume of oxygen liberated from 0.68 gm of\[{{H}_{2}}{{O}_{2}}\]is:
A)
\[112\text{ }ml\]
done
clear
B)
\[224\text{ }ml\]
done
clear
C)
\[56\,ml\]
done
clear
D)
\[336\text{ }ml\]
done
clear
View Answer play_arrow
question_answer 76) \[{{C}_{2}}{{H}_{5}}I\]and\[A{{g}_{2}}O\]reacts to produce:
A)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}-{{C}_{2}}{{H}_{5}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}-O-{{C}_{2}}{{H}_{5}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 77) \[Mg\]burns in CO to produce:
A)
\[Mg{{O}_{2}}\]
done
clear
B)
\[MgC{{O}_{3}}\]
done
clear
C)
\[MgO+CO\]
done
clear
D)
\[MgO+C\]
done
clear
View Answer play_arrow
question_answer 78) The mass of a photon with a wavelength equal to\[1.54\times {{10}^{-8}}cm\]is:
A)
\[0.8268\times {{10}^{-34}}kg\]
done
clear
B)
\[1.2876\times {{10}^{-33}}kg\]
done
clear
C)
\[1.4285\times {{10}^{-32}}kg\]
done
clear
D)
\[1.8884\times {{10}^{-32}}kg\]
done
clear
View Answer play_arrow
question_answer 79) An organic compound on analysis produce \[C=40%,\] \[H=13.33\] and \[N=46.67%.\] The empirical formula of this compound is
A)
\[C{{H}_{5}}N\]
done
clear
B)
\[C{{H}_{4}}N\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}N\]
done
clear
D)
\[{{C}_{2}}{{H}_{4}}N\]
done
clear
View Answer play_arrow
question_answer 80) The most electronegative halogen is:
A)
chlorine
done
clear
B)
bromine
done
clear
C)
fluorine
done
clear
D)
iodine
done
clear
View Answer play_arrow
question_answer 81) The planar structure is represented by:
A)
methane
done
clear
B)
acetylene
done
clear
C)
isobutene
done
clear
D)
benzene
done
clear
View Answer play_arrow
question_answer 82) \[Ca{{(OH)}_{2}}+{{H}_{3}}P{{O}_{4}}\xrightarrow[{}]{{}}CaHP{{O}_{4}}+2{{H}_{2}}O\] the equivalent weight of\[{{H}_{3}}P{{O}_{4}}\]in the above reaction is:
A)
21
done
clear
B)
27
done
clear
C)
38
done
clear
D)
49
done
clear
View Answer play_arrow
question_answer 83) The microcosmic salt is:
A)
\[Na(N{{H}_{4}}){{H}_{2}}O\]
done
clear
B)
\[K(N{{H}_{4}})HP{{O}_{3}}2{{H}_{2}}O\]
done
clear
C)
\[Na(N{{H}_{4}})HP{{O}_{4}}2{{H}_{2}}O\]
done
clear
D)
\[Na(N{{H}_{3}})HP{{O}_{4}}4{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 84) Codon is present in:
A)
\[t-RNA\]
done
clear
B)
\[m-RNA\]
done
clear
C)
\[r-RNA\]
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 85) The bond order in\[CO_{3}^{2-}\]ion between
A)
zero
done
clear
B)
0.88
done
clear
C)
1.33
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 86) At\[25{}^\circ C\]the specific conductivity of a normal solution of\[KCl\]is 0.002765 mho. The resistance of cell is 400 ohms. The cell constant is:
A)
0.815
done
clear
B)
1.016
done
clear
C)
1.106
done
clear
D)
2.016
done
clear
View Answer play_arrow
question_answer 87) Carboxylic acid undergoes ionization due to:
A)
hydrogen bonding
done
clear
B)
absence of\[\alpha -\]hydrogen
done
clear
C)
high reactivity of\[\alpha -\]hydrogen
done
clear
D)
resonance stabilisation of carboxylate ions.
done
clear
View Answer play_arrow
question_answer 88) \[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\xrightarrow[{}]{NaN{{O}_{2}}HCl}X\xrightarrow[{}]{C{{u}_{2}}{{(CN)}_{2}}}\] \[Y\xrightarrow[{}]{{{H}_{2}}O/{{H}^{+}}}Z\] Z is identified as:
A)
\[{{C}_{6}}{{H}_{5}}-NH-C{{H}_{3}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}-COOH\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}-C{{H}_{2}}-N{{H}_{2}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}-C{{H}_{2}}-COOH\]
done
clear
View Answer play_arrow
question_answer 89) The nitration of a compound is due to the:
A)
\[N{{O}_{2}}\]
done
clear
B)
\[N{{O}_{3}}\]
done
clear
C)
\[NO\]
done
clear
D)
\[{{N}^{+}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 90) The reaction of fat and sodium hydroxide is known as:
A)
dehydration
done
clear
B)
hydrogenation
done
clear
C)
saponification
done
clear
D)
esterification
done
clear
View Answer play_arrow
question_answer 91) The molecular weight of a gas is 45. Its density at STP is:
A)
22.4
done
clear
B)
11.2
done
clear
C)
5.7
done
clear
D)
2.0
done
clear
View Answer play_arrow
question_answer 92) Accodring to Arrhenius hypothesis, rate of a reaction increases with:
A)
rise in temperature
done
clear
B)
decrease in temperature
done
clear
C)
rise in pressure
done
clear
D)
decrease in pressure
done
clear
View Answer play_arrow
question_answer 93) Which destroy antigens?
A)
insulin
done
clear
B)
antibodies
done
clear
C)
chromoprotein
done
clear
D)
phosphoprotein
done
clear
View Answer play_arrow
question_answer 94) For a weak acid, the incorrect statement is:
A)
its dissociation constant is low
done
clear
B)
its\[_{p}{{K}_{a}}\]is very low
done
clear
C)
it is partially dissociated
done
clear
D)
solution of its sodium salt is alkaline in water
done
clear
View Answer play_arrow
question_answer 95) The weight of a residue obtained by heating 2.76 g of silver carbonate is:
A)
2.76 g
done
clear
B)
2.98 g
done
clear
C)
2.16 g
done
clear
D)
2.44 g
done
clear
View Answer play_arrow
question_answer 96) The transition metal used as a catalyst is:
A)
nickel
done
clear
B)
platinum
done
clear
C)
cobalt
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 97) The number of moles of oxygen present in one litre of air under standard conditions (it contains 21% oxygen) is:
A)
0.246 mole
done
clear
B)
0.07438 mole
done
clear
C)
2.0078 mole
done
clear
D)
0.0093 mole
done
clear
View Answer play_arrow
question_answer 98) Water gas is a mixture of:
A)
\[CO+{{N}_{2}}+{{H}_{2}}\]
done
clear
B)
\[C{{O}_{2}}+{{N}_{2}}\]
done
clear
C)
\[CO+{{H}_{2}}\]
done
clear
D)
\[C{{O}_{2}}+{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 99) \[BaS{{O}_{4}}\]and carbon on heating reacts to produce:
A)
\[Ba+S{{O}_{2}}+C{{O}_{2}}\]
done
clear
B)
\[BaS+CO\]
done
clear
C)
\[BaS+{{O}_{2}}+S{{O}_{2}}\]
done
clear
D)
\[BaC{{O}_{3}}+S+{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 100) The enzyme present in saliva is:
A)
lipase
done
clear
B)
pepsin
done
clear
C)
ptyalin
done
clear
D)
proteinase
done
clear
View Answer play_arrow
question_answer 101) The study related to the structure and function of a cell is called as:
A)
physiology
done
clear
B)
cytology
done
clear
C)
histology
done
clear
D)
cellology
done
clear
View Answer play_arrow
question_answer 102) The longest phase of meiosis\[I\]is:
A)
metaphase\[I\]
done
clear
B)
prophase\[I\]
done
clear
C)
anaphase\[I\]
done
clear
D)
telophase\[I\]
done
clear
View Answer play_arrow
question_answer 103) Fluid mosaic model was given by:
A)
Knoll and Ruska
done
clear
B)
Singer and Ruska
done
clear
C)
Singer and Nicolson
done
clear
D)
Bateson and Punnet
done
clear
View Answer play_arrow
question_answer 104) Colchicine prevents the mitosis of cell at:
A)
prophase stage
done
clear
B)
anaphase stage
done
clear
C)
telophase stage
done
clear
D)
metaphase stage
done
clear
View Answer play_arrow
question_answer 105) Phylogenetic system of classification was proposed by:
A)
Linneaus
done
clear
B)
Bentham
done
clear
C)
Hutchinson
done
clear
D)
Engler
done
clear
View Answer play_arrow
question_answer 106) The first acceptor of\[C{{O}_{2}}\]in\[{{C}_{4}}\]plant is:
A)
pyruvic acid
done
clear
B)
phosphoenol pyruvic acid
done
clear
C)
acetic acid
done
clear
D)
oxaloacetic acid
done
clear
View Answer play_arrow
question_answer 107) The term mitochondria was given by:
A)
Benda
done
clear
B)
Altmann
done
clear
C)
Palade
done
clear
D)
de Duve
done
clear
View Answer play_arrow
question_answer 108) Auxenometer is used to measure:
A)
length
done
clear
B)
width
done
clear
C)
depth
done
clear
D)
growth
done
clear
View Answer play_arrow
question_answer 109) The term protoplasm was coined by:
A)
Virchov
done
clear
B)
Purkinje
done
clear
C)
DuJardin
done
clear
D)
Kolliker
done
clear
View Answer play_arrow
question_answer 110) In Ulothrix meiosis occurs in:
A)
gamete
done
clear
B)
zygospore
done
clear
C)
zoospore
done
clear
D)
thallus
done
clear
View Answer play_arrow
question_answer 111) Fern gametophyte bears:
A)
archegonia
done
clear
B)
antheridia
done
clear
C)
sporangia
done
clear
D)
both a and b
done
clear
View Answer play_arrow
question_answer 112) Alexender Flamming in 1929 discovered:
A)
penicillin
done
clear
B)
streptomycin
done
clear
C)
tetracyclin
done
clear
D)
chloromycitin
done
clear
View Answer play_arrow
question_answer 113) Lichens are:
A)
sporophyte
done
clear
B)
parasite
done
clear
C)
eymbionts
done
clear
D)
predator
done
clear
View Answer play_arrow
question_answer 114) Parachute mechanism of seed dispersal is seen in:
A)
Poppy
done
clear
B)
Calotorpis
done
clear
C)
Plumbago
done
clear
D)
Lotus
done
clear
View Answer play_arrow
question_answer 115) Lomentum is a kind of:
A)
inflorescence
done
clear
B)
plant
done
clear
C)
fruit
done
clear
D)
insect
done
clear
View Answer play_arrow
question_answer 116) Which of the following is considered as a recessive character of Mendel?
A)
Round seed
done
clear
B)
Wrinkled seed
done
clear
C)
Axial flower
done
clear
D)
Green pod
done
clear
View Answer play_arrow
question_answer 117) In which of the following parthenocarpy makes no sense:
A)
Banana
done
clear
B)
Orange
done
clear
C)
Lemon
done
clear
D)
Pomegranate
done
clear
View Answer play_arrow
question_answer 118) Manmade allopolyploid cereal crop is:
A)
Raphano brassica
done
clear
B)
Triticale
done
clear
C)
Pomato
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 119) Cellular respiration occurs in:
A)
chloroplast
done
clear
B)
golgibodies
done
clear
C)
mitochondria
done
clear
D)
nucleus
done
clear
View Answer play_arrow
question_answer 120) Enzymes are functional at:
A)
\[10-15{}^\circ C\]
done
clear
B)
\[15-25{}^\circ C\]
done
clear
C)
\[25-30{}^\circ C\]
done
clear
D)
\[30-50{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 121) Multiplication of DNA is called:
A)
translation
done
clear
B)
replication
done
clear
C)
duplication
done
clear
D)
transcription
done
clear
View Answer play_arrow
question_answer 122) The pigment sensitive for red and far red light is:
A)
chlorophyll
done
clear
B)
phytochrome
done
clear
C)
cytochrome
done
clear
D)
carotene
done
clear
View Answer play_arrow
question_answer 123) Decomposers are:
A)
autotrophs
done
clear
B)
heterotrophs
done
clear
C)
organotrophs
done
clear
D)
autoheterotrophs
done
clear
View Answer play_arrow
question_answer 124) Acid ram is due to the pollution by:
A)
\[C{{O}_{2}}\]
done
clear
B)
\[S{{O}_{2}}\]and\[N{{O}_{2}}\]
done
clear
C)
dust particles
done
clear
D)
automobiles
done
clear
View Answer play_arrow
question_answer 125) Ovule with funiculus lying close to micropyle is known as:
A)
anatropous
done
clear
B)
campylotropous
done
clear
C)
atropous
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 126) Hormone preventing seed dormancy is:
A)
Auxin
done
clear
B)
Gibberellin
done
clear
C)
Ethylene
done
clear
D)
Cytokinin
done
clear
View Answer play_arrow
question_answer 127) In CAM plants stomata are:
A)
closed to night and open during the day
done
clear
B)
closed during the day & open at night
done
clear
C)
never closes
done
clear
D)
never opens
done
clear
View Answer play_arrow
question_answer 128) Which of the following is biodegradable pollutant?
A)
Sewage
done
clear
B)
Plastic
done
clear
C)
Polythene
done
clear
D)
Asbestos
done
clear
View Answer play_arrow
question_answer 129) In dicot stem secondary growth is due to the activity of:
A)
apical meristem
done
clear
B)
lateral meristem
done
clear
C)
cork
done
clear
D)
bark
done
clear
View Answer play_arrow
question_answer 130) The real force responsible for the movement of water from cell to cell is:
A)
OP
done
clear
B)
TP
done
clear
C)
DPD
done
clear
D)
BP
done
clear
View Answer play_arrow
question_answer 131) The inflorescence found in Ficus is known as:
A)
cyathium
done
clear
B)
catkin
done
clear
C)
syconus
done
clear
D)
hypanthodium
done
clear
View Answer play_arrow
question_answer 132) In Opuntia the spines are modification of:
A)
leaf
done
clear
B)
branch
done
clear
C)
epidermis
done
clear
D)
flower
done
clear
View Answer play_arrow
question_answer 133) Witches broom disease is caused by:
A)
Pungus
done
clear
B)
Mycoplasma
done
clear
C)
Bacteria
done
clear
D)
mineral
done
clear
View Answer play_arrow
question_answer 134) Antiviral substance is:
A)
antigen
done
clear
B)
antibody
done
clear
C)
interferon
done
clear
D)
interon
done
clear
View Answer play_arrow
question_answer 135) Which of the following is symbiotic bacteria?
A)
Rhizobhim
done
clear
B)
Azotobactor
done
clear
C)
Clostridium
done
clear
D)
Streptomyces
done
clear
View Answer play_arrow
question_answer 136) The thallus of Volvox is called:
A)
trichome
done
clear
B)
coenobium
done
clear
C)
coenocyte
done
clear
D)
parenchymatous
done
clear
View Answer play_arrow
question_answer 137) Resin duct of gymnospermous stem is an example of:
A)
lysigenous cavity
done
clear
B)
lysogenous cavity
done
clear
C)
shizogenous cavity
done
clear
D)
shizolysigenous cavity
done
clear
View Answer play_arrow
question_answer 138) During food chain the maximum energy is stored in:
A)
producers
done
clear
B)
decomposers
done
clear
C)
herbivores
done
clear
D)
carnivores
done
clear
View Answer play_arrow
question_answer 139) Insectivorous plants usually survive in :
A)
water rich soil
done
clear
B)
\[{{N}_{2}}\]deficient soil
done
clear
C)
\[{{N}_{2}}\]rich soil
done
clear
D)
sugar deficient medium
done
clear
View Answer play_arrow
question_answer 140) Spore of Funaria on germination give rise to:
A)
protonema
done
clear
B)
embryo
done
clear
C)
antheridia
done
clear
D)
archegonia
done
clear
View Answer play_arrow
question_answer 141) The function of a vessel is:
A)
conduction of food
done
clear
B)
conduction of water and minerals
done
clear
C)
conduction of hormones
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 142) In the phosphorus cycle, weathering makes phosphate available first to:
A)
decomposers
done
clear
B)
consumers
done
clear
C)
producers
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 143) Periderm includes:
A)
cork, cork cambium
done
clear
B)
secondary cortex
done
clear
C)
cork
done
clear
D)
cork, cork cambium and secondary cortex
done
clear
View Answer play_arrow
question_answer 144) In a food chain, the total amount of living material is depicted by:
A)
pyramid of biomass
done
clear
B)
pyramid of energy
done
clear
C)
pyramid of number
done
clear
D)
trophic levels
done
clear
View Answer play_arrow
question_answer 145) In the process of photosynthesis water molecule breaks in:
A)
red drop
done
clear
B)
photolysis
done
clear
C)
phosphorylation
done
clear
D)
carbon assimilation
done
clear
View Answer play_arrow
question_answer 146) Syngamy is the process in which:
A)
male gamete fuses with female gamete
done
clear
B)
pollen tube enters into the ovule through micropyle
done
clear
C)
pollen tube enter into the ovule through chalaza
done
clear
D)
vegetative cell and tube cell fuse
done
clear
View Answer play_arrow
question_answer 147) The\[1:2:1\]ratio with the pink flower in the\[{{F}_{2}}\] generation indicate the phenomenon of:
A)
dominance
done
clear
B)
codominance
done
clear
C)
incomplete dominance
done
clear
D)
segregation
done
clear
View Answer play_arrow
question_answer 148) The chemical produced by the host plant to protect themselves against fungal infection is:
A)
toxin
done
clear
B)
phytoalexin
done
clear
C)
phytotoxin
done
clear
D)
hormone
done
clear
View Answer play_arrow
question_answer 149) The phenomenon ontogeny repeats phylogeny is explained by:
A)
natural selection
done
clear
B)
inheritance theory
done
clear
C)
mutation theory
done
clear
D)
recapitulation theory
done
clear
View Answer play_arrow
question_answer 150) Nitrates are converted to nitrogen by:
A)
iiitrogen fixing bacteria
done
clear
B)
ammonification bacteria
done
clear
C)
denitrifying bacteria
done
clear
D)
nitrifying bacteria
done
clear
View Answer play_arrow
question_answer 151) The continuous excretion of watery substance from stump of a well watered pot plant after cutting off the shoot slightly above the base is due to:
A)
root pressure
done
clear
B)
guttation
done
clear
C)
transpiration
done
clear
D)
imbibition
done
clear
View Answer play_arrow
question_answer 152) The organelle which bear anthocyanins is:
A)
cholroplast
done
clear
B)
chromoplast
done
clear
C)
vacuole
done
clear
D)
itioplast
done
clear
View Answer play_arrow
question_answer 153) Which of the following hormone is gaseous in nature?
A)
ethylene
done
clear
B)
auxin
done
clear
C)
gibberellin
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 154) A nucleoside is:
A)
sugar + base
done
clear
B)
sugar + phosphate
done
clear
C)
base + phosphate
done
clear
D)
sugar + base + phosphate
done
clear
View Answer play_arrow
question_answer 155) A lubricant, mucin in saliva is made up of:
A)
glycoprotein
done
clear
B)
polysaccharides
done
clear
C)
phospholipids
done
clear
D)
myosin
done
clear
View Answer play_arrow
question_answer 156) At which phase of meiosis, the two cells, each with separated sister chromatids move towards opposite poles:
A)
metaphase I
done
clear
B)
metaphase II
done
clear
C)
anaphase I
done
clear
D)
anaphase II
done
clear
View Answer play_arrow
question_answer 157) A metazoa without tissue organisation is called:
A)
parazoa
done
clear
B)
protozoa
done
clear
C)
eumetazoa
done
clear
D)
dermatozoa
done
clear
View Answer play_arrow
question_answer 158) The nucleus is separated from surrounding cytoplasm by a nuclear membrane, which is:
A)
single layered with pores
done
clear
B)
single layered without pores
done
clear
C)
double layered with pores
done
clear
D)
double layered without pores
done
clear
View Answer play_arrow
question_answer 159) During conjugation in Pammaecium:
A)
out of the four microhuclei formed degenerate
done
clear
B)
out of six macronuclei formed, four degenerate
done
clear
C)
zygote nucleus undergoes eight successive division in each conjugant
done
clear
D)
out of 16 nuclei only 4 degenerate
done
clear
View Answer play_arrow
question_answer 160) Which of the following pair is correct:
A)
protozoa - Hydra
done
clear
B)
arthropoda - Crustacea-cockroach
done
clear
C)
mollusca - Cephalopoda Octopus
done
clear
D)
Annelide-Polychaeta-leech
done
clear
View Answer play_arrow
question_answer 161) Ecosystem consists of:
A)
producers
done
clear
B)
consumers
done
clear
C)
decomposers
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 162) The correct sequence of food chain is:
A)
grass-insect-bird-snake
done
clear
B)
grass-bird-insect-snake
done
clear
C)
snake-bird-insect-grass
done
clear
D)
grass-snake-bird-insect
done
clear
View Answer play_arrow
question_answer 163) The terminating codon are:
A)
UAA
done
clear
B)
UAG
done
clear
C)
UGA
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 164) Tocopherol stands for:
A)
vit. K
done
clear
B)
vit. A
done
clear
C)
vit. E
done
clear
D)
vit. C
done
clear
View Answer play_arrow
question_answer 165) The outer membranee of lung is called:
A)
pericardium
done
clear
B)
peritonium
done
clear
C)
pleural membrane
done
clear
D)
perichondrium
done
clear
View Answer play_arrow
question_answer 166) Blood group O has:
A)
no antibodies
done
clear
B)
no antigens
done
clear
C)
A or B antibodies
done
clear
D)
A or B antigens
done
clear
View Answer play_arrow
question_answer 167) Darwins finches represents:
A)
morphological variation
done
clear
B)
geographical isolation
done
clear
C)
climatic variation
done
clear
D)
reproductive isolation
done
clear
View Answer play_arrow
question_answer 168) DPT vaccine is given for:
A)
tetanus, polio, plague
done
clear
B)
diphtheria, whooping cough and leprosy
done
clear
C)
diphtheria, pneumonia, tetanus
done
clear
D)
diphtheria, whooping cough, tetanus
done
clear
View Answer play_arrow
question_answer 169) Abiogenetic theory of origin states:
A)
spontaneous generation
done
clear
B)
organic evolution due to chemical reaction
done
clear
C)
origin of life due to pre-existing organisms
done
clear
D)
origin of life from blue green algae
done
clear
View Answer play_arrow
question_answer 170) What is the scientific name of pinworm of man?
A)
Trichmella spiralis
done
clear
B)
Dracuncuhis medinensis
done
clear
C)
Trichuris irichuria
done
clear
D)
Enterobius vermiciuaris
done
clear
View Answer play_arrow
question_answer 171) Which of the following is enterocoelomate invertebrate?
A)
Echinodermata
done
clear
B)
Arthropoda
done
clear
C)
Annelida
done
clear
D)
Mollusca
done
clear
View Answer play_arrow
question_answer 172) The egg of a frog is:
A)
centrolecithal
done
clear
B)
macrolecithal
done
clear
C)
microlecithal
done
clear
D)
mesolecithal
done
clear
View Answer play_arrow
question_answer 173) Sub class Prototheria is related with egg laying mammal such as:
A)
Kangaroo
done
clear
B)
Echidna
done
clear
C)
Primate
done
clear
D)
none
done
clear
View Answer play_arrow
question_answer 174) Regeneration of tail in lizards is an example of:
A)
epimorphosis
done
clear
B)
morpholaxis
done
clear
C)
heteromrophosis
done
clear
D)
parthenogenesis
done
clear
View Answer play_arrow
question_answer 175) Territoriality occurs as a result of:
A)
predation
done
clear
B)
parasitism
done
clear
C)
symbiotism
done
clear
D)
competition
done
clear
View Answer play_arrow
question_answer 176) Which of the following metal is a water pollutant and causes sterility in human being?
A)
\[As\]
done
clear
B)
\[Mn\]
done
clear
C)
\[Mg\]
done
clear
D)
\[Hg\]
done
clear
View Answer play_arrow
question_answer 177) Snake has:
A)
movable eyelids
done
clear
B)
immovable eyelids
done
clear
C)
no eyelids
done
clear
D)
eyelids in pouches
done
clear
View Answer play_arrow
question_answer 178) Cockroach and earthworm have common type of:
A)
heart
done
clear
B)
nerve cord
done
clear
C)
nephridia
done
clear
D)
spermathecae
done
clear
View Answer play_arrow
question_answer 179) Which of the following cell type is capable of giving rise to other cell type in sponges?
A)
Archaeocytes
done
clear
B)
Collenocytes
done
clear
C)
Therocytes
done
clear
D)
Pinacocytes
done
clear
View Answer play_arrow
question_answer 180) Which of the following is characteristic of fishes?
A)
Tail and venous heart
done
clear
B)
Venous heart and gills
done
clear
C)
Gills and tail
done
clear
D)
Scales and gills
done
clear
View Answer play_arrow
question_answer 181) The main function of Henles loop is:
A)
conservation of water
done
clear
B)
filtration of blood
done
clear
C)
passage of urine
done
clear
D)
formation of urine
done
clear
View Answer play_arrow
question_answer 182) Transmission of nerve impulse, across the synapse is accomplished by:
A)
release of ions
done
clear
B)
release of neurotransmitters
done
clear
C)
movement of water
done
clear
D)
movement of\[N{{a}^{+}}\]and\[{{K}^{+}}\]
done
clear
View Answer play_arrow
question_answer 183) Pacemaker is:
A)
AV node
done
clear
B)
SA node
done
clear
C)
AS node
done
clear
D)
SV node
done
clear
View Answer play_arrow
question_answer 184) A sesamoid bone is:
A)
palatine
done
clear
B)
pterygoid
done
clear
C)
patella
done
clear
D)
presphenoid
done
clear
View Answer play_arrow
question_answer 185) Peyers patches produce:
A)
trypsin
done
clear
B)
mucous
done
clear
C)
lymphocyte
done
clear
D)
leucocyte
done
clear
View Answer play_arrow
question_answer 186) The purely motor cranial nerve is:
A)
facial
done
clear
B)
vagus
done
clear
C)
trigerminal
done
clear
D)
spinal accessory
done
clear
View Answer play_arrow
question_answer 187) The type of joint between humerus and radius bone is called:
A)
gliding joint
done
clear
B)
shaddle joint
done
clear
C)
pivot joint
done
clear
D)
hinge joint
done
clear
View Answer play_arrow
question_answer 188) Tendon or ligaments are:
A)
connective tissue
done
clear
B)
vascular tissue
done
clear
C)
epithelial tissue
done
clear
D)
skeletal tissues
done
clear
View Answer play_arrow
question_answer 189) Maximum expiratory volume is:
A)
100 ml
done
clear
B)
1000 ml
done
clear
C)
1500 ml
done
clear
D)
3000 ml
done
clear
View Answer play_arrow
question_answer 190) The function and unit of nervous system is:
A)
neuron
done
clear
B)
axon
done
clear
C)
dendrite
done
clear
D)
cyton
done
clear
View Answer play_arrow
question_answer 191) Ovulation takes place in a month during:
A)
11-14 days
done
clear
B)
14-16 days
done
clear
C)
15-28 days
done
clear
D)
21-26 days
done
clear
View Answer play_arrow
question_answer 192) After 14 years of age among men, the gland that disappears is:
A)
pineal
done
clear
B)
thyroid
done
clear
C)
para thyroid
done
clear
D)
thymus
done
clear
View Answer play_arrow
question_answer 193) A cross between donkey and horse is a:
A)
poly hybrid cross
done
clear
B)
monohybrid cross
done
clear
C)
dihybrid cross
done
clear
D)
receprocal cross
done
clear
View Answer play_arrow
question_answer 194) Common feature between house fly and honey bee is:
A)
head
done
clear
B)
mouthparts
done
clear
C)
abdomen
done
clear
D)
three pairs of jointed legs
done
clear
View Answer play_arrow
question_answer 195) ELISA test is done for the diagnosis of:
A)
AIDS
done
clear
B)
HIV
done
clear
C)
hepatitis
done
clear
D)
malaria
done
clear
View Answer play_arrow
question_answer 196) Fossils of Austral opithecus were first found in:
A)
America
done
clear
B)
Australia
done
clear
C)
S. Africa
done
clear
D)
E. Africa
done
clear
View Answer play_arrow
question_answer 197) Cholera, leprosy and diphtheria are:
A)
bacterial diseases
done
clear
B)
viral diseases
done
clear
C)
fungal diseases
done
clear
D)
functional diseases
done
clear
View Answer play_arrow
question_answer 198) Which of the following is common in Annelida and Arthropoda?
A)
basal nerve cord
done
clear
B)
dorsal nerve cord
done
clear
C)
ventral nerve cord
done
clear
D)
anterior nerve cord
done
clear
View Answer play_arrow
question_answer 199) Hydra captures the victim by injectinig the chemical:
A)
kaliotoxin
done
clear
B)
hypnotoxin
done
clear
C)
toxoplasmin
done
clear
D)
sarafotoxin
done
clear
View Answer play_arrow
question_answer 200) The hormone that regulates the calcium level of blood is:
A)
parathormone
done
clear
B)
estrogen
done
clear
C)
insulin
done
clear
D)
thyroxine
done
clear
View Answer play_arrow