question_answer 1) A heavy uniform chain lies on a horizontal top of table. If the coefficient of friction between the chain and the table is 0.25 sec, then the maximum percentage of the length of the chain that can hang over one edge of the table is:
A)
20%
done
clear
B)
25%
done
clear
C)
35 %
done
clear
D)
15%
done
clear
View Answer play_arrow
question_answer 2) A weight\[\omega \]is suspended from the midpoint of a rope, whose ends are at the same level. In order to make the rope perfectly horizontal, the force applied to each of its ends must be:
A)
less than \[\omega \]
done
clear
B)
equal to \[\omega \]
done
clear
C)
equal to \[2\omega \]
done
clear
D)
infinitely large
done
clear
View Answer play_arrow
question_answer 3) A disc of mass 100 g is kept floating horizontally in air by firing bullets, each of mass 5 g with the same velocity at the same rate of 10 bullets per second. The bullets rebound with the same speed in opposite direction, the velocity of each bullet at the time of impact is:
A)
196 cm/s
done
clear
B)
9.8 cm/s
done
clear
C)
98 cm/s
done
clear
D)
980 cm/s
done
clear
View Answer play_arrow
question_answer 4) The velocity of a particle at an instant is 10 m/s. After 3 s its velocity will become 16 m/s. The velocity at 2 s, before the given instant will be:
A)
6 m/s
done
clear
B)
4 m/s
done
clear
C)
2m/s
done
clear
D)
1 m/s
done
clear
View Answer play_arrow
question_answer 5) A heavy stone hanging from a massless string of length 15m is projected horizontally with speed 147 m/s. The speed of the particle at the point where the tension in the string equals the weight of the particle is?
A)
10 m/s
done
clear
B)
7 m/s
done
clear
C)
12 m/s
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 6) When a body moves with constant speed in a circular path, then:
A)
work done will be zero
done
clear
B)
acceleration will be zero
done
clear
C)
no force acts on the body
done
clear
D)
its velocity remains constant
done
clear
View Answer play_arrow
question_answer 7) Two stones are projected with same velocity\[\upsilon \] at an angle\[\theta \]&\[(90-\theta )\]. If H and hi are the greatest heights in the two paths, what is the relation between\[R,H\]and\[{{H}_{1}}\]?
A)
\[R=4\sqrt{H{{H}_{1}}}\]
done
clear
B)
\[R=\sqrt{H{{H}_{1}}}\]
done
clear
C)
\[R=4\,H{{H}_{1}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 8) A body initially at rest is moving with uniform acceleration a. Its velocity after n second is\[\upsilon \]. The displacement of the body in 2 s is:
A)
\[\frac{2\upsilon (n-1)}{n}\]
done
clear
B)
\[\frac{\upsilon (n-1)}{n}\]
done
clear
C)
\[\frac{\upsilon (n+1)}{n}\]
done
clear
D)
\[\frac{2\upsilon (n+1)}{n}\]
done
clear
View Answer play_arrow
question_answer 9) The magnetic force on a point charge is \[\overrightarrow{F}=q(\overrightarrow{v}\times \overrightarrow{B})\] Here,\[q=\]electric charge \[\overrightarrow{v}=\]velocity of point charge \[\overrightarrow{B}=\]magnetic field The dimensions of B is:
A)
\[[ML{{T}^{-1}}A]\]
done
clear
B)
\[[{{M}^{2}}L{{T}^{-2}}{{A}^{-1}}]\]
done
clear
C)
\[[M{{T}^{-2}}{{A}^{-1}}]\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 10) The first diffraction minimum due to single slit diffraction is\[\theta ,\]for a light of wave length\[5000\overset{o}{\mathop{\text{A}}}\,\]. If the width of the slit is\[1\times {{10}^{-4}}cm,\] then the value of\[\theta \]is:
A)
\[30{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
\[15{}^\circ \]
done
clear
View Answer play_arrow
question_answer 11) A stone of mass m tied to a string of length / is rotated in a circle with the other end of the string as the centre. The speed of the stone is\[\upsilon \]. If the string breaks, the stone will:
A)
move towards the centre
done
clear
B)
move away from the centre
done
clear
C)
move along tangent
done
clear
D)
stop
done
clear
View Answer play_arrow
question_answer 12) A body of mass 2 kg is placed on rough r horizontal plane. The coefficient of friction between body and plane is 0.2. Then:
A)
body will move in forward direction if\[F=5N\]
done
clear
B)
body will move in backward direction with acceleration\[0.5\text{ }m/{{s}^{2}},\]if force\[F=3N\]
done
clear
C)
If\[F=3N,\]then body will be in rest condition
done
clear
D)
both [a] & [c] are correct
done
clear
View Answer play_arrow
question_answer 13) A particle moves with ,a velocity\[(5\hat{i}-3\hat{j}+6\hat{k})\] m/s under the influence of a constant force \[\overrightarrow{F}=10\hat{i}+10\hat{j}+12\hat{k}N.\]The instantaneous power applied to the particle is:
A)
200 J/s
done
clear
B)
40 J/s
done
clear
C)
140 J/s
done
clear
D)
170 J/s
done
clear
View Answer play_arrow
question_answer 14) The potential energy of a particle of 5 kg moving in the\[x-y\] plane is given by\[U=(-7x+24y)J.x\And y\]being in metre. If the particle starts from rest from origin, then speed of particle at\[t=2\text{ }s\]is:
A)
5 m/s
done
clear
B)
14 m/s
done
clear
C)
175 m/s
done
clear
D)
10 m/s
done
clear
View Answer play_arrow
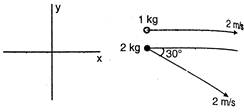
question_answer 15)
Find the velocity of centre of mass of the system shown in the figure?
A)
\[\left( \frac{2+2\sqrt{3}}{3} \right)\hat{i}-\frac{2}{3}\hat{j}\]
done
clear
B)
\[4\hat{i}\]
done
clear
C)
\[\left( \frac{2-2\sqrt{3}}{3} \right)\hat{i}-\frac{1}{3}\hat{j}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 16) The ratio of radii of gyration of a circular disc and a circular ring of the same radii and same mass about a tangential axis in the plane is:
A)
\[1:2\]
done
clear
B)
\[\sqrt{5}:\sqrt{6}\]
done
clear
C)
\[2:3\]
done
clear
D)
\[2:1\]
done
clear
View Answer play_arrow
question_answer 17) A particle performs uniform circular motion with an angular momentum L. If the frequency of particle motion is doubled and its KE is halved, the angular momentum becomes:
A)
\[2L\]
done
clear
B)
\[4L\]
done
clear
C)
\[\frac{L}{2}\]
done
clear
D)
\[\frac{L}{4}\]
done
clear
View Answer play_arrow
question_answer 18) If a particle of mass m is projected at an angle a with the horizontal, then:
A)
the angular momentum remains constant
done
clear
B)
the linear momentum of particle remains constant
done
clear
C)
total mechanical energy remains constant in the absence of air resistance
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 19) The minimum energy required to launch a\[m\text{ }kg\]satellite from earths surface in a circular orbit at an altitude of\[2R/R\]is the radius of earth, will be:
A)
\[3\,mgR\]
done
clear
B)
\[\frac{5}{6}\,mgR\]
done
clear
C)
\[2\,mgR\]
done
clear
D)
\[\frac{1}{5}\,mgR\]
done
clear
View Answer play_arrow
question_answer 20) A satellite is moving on a circular path of radius r around the earth has a time period T. If its radius slightly increases by\[\Delta r,\]the change in its time period is:
A)
\[\frac{3}{2}\left( \frac{T}{r} \right)\Delta r\]
done
clear
B)
\[\left( \frac{T}{r} \right)\Delta r\]
done
clear
C)
\[\frac{3}{2}\left( \frac{{{T}^{2}}}{{{r}^{2}}} \right)\Delta r\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
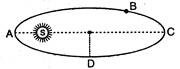
question_answer 21)
A planet revloves in elliptical orbit around the sun. The linear speed of the planet will be maximum at:
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
View Answer play_arrow
question_answer 22) A particle executes SHM, its time period is 16 s. If it passes through the centre of oscillation then its velocity is 2 m/s at time 2 s. The amplitude will be:
A)
7.2 m
done
clear
B)
4 cm
done
clear
C)
6 cm
done
clear
D)
0.72 m
done
clear
View Answer play_arrow
question_answer 23) A simple harmonic oscillator has amplitude\[A,\]angular velocity\[\omega ,\]and mass\[m\]. Then average energy in one time period will be:
A)
\[\frac{1}{4}m{{\omega }^{2}}{{A}^{2}}\]
done
clear
B)
\[\frac{1}{2}m{{\omega }^{2}}{{A}^{2}}\]
done
clear
C)
\[m{{\omega }^{2}}{{A}^{2}}\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 24) A particle executes simple harmonic motion with a frequency/. The frequency with which the potential energy oscillates is:
A)
\[f\]
done
clear
B)
\[f/2\]
done
clear
C)
\[2f\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 25) The wave front due to a source situated at infinity is:
A)
spherical
done
clear
B)
cylindrical
done
clear
C)
plemar
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 26) An astronaut is approaching the moon. He sends out a radio signal of frequency 5000 MHz and the frequency of echo is different from that of the, original frequency by 100 kHz. His velocity of approach with respect to the moon is:
A)
2 km/s
done
clear
B)
3 km/s
done
clear
C)
4 km/s
done
clear
D)
5 km/s
done
clear
View Answer play_arrow
question_answer 27) When a sphere is taken to bottom of sea 1 km deep, it contracts by 0.01%. The bulk modulus of elasticity of the material of sphere is: (Given : Density of water\[=1\text{ }g/c{{m}^{3}}\])
A)
\[9.8\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
B)
\[10.2\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
C)
\[0.98\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
D)
\[8.4\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 28) The temperature of\[{{H}_{2}}\]at which the rms velocity of its molecules is seven times the rms velocity of the molecules of nitrogen at 300 K is:
A)
2100 K
done
clear
B)
1700 K
done
clear
C)
1350 K
done
clear
D)
1050 K
done
clear
View Answer play_arrow
question_answer 29) Gas exerts pressure on the walls of the container because:
A)
gas has weight
done
clear
B)
gas molecules have momentum
done
clear
C)
gas molecules collide with each other
done
clear
D)
gas molecules collide with the walls of the container
done
clear
View Answer play_arrow
question_answer 30) The inside and outside temperatures of a refrigerator are 273 K and 303 K respectively. Assuming that refrigerator cycle is reversible, for every joule of work done, the heat delivered to the surrounding will be:
A)
10 J
done
clear
B)
20 J
done
clear
C)
30 J
done
clear
D)
50 J
done
clear
View Answer play_arrow
question_answer 31) In an energy recycling process,\[X\text{ }g\]of steam at \[100{}^\circ C\]becomes water at\[100{}^\circ C\] which converts V g of ice at\[0{}^\circ C\]into water at\[100{}^\circ C\]. The ratio of X & Y will be:
A)
\[\frac{1}{3}\]
done
clear
B)
\[\frac{2}{3}\]
done
clear
C)
3
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 32) The surface temperature of the sun is T K and the solar constant for a plate is S. The sun subtends an angle\[\theta \]at the planet Then:
A)
\[S\propto {{T}^{4}}\]
done
clear
B)
\[S\propto {{T}^{2}}\]
done
clear
C)
\[S\propto {{\theta }^{2}}\]
done
clear
D)
\[S\propto {{\theta }^{2}}\]
done
clear
View Answer play_arrow
question_answer 33) A body at a temperature of\[728{}^\circ C\]and has surface area 5 cm, radiates 300 J of energy each minute. The emissivity is: (Given: Boltzmann constant\[=5.67\times {{10}^{-8}}\]watt\[{{m}^{2}}{{K}^{4}}\])
A)
\[e=0.18\]
done
clear
B)
\[e=0.02\]
done
clear
C)
\[e=0.2\]
done
clear
D)
\[e=0.15\]
done
clear
View Answer play_arrow
question_answer 34) If at NTP velocity of sound in a gas is 1150 m/s, then the rms velocity of gas molecules at NTP is: (Given\[R=8.3\text{ }joule/mol/K,\]\[{{C}_{p}}=4.8cal/mol/K\])
A)
1600 m/s
done
clear
B)
1532.19 m/s
done
clear
C)
160 m/s
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 35) If\[\sigma =\]surface charge density,\[\varepsilon =\]electric permittivity, the dimensions of\[\frac{\sigma }{\varepsilon }\]are same as:
A)
electric force
done
clear
B)
electric field intensity
done
clear
C)
pressure
done
clear
D)
electric charge
done
clear
View Answer play_arrow
question_answer 36)
For circuit the equivalent capacitance between P & Q is:
A)
\[6C\]
done
clear
B)
\[4C\]
done
clear
C)
\[\frac{3C}{2}\]
done
clear
D)
\[\frac{6C}{11}\]
done
clear
View Answer play_arrow
question_answer 37) A wire has resistance \[12\,\Omega \]. It is bent in the form of a circle. The effective resistance between the two points on any diameter of the circle is:
A)
\[12\,\Omega \]
done
clear
B)
\[24\,\Omega \]
done
clear
C)
\[6\,\Omega \]
done
clear
D)
\[3\,\Omega \]
done
clear
View Answer play_arrow
question_answer 38) If two identical heaters each rated as (1000 W, 220 V) are connected in parallel to 220 volt, then the total power consumed is:
A)
200 W
done
clear
B)
2500 W
done
clear
C)
250 W
done
clear
D)
2000 W
done
clear
View Answer play_arrow
question_answer 39) A conducting circular loop of radius r carries a constant current\[I\]. It is placed in a uniform magnetic field\[{{B}_{0}}\]such that\[{{B}_{0}}\]is perpendicular to the plane of the loop. The magnetic force acting on the loop is:
A)
\[Ir\,{{B}_{0}}\]
done
clear
B)
\[2\pi Ir\,{{B}_{0}}\]
done
clear
C)
\[\pi Ir\,{{B}_{0}}\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
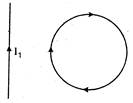
question_answer 40)
In the given figure, the loop is fixed but straight wire can move. The straight wire will:
A)
remain stationary
done
clear
B)
move towards the loop
done
clear
C)
move away from the loop
done
clear
D)
rotates about the axis
done
clear
View Answer play_arrow
question_answer 41) At a point on the right bisector of a magnetic dipole, the magnetic:
A)
potential varies as\[\frac{1}{{{r}^{2}}}\]
done
clear
B)
potential is zero at all points on the right bisector
done
clear
C)
field varies as\[{{r}^{3}}\]
done
clear
D)
field is perpendicular to the axis of dipole
done
clear
View Answer play_arrow
question_answer 42) The couple acting on a magnet of length 10 cm and pole strength 15 Am, kept in a field of \[B=2\times {{10}^{-5}}T,\]at an angle of\[30{}^\circ ,\]is:
A)
\[1.5\times {{10}^{-5}}Nm\]
done
clear
B)
\[1.5\times {{10}^{-3}}Nm\]
done
clear
C)
\[1.5\times {{10}^{-2}}Nm\]
done
clear
D)
\[1.5\times {{10}^{-6}}Nm\]
done
clear
View Answer play_arrow
question_answer 43) In a step-up transformer, the number of turns in:
A)
primary are less
done
clear
B)
primary are more
done
clear
C)
primary & secondary are equal
done
clear
D)
primary are infinite
done
clear
View Answer play_arrow
question_answer 44) 1 If a circuit made up of a resistance 10 and inductance 0.01H, an alternating emf 200 volt at 50 Hz is connected, then the phase difference between the current and the emf in the circuit is:
A)
\[{{\tan }^{-1}}(\pi )\]
done
clear
B)
\[{{\tan }^{-1}}\left( \frac{\pi }{2} \right)\]
done
clear
C)
\[{{\tan }^{-1}}\left( \frac{\pi }{4} \right)\]
done
clear
D)
\[{{\tan }^{-1}}\left( \frac{\pi }{3} \right)\]
done
clear
View Answer play_arrow
question_answer 45) An a. c. is represented by\[e=220\text{ }sin(100\pi )t\]volt and is applied over a resistance of 110 ohm. The heat produced in 7 minute is:
A)
\[11\times {{10}^{3}}cal\]
done
clear
B)
\[22\times {{10}^{3}}cal\]
done
clear
C)
\[33\times {{10}^{3}}cal\]
done
clear
D)
\[25\times {{10}^{3}}cal\]
done
clear
View Answer play_arrow
question_answer 46) The wave length of a radio wave of frequency of 1 MHz is:
A)
400m
done
clear
B)
300m
done
clear
C)
350m
done
clear
D)
200m
done
clear
View Answer play_arrow
question_answer 47) The correct option, if speed of gamma rays,\[x-\]rays and micro waves are \[{{\upsilon }_{g}},{{\upsilon }_{x}}\]\[\And \,{{\upsilon }_{m}}\] respectively will be:
A)
\[{{\upsilon }_{g}}>{{\upsilon }_{x}}>\,{{\upsilon }_{m}}\]
done
clear
B)
\[{{\upsilon }_{g}}<{{\upsilon }_{x}}<\,{{\upsilon }_{m}}\]
done
clear
C)
\[{{\upsilon }_{g}}>{{\upsilon }_{x}}>\,{{\upsilon }_{m}}\]
done
clear
D)
\[{{\upsilon }_{g}}={{\upsilon }_{x}}=\,{{\upsilon }_{m}}\]
done
clear
View Answer play_arrow
question_answer 48) The electric field E and magnetic field B in electromagnetic waves are:
A)
parallel to each other
done
clear
B)
inclined at an angle of \[45{}^\circ \]
done
clear
C)
perpendicular to each other
done
clear
D)
opposite to each other
done
clear
View Answer play_arrow
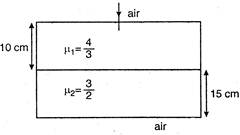
question_answer 49)
Considering normal incidence of ray, the equivalent refractive index of combination of two slabs shown in figure is:
A)
1.8
done
clear
B)
1.43
done
clear
C)
2
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 50) To obtain a good photographic print, an exposure of 2 s at a distance of 1 m from a 75 cd bulb is done. To obtain an equally satisfactory result, what should be the distance, if time of exposure is 12 s from a 50 cd bulb:
A)
1 m
done
clear
B)
2 m
done
clear
C)
3 m
done
clear
D)
4m
done
clear
View Answer play_arrow
question_answer 51) In Youngs double slit experiment, the spacing between the slits is d and wave length of light used is\[6000\overset{o}{\mathop{\text{A}}}\,\]. If the angular width of a fringe formed on a distant screen is\[1{}^\circ ,\] then value of d is:
A)
1 mm
done
clear
B)
0.0.5 mm
done
clear
C)
0.03 mm
done
clear
D)
0.01 mm
done
clear
View Answer play_arrow
question_answer 52) In a Youngs experiment, one of the slit is covered with a transparent sheet of thickness \[3.6\times {{10}^{-3}}cm\]due to which position of central fringe shifts to a position originally occupied by 30th bright fringe. The refractive index of the sheet, if\[\lambda \text{= }6000\overset{o}{\mathop{\text{A}}}\,\]is:
A)
1.5
done
clear
B)
1.2
done
clear
C)
1.3
done
clear
D)
1.7
done
clear
View Answer play_arrow
question_answer 53) Polarisation of light proves:
A)
corpuscular nature of light
done
clear
B)
quantum nature of light
done
clear
C)
transverse wave nature of light
done
clear
D)
longitudinal wave nature of light
done
clear
View Answer play_arrow
question_answer 54) Three particles having charges in the ratio of \[2:3:5,\]produce the same point on the photographic film in Thomson experiment. Their masses are in the ratio of:
A)
\[2:3:5\]
done
clear
B)
\[5:3:2\]
done
clear
C)
\[15:10:6\]
done
clear
D)
\[3:5:2\]
done
clear
View Answer play_arrow
question_answer 55) In terms of Rydberg constant R, the wave number of the first Balmer line is:
A)
\[R\]
done
clear
B)
\[3R\]
done
clear
C)
\[\frac{5R}{36}\]
done
clear
D)
\[\frac{8R}{9}\]
done
clear
View Answer play_arrow
question_answer 56) In nuclear reaction: \[_{2}H{{e}^{4}}{{+}_{Z}}{{X}^{4}}{{\xrightarrow[{}]{{}}}_{Z+2}}{{Y}^{A+3}}{{+}_{Z}}{{M}^{A}}\] where M denotes
A)
electron
done
clear
B)
positron
done
clear
C)
proton
done
clear
D)
neutron
done
clear
View Answer play_arrow
question_answer 57) If the de-Broglie wavelength of a proton is \[{{10}^{-13}}m,\]the electric potential through which it must have been accelerated is:
A)
\[4.07\times {{10}^{4}}\,V\]
done
clear
B)
\[8.2\times {{10}^{4}}\,V\]
done
clear
C)
\[8.2\times {{10}^{3}}\,V\]
done
clear
D)
\[4.07\times {{10}^{5}}\,V\]
done
clear
View Answer play_arrow
question_answer 58) \[n\]alpha particles per second are emitted from N atoms of a radioactive element. The half-life of radioactive element is:
A)
\[\frac{n}{N}\sec \]
done
clear
B)
\[\frac{N}{n}\sec \]
done
clear
C)
\[\frac{0.693}{n}\sec \]
done
clear
D)
\[\frac{0.693n}{N}\sec \]
done
clear
View Answer play_arrow
question_answer 59) Depletion layer consists of:
A)
electrons
done
clear
B)
protons
done
clear
C)
mobile ions
done
clear
D)
immobile ions
done
clear
View Answer play_arrow
question_answer 60) In a semiconductor diode, the barrier potential offers opposition to only:
A)
majority carrier in both regions
done
clear
B)
minority carrier in both regions
done
clear
C)
free electrons in the n-region
done
clear
D)
holes in the p-region
done
clear
View Answer play_arrow
question_answer 61) Maximum entropy will be in which of the following?
A)
Ice
done
clear
B)
Liquid water
done
clear
C)
Snow
done
clear
D)
Water vapours
done
clear
View Answer play_arrow
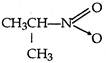
question_answer 62) What is obtained when chlorine is passed in boiling toluene and product is hydrolysed?
A)
o-Cresol
done
clear
B)
p-Cresol
done
clear
C)
2, 4-DihydroxytoIuene
done
clear
D)
Benzyl alcohol
done
clear
View Answer play_arrow
question_answer 63) Which of the following has covalent bond?
A)
\[N{{a}_{2}}S\]
done
clear
B)
\[AlC{{l}_{3}}\]
done
clear
C)
\[NaH\]
done
clear
D)
\[MgC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 64) Which of the following acts as an oxidising as well as reducing agent?
A)
\[N{{a}_{2}}O\]
done
clear
B)
\[N{{a}_{2}}{{O}_{2}}\]
done
clear
C)
\[NaN{{O}_{3}}\]
done
clear
D)
\[NaN{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 65) What is the oxidation state of P in\[Ba{{({{H}_{2}}P{{O}_{2}})}_{2}}?\]
A)
\[+1\]
done
clear
B)
\[+2\]
done
clear
C)
\[+3\]
done
clear
D)
\[-1\]
done
clear
View Answer play_arrow
question_answer 66) Which of the following molecules has pyramidal shape?
A)
\[PC{{l}_{3}}\]
done
clear
B)
\[S{{O}_{3}}\]
done
clear
C)
\[CO_{3}^{2-}\]
done
clear
D)
\[NO_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 67) Maximum number of hydrogen bonding in\[{{H}_{2}}O\]is:
A)
\[1\]
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 68) Number of isomers possible for\[{{C}_{4}}{{H}_{8}}O\]is:
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 69) \[{{C}_{6}}{{H}_{5}}-CH=CHCHO\xrightarrow[{}]{X}\] \[{{C}_{6}}{{H}_{5}}CH=\text{ }CHC{{H}_{2}}OH\] In the above sequence X can be:
A)
\[{{H}_{2}}/Ni\]
done
clear
B)
\[NaB{{H}_{4}}\]
done
clear
C)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}^{+}}\]
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 70) For a reaction\[{{H}_{2}}+{{I}_{2}}2HI\]at 721 K, the value of equilibrium constant is 50. If 0.5 mols each of\[{{H}_{2}}\]and\[{{I}_{2}}\]is added to the system the value of equilibrium constant will be:
A)
40
done
clear
B)
60
done
clear
C)
50
done
clear
D)
30
done
clear
View Answer play_arrow
question_answer 71) Schottky defect generally appears in:
A)
\[NaCl\]
done
clear
B)
\[KCl\]
done
clear
C)
\[CsCl\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 72) The ability of a given substance to assume two or more crystalline structure is called:
A)
amorphism
done
clear
B)
isomorphism
done
clear
C)
Polymorphism
done
clear
D)
isomerism
done
clear
View Answer play_arrow
question_answer 73) A cricket ball of 0.5 kg is moving with a velocity of 100 m/sec. The wavelength associated with its motion is:
A)
1/100 cm
done
clear
B)
\[6.6\times {{10}^{-34}}m\]
done
clear
C)
\[1.32\times {{10}^{-35}}m\]
done
clear
D)
\[6.6\times {{10}^{-28}}m\]
done
clear
View Answer play_arrow
question_answer 74) Which among the following species have the same number of electrons in its outermost as well as penultimate shell?
A)
\[M{{g}^{2+}}\]
done
clear
B)
\[{{O}^{2-}}\]
done
clear
C)
\[{{F}^{-}}\]
done
clear
D)
\[C{{a}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 75) \[\Delta E{}^\circ \]of combustion of isobutylene is \[-X\text{ }kJ\text{ }mo{{l}^{-1}}\]. The value of\[\Delta H{}^\circ \]is:
A)
\[=\Delta E{}^\circ \]
done
clear
B)
\[>\Delta E{}^\circ \]
done
clear
C)
\[=0\]
done
clear
D)
\[<\Delta E{}^\circ \]
done
clear
View Answer play_arrow
question_answer 76) At\[90{}^\circ C,\]pure water has\[{{H}_{3}}{{O}^{+}}\]ion concentration of \[{{10}^{-6}}mol/{{L}^{-1}}\]. The\[{{K}_{\omega }}\]at \[90{}^\circ C\]is:
A)
\[{{10}^{-6}}\]
done
clear
B)
\[{{10}^{-14}}\]
done
clear
C)
\[{{10}^{-12}}\]
done
clear
D)
\[{{10}^{-8}}\]
done
clear
View Answer play_arrow
question_answer 77) A gas is found to have a formula\[{{[CO]}_{x}}\].If its vapour density is 70, the value of\[x\]is:
A)
2.5
done
clear
B)
3.0
done
clear
C)
5.0
done
clear
D)
6.0
done
clear
View Answer play_arrow
question_answer 78) Which of the following represents soap?
A)
\[{{C}_{17}}{{H}_{35}}COOK\]
done
clear
B)
\[{{C}_{17}}{{H}_{35}}COOH\]
done
clear
C)
\[{{C}_{15}}{{H}_{31}}COOH\]
done
clear
D)
\[{{({{C}_{17}}{{H}_{35}}COO)}_{2}}Ca\]
done
clear
View Answer play_arrow
question_answer 79) Aspirin is chemically:
A)
methyl benzoate
done
clear
B)
ethyl salicylate
done
clear
C)
acetyl salicylic acid
done
clear
D)
o-hydroxy benzoic acid
done
clear
View Answer play_arrow
question_answer 80) Vitamin\[{{B}_{6}}\]is known as :
A)
Pyridoxin
done
clear
B)
Thiamine
done
clear
C)
Tocopherol
done
clear
D)
Riboflavin
done
clear
View Answer play_arrow
question_answer 81) Which of the following compounds is found abundantly in nature?
A)
Fructose
done
clear
B)
Starch
done
clear
C)
Glucose
done
clear
D)
Cellulose
done
clear
View Answer play_arrow
question_answer 82) Synthetic polymer which resembles natural rubber is:
A)
neoprene
done
clear
B)
chloroprene
done
clear
C)
glyptal
done
clear
D)
nylon
done
clear
View Answer play_arrow
question_answer 83) Which of the following is not a nitro-derivative?
A)
\[{{C}_{6}}{{H}_{5}}N{{O}_{2}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}ONO\]
done
clear
C)
done
clear
D)
\[{{C}_{6}}{{H}_{4}}(OH)N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 84) The reduction of which of the following compound would yield secondary amine?
A)
Alkyl nitrile
done
clear
B)
Carbylamine
done
clear
C)
Primary amine
done
clear
D)
Secondary nitro compound
done
clear
View Answer play_arrow
question_answer 85) Which of the aldehyde is most reactive?
A)
\[{{C}_{6}}{{H}_{5}}CHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[HCHO\]
done
clear
D)
All the equally reactive
done
clear
View Answer play_arrow
question_answer 86) Which of the following does not contain\[-COOH\] group?
A)
Aspirin
done
clear
B)
Benzole acid
done
clear
C)
Picric acid
done
clear
D)
All have\[-COOH\]group
done
clear
View Answer play_arrow
question_answer 87) Which of the following is dihydric alcohol?
A)
Glycerol
done
clear
B)
Ethylene glycol
done
clear
C)
Catechol
done
clear
D)
Resorcmol
done
clear
View Answer play_arrow
question_answer 88) Ethyl alcohol is heated with cone.\[{{H}_{2}}S{{O}_{4}}\]. The product formed is:
A)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ |\,\,| \end{smallmatrix}}{\mathop{C}}\,-O{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 89) In the first order reaction, the concentration of the reactants is reduced to 25% in one hour. The half-life period of the reaction is:
A)
2 hr
done
clear
B)
4 hr
done
clear
C)
1/2 hr
done
clear
D)
1/4 hr
done
clear
View Answer play_arrow
question_answer 90) For a reaction, \[X(g)\xrightarrow[{}]{{}}Y(g)+Z(g)\] the half life period is 10 min. In what period of time would the concentration of X be reduced to 10% of original conceiitration?
A)
20 min
done
clear
B)
33 min
done
clear
C)
15 min
done
clear
D)
25 min
done
clear
View Answer play_arrow
question_answer 91) The molar freezing point constant for water is\[1.86{}^\circ C/mole\]. If 342 g of cane sugar\[({{C}_{12}}{{H}_{22}}{{O}_{11}})\]is dissolved in 1000 g of water, the solution will freeze at:
A)
\[-1.86{}^\circ C\]
done
clear
B)
\[1.86{}^\circ C\]
done
clear
C)
\[-3.92{}^\circ C\]
done
clear
D)
\[2.42{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 92) The movement of solvent molecules through a semipermeable membrane called:
A)
electrolysis
done
clear
B)
electrophoresis
done
clear
C)
osmosis
done
clear
D)
cataphoresis
done
clear
View Answer play_arrow
question_answer 93) Which of the following is a primary halide?
A)
Isopropyl iodide
done
clear
B)
Secondary butyl iodide
done
clear
C)
Tertiary butyl bromide
done
clear
D)
Neo hexyl chloride
done
clear
View Answer play_arrow
question_answer 94) A gas is found to have the formula\[{{(CO)}_{n}}\]. If its vapour density is 56, the value of n will be:
A)
7
done
clear
B)
5
done
clear
C)
4
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 95) Aromatisation of\[n-\]heptane by passing over \[(A{{l}_{2}}{{O}_{3}}+C{{r}_{2}}{{O}_{3}})\]catalyst at 773 K gives:
A)
benzene
done
clear
B)
toluene
done
clear
C)
mixture of both
done
clear
D)
heptylene
done
clear
View Answer play_arrow
question_answer 96) \[{{C}_{6}}{{H}_{5}}C{{H}_{3}}\xrightarrow[{}]{Cr{{O}_{2}}C{{l}_{2}}}Z\] In the given sequence Z is:
A)
benzaldehyde
done
clear
B)
toluic acid
done
clear
C)
phenyl acetic acid
done
clear
D)
benzoic acid
done
clear
View Answer play_arrow
question_answer 97) Nitroethane can exhibit one of the following kind of isomerism:
A)
metamerism
done
clear
B)
optical activity
done
clear
C)
tautomerism
done
clear
D)
position isomerism
done
clear
View Answer play_arrow
question_answer 98) A compound has 3 chiral carbon atoms. The number of possible optical isomers it can have:
A)
3
done
clear
B)
2
done
clear
C)
8
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 99) \[4K{{ }_{2}}C{{r}_{2}}{{O}_{7}}\xrightarrow[{}]{Heat}4{{K}_{2}}Cr{{O}_{4}}+3{{O}_{2}}+X.\] In the above reaction X is:
A)
\[Cr{{O}_{3}}\]
done
clear
B)
\[C{{r}_{2}}{{O}_{7}}\]
done
clear
C)
\[C{{r}_{2}}{{O}_{3}}\]
done
clear
D)
\[Cr{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 100) The co-ordination number and oxidation number of X in the following compound \[[X(S{{O}_{4}}){{(N{{H}_{3}})}_{5}}]Cl\]will be:
A)
10 and 3
done
clear
B)
2 and 6
done
clear
C)
6 and 3
done
clear
D)
6 and 4
done
clear
View Answer play_arrow
question_answer 101) In the electrolysis of water, one faraday of electrical energy would evolve:
A)
one mole of oxygen
done
clear
B)
one g atom of oxygen
done
clear
C)
8 g of oxygen
done
clear
D)
22.4 litres of oxygen
done
clear
View Answer play_arrow
question_answer 102) In which of these processes platinum is used as a catalyst?
A)
Oxidation of ammonia to form\[HN{{O}_{3}}\]
done
clear
B)
Hardening of oils
done
clear
C)
Production of synthetic rubber
done
clear
D)
Synthesis of methanol
done
clear
View Answer play_arrow
question_answer 103) If the half life of an isotope X is 10 years, its decay constant is:
A)
\[6.932\text{ }y{{r}^{-1}}\]
done
clear
B)
\[0.6932\text{ }y{{r}^{-1}}\]
done
clear
C)
\[0.06932\text{ }y{{r}^{-1}}\]
done
clear
D)
\[0.006932\text{ }y{{r}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 104) On strong heating sodium bicarbonate changes into:
A)
sodium monoxide
done
clear
B)
sodium hydroxide
done
clear
C)
sodium carbonate
done
clear
D)
sodium peroxide
done
clear
View Answer play_arrow
question_answer 105) Which of the following is not an ore of magnesium?
A)
Carnallite
done
clear
B)
Magnesite
done
clear
C)
Dolomite
done
clear
D)
Gypsum
done
clear
View Answer play_arrow
question_answer 106) Aluminium reacts with caustic soda to form:
A)
aluminium hydroxide
done
clear
B)
aluminium oxide
done
clear
C)
sodium meta-aluminate
done
clear
D)
sodium tetra aluminate
done
clear
View Answer play_arrow
question_answer 107) In laboratory burners, we use:
A)
producer gas
done
clear
B)
oil gas
done
clear
C)
gobar gas
done
clear
D)
coal gas
done
clear
View Answer play_arrow
question_answer 108) Iron is dropped in dil.\[HN{{O}_{3}},\]it gives:
A)
ferric nitrate
done
clear
B)
ferric nitrate and\[N{{O}_{2}}\]
done
clear
C)
ferrous nitrate and animonium nitrate
done
clear
D)
ferrous nitrate and nitric oxide
done
clear
View Answer play_arrow
question_answer 109) When tin is treated with concentrated nitric acid:
A)
it is converted into stannous nitrate
done
clear
B)
it is converted into stannic nitrate
done
clear
C)
it is converted into metastannic acid
done
clear
D)
it becomes passive
done
clear
View Answer play_arrow
question_answer 110) The chief impurity present in red bauxite is:
A)
\[Si{{O}_{2}}\]
done
clear
B)
\[F{{e}_{2}}{{O}_{3}}\]
done
clear
C)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
D)
\[NaF\]
done
clear
View Answer play_arrow
question_answer 111) The extraction of which of the following metals involves bessemerisation?
A)
\[Fe\]
done
clear
B)
\[Ag\]
done
clear
C)
\[Al\]
done
clear
D)
\[Cu\]
done
clear
View Answer play_arrow
question_answer 112) The composition of the common glass is:
A)
\[N{{a}_{2}}O.\text{ }CaO\text{ }.\text{ }6Si{{O}_{3}}\]
done
clear
B)
\[N{{a}_{2}}\text{O }.\text{ }A{{l}_{2}}{{O}_{3}}\text{ }.\text{ }Si{{O}_{2}}\]
done
clear
C)
\[CaO\text{ }.\text{ }A{{l}_{2}}{{O}_{3}}\text{ }.\text{ }Si{{O}_{2}}\]
done
clear
D)
\[N{{a}_{2}}\text{O }.\text{ }CaO\text{ }.\text{ }6Si{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 113) \[{{H}_{2}}{{O}_{2}}\]is manufactured these days:
A)
by the action of\[{{H}_{2}}{{O}_{2}}\]on \[Ba{{O}_{2}}\]
done
clear
B)
by the action of\[{{H}_{2}}S{{O}_{4}}\]on \[N{{a}_{2}}{{O}_{2}}\]
done
clear
C)
by electrolysis of 50% \[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
by burning hydrogen in excess of oxygen
done
clear
View Answer play_arrow
question_answer 114) \[{{K}_{a}}\]of\[{{H}_{2}}{{O}_{2}}\]is of the order of:
A)
\[{{10}^{-12}}\]
done
clear
B)
\[{{10}^{-14}}\]
done
clear
C)
\[{{10}^{-16}}\]
done
clear
D)
\[{{10}^{-10}}\]
done
clear
View Answer play_arrow
question_answer 115) Which of the following oxy acids of phosphorus is a reducing agent and monobasic?
A)
\[{{H}_{3}}P{{O}_{2}}\]
done
clear
B)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
C)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
D)
\[{{H}_{4}}{{P}_{2}}{{O}_{6}}\]
done
clear
View Answer play_arrow
question_answer 116) The hybrid state of sulphur in\[S{{O}_{3}}\]molecule is:
A)
\[s{{p}^{3}}d\]
done
clear
B)
\[s{{p}^{3}}\]
done
clear
C)
\[s{{p}^{3}}{{d}^{2}}\]
done
clear
D)
\[s{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 117) Solubility of iodine in water may be increased by adding:
A)
chloroform
done
clear
B)
potassium iodide
done
clear
C)
carbon disulphide
done
clear
D)
sodium thiosulphate
done
clear
View Answer play_arrow
question_answer 118) Noble gases are adsorbed by:
A)
anhydrous calcium chloride
done
clear
B)
ferric hydroxide
done
clear
C)
cone. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
activated coconut charcoal
done
clear
View Answer play_arrow
question_answer 119) Which one of the following is not present in R.N.A.?
A)
Uracil
done
clear
B)
Thymine
done
clear
C)
Ribose
done
clear
D)
Phosphate
done
clear
View Answer play_arrow
question_answer 120) An organic compound has an empirical formula \[C{{H}_{2}}O,\]its vapour density is 45. The molecular formula of the compound is:
A)
\[C{{H}_{2}}O\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}O\]
done
clear
C)
\[{{C}_{2}}{{H}_{2}}O\]
done
clear
D)
\[{{C}_{3}}{{H}_{6}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 121) Spiracles found in cockroach are:
A)
2 pairs in thorax and 100 pairs in abdomen
done
clear
B)
2 pairs in thorax and 6 pairs in abdomen
done
clear
C)
2 pairs in thorax and 8 pairs in abdomen
done
clear
D)
2 pairs in thorax and 4 pairs in abdomen
done
clear
View Answer play_arrow
question_answer 122) Common bacteria used in genetic engineering is:
A)
E. coli
done
clear
B)
Diplococcus
done
clear
C)
Rhizobium
done
clear
D)
Spirillum
done
clear
View Answer play_arrow
question_answer 123) Characteristic of fern is:
A)
circmate vernation
done
clear
B)
reticulate venation
done
clear
C)
parallel venation
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 124) Transfusion tissue is found in:
A)
Cycas
done
clear
B)
Ginkgo
done
clear
C)
Pinus
done
clear
D)
Both a and c
done
clear
View Answer play_arrow
question_answer 125) Fats are emulsified by the bile juice because it contains:
A)
enzyme
done
clear
B)
esterase
done
clear
C)
bile salt
done
clear
D)
bile pigment
done
clear
View Answer play_arrow
question_answer 126) Arbor vitae is part of:
A)
cerebrum
done
clear
B)
cerebellum
done
clear
C)
mid brain
done
clear
D)
fore brain
done
clear
View Answer play_arrow
question_answer 127) The number of cranial nerves in frog and man is:
A)
10 and 12
done
clear
B)
12 and 10
done
clear
C)
10 and 8
done
clear
D)
8 and 10
done
clear
View Answer play_arrow
question_answer 128) Structure connecting the foetus to placeiita is:
A)
umbilical cord
done
clear
B)
amnion
done
clear
C)
yolk sac
done
clear
D)
chorion
done
clear
View Answer play_arrow
question_answer 129) Slipper animalcule is:
A)
Paramecium
done
clear
B)
Trypanosoma
done
clear
C)
Entamoeba
done
clear
D)
Protozoa
done
clear
View Answer play_arrow
question_answer 130) Hormone responsible for metamorphosis in tadpole is:
A)
adrenaline
done
clear
B)
thyroxine
done
clear
C)
aldosterone
done
clear
D)
vasopressin
done
clear
View Answer play_arrow
question_answer 131) Corpus callosum connects:
A)
two cerebral hemispheres
done
clear
B)
two ventricles of brain
done
clear
C)
two cerebellar hemispheres
done
clear
D)
two optic thalamus
done
clear
View Answer play_arrow
question_answer 132) Hormone responsible for ovulation is:
A)
LH
done
clear
B)
FSH
done
clear
C)
progesterone
done
clear
D)
testosterone
done
clear
View Answer play_arrow
question_answer 133) Blastula of frog is:
A)
amphiblastula
done
clear
B)
coeloblastula
done
clear
C)
holoblastula
done
clear
D)
stereoblastula
done
clear
View Answer play_arrow
question_answer 134) Nerve cells do not possess:
A)
neurilemma
done
clear
B)
sarcolemma
done
clear
C)
dendrite
done
clear
D)
axon
done
clear
View Answer play_arrow
question_answer 135) Which of the following is not syncytial?
A)
Cardiac muscle
done
clear
B)
Skeletal muscle
done
clear
C)
Smooth muscle
done
clear
D)
Interstitial muscle
done
clear
View Answer play_arrow
question_answer 136) What do you mean by the term spermateleosis?
A)
Conversion of spermatids to sperm
done
clear
B)
Conversion of spermogonium to spermatid
done
clear
C)
Conversion of spermatid to spermogonium
done
clear
D)
Conversion of primay spermatocyte to secondary spermatocyte
done
clear
View Answer play_arrow
question_answer 137) Gonorrhoea is caused by:
A)
Treponema pallidum
done
clear
B)
Entamoeba gingivalis
done
clear
C)
Mycobaciemim leprae
done
clear
D)
Neisseria gonorrhoeae
done
clear
View Answer play_arrow
question_answer 138) The diagrammatic representation of the chromosomes of an individual is called:
A)
idiogram
done
clear
B)
karyotype
done
clear
C)
phenotype
done
clear
D)
diploidy
done
clear
View Answer play_arrow
question_answer 139) Elephantiasis causing organism belongs to:
A)
aschelminthes
done
clear
B)
platyhelminthes
done
clear
C)
cnidarian
done
clear
D)
porifera
done
clear
View Answer play_arrow
question_answer 140) Right aortic arch is present in:
A)
reptiles only
done
clear
B)
mammals only
done
clear
C)
birds only
done
clear
D)
both b and c
done
clear
View Answer play_arrow
question_answer 141) Kwashiorkar is caused due to deficiency of:
A)
calories
done
clear
B)
hormone
done
clear
C)
zwitterion
done
clear
D)
essential amino acid
done
clear
View Answer play_arrow
question_answer 142) Darwin travelled in which ship?
A)
H.N.S. Eagle
done
clear
B)
D. Matrica
done
clear
C)
H.M.S. Beagle
done
clear
D)
Titanic
done
clear
View Answer play_arrow
question_answer 143) First hormone prepared by genetic engineering is:
A)
oxytocin
done
clear
B)
somatotropin
done
clear
C)
adrenaline
done
clear
D)
insulin
done
clear
View Answer play_arrow
question_answer 144) Kidney stones are produced due to deposition of uric acid and:
A)
silicates
done
clear
B)
minerals
done
clear
C)
calcium carbonate
done
clear
D)
calcium oxalate
done
clear
View Answer play_arrow
question_answer 145) Aldosterone is secreted by:
A)
zona glomerulosa
done
clear
B)
zona fasciculata
done
clear
C)
zona reticularis
done
clear
D)
zona pellucida
done
clear
View Answer play_arrow
question_answer 146) Mammary gland is a modified:
A)
sebaceous gland
done
clear
B)
mucous gland
done
clear
C)
sweat gland
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 147) Pacinian corpuscles are abundantly found in:
A)
skin
done
clear
B)
blood
done
clear
C)
tissue
done
clear
D)
kidney
done
clear
View Answer play_arrow
question_answer 148) Which is not true for glycolysis?
A)
End product is\[C{{O}_{2}},{{H}_{2}}O\]
done
clear
B)
Substrate level phosphorylation
done
clear
C)
Production of ATP
done
clear
D)
Expenditure of ATP
done
clear
View Answer play_arrow
question_answer 149) Bordeaux mixture can be prepared by mixing the copper sulphate with:
A)
sodium chloride
done
clear
B)
milk of calcium hydroxide
done
clear
C)
calcium sulphate
done
clear
D)
lime stone
done
clear
View Answer play_arrow
question_answer 150) Law of limiting factor was given by:
A)
Robert Hill
done
clear
B)
Kelvin
done
clear
C)
Blackmann
done
clear
D)
Arnon
done
clear
View Answer play_arrow
question_answer 151) Radioactive\[{{C}^{14}}\]is given to\[C{{O}_{2}}\]and released to atmosphere. This\[C{{O}_{2}}\]is taken by\[RuBP\]in a\[{{C}_{3}}\] plant. First radioactive\[{{C}^{14}}\]is seen in which compound?
A)
PGAL
done
clear
B)
PEP
done
clear
C)
RMP
done
clear
D)
PGA
done
clear
View Answer play_arrow
question_answer 152) Photosynthetically least efficient radiation is:
A)
blue
done
clear
B)
yellow
done
clear
C)
green
done
clear
D)
red
done
clear
View Answer play_arrow
question_answer 153) In non-cyclic photophosphorylation, there are photolysis of 12 water molecules. How many\[{{H}^{+}}\]are formed?
A)
\[24{{H}^{+}}\]
done
clear
B)
\[36{{H}^{+}}\]
done
clear
C)
\[12{{H}^{+}}\]
done
clear
D)
\[32{{H}^{+}}\]
done
clear
View Answer play_arrow
question_answer 154) Compensation point refers to:
A)
little photosynthesis
done
clear
B)
beginning of photosynthesis
done
clear
C)
rate of photosynthesis equals to the rate of respiration
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 155) The hardest part of drupe is:
A)
mesocarp
done
clear
B)
endocarp
done
clear
C)
pericarp
done
clear
D)
epicarp
done
clear
View Answer play_arrow
question_answer 156) Seedless grapes are produced due to:
A)
parthenocarpy
done
clear
B)
crossing over
done
clear
C)
parthenogenesis
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 157) Which is used as weedicide?
A)
2, 4-D
done
clear
B)
\[IBA\]
done
clear
C)
\[IAA\]
done
clear
D)
\[ABA\]
done
clear
View Answer play_arrow
question_answer 158) Muscle fatigue is due to:
A)
lactic acid
done
clear
B)
citric acid
done
clear
C)
Na
done
clear
D)
K
done
clear
View Answer play_arrow
question_answer 159) As per NeoDarwinism, which is mainly responsible for evolution?
A)
Mutation
done
clear
B)
Natural selection
done
clear
C)
Both a and b
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 160) The mechanism of ATP formation both in chloroplast and mitochondria is explained by:
A)
Relay pump theory of Godlewski
done
clear
B)
Cholodny-Wents model
done
clear
C)
Chemiosmotic theory
done
clear
D)
Munchs mass flow hypothesis
done
clear
View Answer play_arrow
question_answer 161) What are episomes?
A)
Hereditary DNA of bacterial cell
done
clear
B)
Extrachromosomal hereditary material of bacteria associated with nucleoid
done
clear
C)
Modification of the cell membrane performing respiration
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 162) Salivary amylase is also known as:
A)
ptyalin
done
clear
B)
gastrin
done
clear
C)
glyoxylase
done
clear
D)
pepsin
done
clear
View Answer play_arrow
question_answer 163) Yeast is not included in protozoans but in fungi because:
A)
it has no chlorophyll
done
clear
B)
some fungal hyphae grow in such a way that they give the appearance of pseudomycelium
done
clear
C)
it has eukaryotic organisation
done
clear
D)
cell wall is made up of cellulose and reserve food material as starch
done
clear
View Answer play_arrow
question_answer 164) Which can accept a hydride ion during electron transduction system?
A)
FADH, NADH
done
clear
B)
\[FA{{D}^{+}},\text{ }NAD{{P}^{+}}\]
done
clear
C)
\[FA{{D}^{+}},\text{ }NADH\]
done
clear
D)
\[FADH,NA{{D}^{+}}\]
done
clear
View Answer play_arrow
question_answer 165) Which hormone is maximum in coconut milk?
A)
Gibberellin
done
clear
B)
Ethylene
done
clear
C)
Cytokmin
done
clear
D)
Auxin
done
clear
View Answer play_arrow
question_answer 166) Gibberellic acid induces flowering:
A)
in some gymnospermic plants only
done
clear
B)
in long day plants under short day conditions
done
clear
C)
in short day plants under long day conditions
done
clear
D)
in day-neutral plants under dark conditions
done
clear
View Answer play_arrow
question_answer 167) Root caps are absent in:
A)
mesophytes
done
clear
B)
xerophytes
done
clear
C)
hydrophytes
done
clear
D)
lithophytes
done
clear
View Answer play_arrow
question_answer 168) Somatic hybrids are produced by:
A)
protoplast fusion
done
clear
B)
tissue culture
done
clear
C)
pollen culture
done
clear
D)
hybridoma process
done
clear
View Answer play_arrow
question_answer 169) Leaf blade is spinous in case of:
A)
Nerium
done
clear
B)
Zizipus
done
clear
C)
Argemore
done
clear
D)
Cannabis
done
clear
View Answer play_arrow
question_answer 170) The histogens are classified on the basis of:
A)
cells they contain
done
clear
B)
cells they give rise to future tissue
done
clear
C)
meristematic activity
done
clear
D)
cell division
done
clear
View Answer play_arrow
question_answer 171) During cell division, chromosome attaches with spindles:
A)
kinetochore
done
clear
B)
centrosome
done
clear
C)
centriole
done
clear
D)
secondary constriction
done
clear
View Answer play_arrow
question_answer 172) Inverted pyramid is found in:
A)
biomass pyramid of aquatic system
done
clear
B)
energy pyramid of grassland
done
clear
C)
biomass pyramid of grassland
done
clear
D)
pyramid of number of aquatic system
done
clear
View Answer play_arrow
question_answer 173) Father of green revolution in India is:
A)
M.S. Swaminathan
done
clear
B)
N. Borlaug
done
clear
C)
R. Mishra
done
clear
D)
P. Maheswari
done
clear
View Answer play_arrow
question_answer 174) Stanley Miller proposed origin of life by:
A)
chemicaL synthesis
done
clear
B)
abiogenesis
done
clear
C)
biogenesis
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 175) Anemophilly is pollination by:
A)
wind
done
clear
B)
air
done
clear
C)
insects
done
clear
D)
birds
done
clear
View Answer play_arrow
question_answer 176) Conglobate gland is found in:
A)
female cockroach
done
clear
B)
male cockroach
done
clear
C)
Anopheles mosquito
done
clear
D)
Culex mosquito
done
clear
View Answer play_arrow
question_answer 177) Parenchymatous cells are found in:
A)
pulp of fruit
done
clear
B)
seeds
done
clear
C)
endocarp
done
clear
D)
skin of fruit
done
clear
View Answer play_arrow
question_answer 178) Quiescent centre is a:
A)
weak zone
done
clear
B)
active zone
done
clear
C)
inactive zone
done
clear
D)
strong base
done
clear
View Answer play_arrow
question_answer 179) The vacuole in the plant cell contains:
A)
tonoplast
done
clear
B)
cell sap
done
clear
C)
stroma
done
clear
D)
matrix
done
clear
View Answer play_arrow
question_answer 180) Water reaches the top of a plant due to:
A)
root pressure
done
clear
B)
capillarity
done
clear
C)
transpiration
done
clear
D)
diffusion
done
clear
View Answer play_arrow
question_answer 181) Germplasm theory was proposed by:
A)
Weismann
done
clear
B)
Darwin
done
clear
C)
Lamarck
done
clear
D)
Haeckel
done
clear
View Answer play_arrow
question_answer 182) The animal which has oval RBCs:
A)
humans
done
clear
B)
camel
done
clear
C)
dog
done
clear
D)
fish
done
clear
View Answer play_arrow
question_answer 183) Which of the following represents the condition seen in the family composite?
A)
Superior ovary, syngenesious, single basal ovule
done
clear
B)
Inferior ovary, monoadelphous, basal placentation
done
clear
C)
Inferior ovary, syngenesious, axile placentation
done
clear
D)
Syngenesious, basal placentation and epigynous
done
clear
View Answer play_arrow
question_answer 184) Maltose consists of which one of the following?
A)
\[\beta -\]glucose and\[\beta -\]galactose
done
clear
B)
\[\alpha -\]glucose and\[\alpha -\]fructose
done
clear
C)
\[\alpha -\]sucrose and\[\beta -\]glucose
done
clear
D)
glucose and glucose
done
clear
View Answer play_arrow
question_answer 185) Which of these is not essential for allogamy?
A)
Self sterility
done
clear
B)
Dichogamy
done
clear
C)
Heterogamy
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 186) If turgidity of a cell surrounded by water increases, the wall pressure will:
A)
increase
done
clear
B)
decrease
done
clear
C)
fluctuate
done
clear
D)
remain unchanged
done
clear
View Answer play_arrow
question_answer 187) Secondary cells cant divide because:
A)
they lose the ability to divide
done
clear
B)
they do not have nucleus
done
clear
C)
they undergo certain irreversible changes during differentiation
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 188) Mesosome in a bacterial cell is:
A)
plasmid
done
clear
B)
connection between two cells
done
clear
C)
plasma membrane infolded for respiration
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 189) First cell produced on earth is:
A)
protobiont
done
clear
B)
Protozoa
done
clear
C)
Metazoa
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 190) What is the botanical name of mulberry?
A)
Moms
done
clear
B)
Antherea
done
clear
C)
Attacus
done
clear
D)
Solanum
done
clear
View Answer play_arrow
question_answer 191) Meristematic cells have:
A)
thick cell wall and large intercellular spaces
done
clear
B)
thick cell wall and no intercellular spaces
done
clear
C)
thin cell wall and large intercellular spaces
done
clear
D)
thin cell wall and no intercellular spaces
done
clear
View Answer play_arrow
question_answer 192) Which has H-shaped grey matter?
A)
Cerebrum
done
clear
B)
Medulla oblongata
done
clear
C)
Cerebellum
done
clear
D)
Spinal cord
done
clear
View Answer play_arrow
question_answer 193) Phenomenon of Industrial melanism demonstrates:
A)
reproductive isolation
done
clear
B)
induced mutation
done
clear
C)
natural selection
done
clear
D)
geographical isolation
done
clear
View Answer play_arrow
question_answer 194) In lac operon model represser protein binds to which site?
A)
Regulator
done
clear
B)
Promoter
done
clear
C)
Operator
done
clear
D)
Structural genes
done
clear
View Answer play_arrow
question_answer 195) The chemical fertilizin is present in:
A)
ovum
done
clear
B)
sperm
done
clear
C)
immature ovum
done
clear
D)
fertilized ovum
done
clear
View Answer play_arrow
question_answer 196) The eukaryotic genome differs from the prokaryotic genome because:
A)
DNA is complexed with histones in prokaryotes
done
clear
B)
repetitive sequences are present in eukaryotes
done
clear
C)
genes in the former cases are organised into operons
done
clear
D)
DNA is circular and single stranded in prokaryotes
done
clear
View Answer play_arrow
question_answer 197) Temperature changes in the environment affect most of the animals which are:
A)
homoeothermic
done
clear
B)
aquatic
done
clear
C)
poikilothermic
done
clear
D)
desert living
done
clear
View Answer play_arrow
question_answer 198)
Match column-I with column-IL Column-I Column-II A. Bulliform cells 1. Stomata B. Guard cells 2. Aerating pore C. Lenticel 3. Accessory cells D. Subsidiary cell 4. Isobilateral leaf
A)
A-4, B-1, C-2, D-3
done
clear
B)
A-1, B-4, C-2, D-3
done
clear
C)
A-4, B-2, C-3, D-1
done
clear
D)
A-1, B-2, C-3, D-4
done
clear
View Answer play_arrow
question_answer 199) Tablets to prevent contraception contain:
A)
progesterone
done
clear
B)
FSH
done
clear
C)
LH
done
clear
D)
both b and c
done
clear
View Answer play_arrow
question_answer 200) Sperms acrosome has:
A)
hyaluronic acid and proacrosine
done
clear
B)
hyaluronic acid and fertilizin
done
clear
C)
hyaluronidase and proacrosin
done
clear
D)
fertilizin and proacrosin
done
clear
View Answer play_arrow
question_answer 201) What did the two persons do after robbing the man in. the car?
A)
They took away the car
done
clear
B)
They stabbed him in the back
done
clear
C)
They fled from the spot
done
clear
D)
They took him to the hospital
done
clear
View Answer play_arrow
question_answer 202) Why did the man not respond the two persons?
A)
He was not brave
done
clear
B)
He did not get enough time to understand what was going on
done
clear
C)
He was all alone
done
clear
D)
He had no weapon
done
clear
View Answer play_arrow
question_answer 203) Many patients do not like:
A)
strong smell
done
clear
B)
too many visitors
done
clear
C)
the colour of flowers
done
clear
D)
modem sick rooms
done
clear
View Answer play_arrow
question_answer 204) Many people think that flowers should not be kept:
A)
near an oxygen cylinder
done
clear
B)
for the night
done
clear
C)
near a sick mans bed
done
clear
D)
in an old room
done
clear
View Answer play_arrow
question_answer 205) Plants breathe oxygen:
A)
through their roots
done
clear
B)
in well-ventilated rooms
done
clear
C)
in a large quantity
done
clear
D)
after sunset
done
clear
View Answer play_arrow
question_answer 206) Directions: Select the meaning of the words printed in bold. Sagacity increases with age.
A)
maturity
done
clear
B)
wisdom
done
clear
C)
love
done
clear
D)
kindness
done
clear
View Answer play_arrow
question_answer 207) Directions: Select the meaning of the words printed in bold. He entertained a sense of rancor against the colleague who had superseded him.
A)
ill-will
done
clear
B)
disgust
done
clear
C)
hatred
done
clear
D)
malice
done
clear
View Answer play_arrow
question_answer 208) Directions: Select the meaning of the words printed in bold. A feeling of stillness, almost of suffering came over him.
A)
sorrow
done
clear
B)
grief
done
clear
C)
bereavement
done
clear
D)
agony
done
clear
View Answer play_arrow
question_answer 209) Directions: Select the meaning of the words printed in bold. The man vehemently denied all the charges of corruption that were levelled against him.
A)
hysterically
done
clear
B)
devoutly
done
clear
C)
serenely
done
clear
D)
forcefully
done
clear
View Answer play_arrow
question_answer 210) Directions: Select the meaning of the words printed in bold. This was her maiden speech and she did very well.
A)
primary
done
clear
B)
opening
done
clear
C)
first
done
clear
D)
girlish
done
clear
View Answer play_arrow
question_answer 211) Directions: Select the opposite meanings of the words printed in bold. The murderers guilt has been proved beyond doubt.
A)
excuse
done
clear
B)
innocence
done
clear
C)
independence
done
clear
D)
efficiency
done
clear
View Answer play_arrow
question_answer 212) Directions: Select the opposite meanings of the words printed in bold. He had a delectable meal yesterday.
A)
nice
done
clear
B)
unsavoury
done
clear
C)
heavy
done
clear
D)
tasty
done
clear
View Answer play_arrow
question_answer 213) Directions: Select the opposite meanings of the words printed in bold. The more you try to dissuade him, the more will he pursue the path.
A)
pursue
done
clear
B)
advise
done
clear
C)
persuade
done
clear
D)
assure
done
clear
View Answer play_arrow
question_answer 214) The lowest temperature recorded for any landmass in the world is:
A)
\[-70{}^\circ C\]
done
clear
B)
\[-100{}^\circ C\]
done
clear
C)
\[-90{}^\circ C\]
done
clear
D)
\[-40{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 215) Time is to watch as pressure is to.
A)
Barometer
done
clear
B)
Hydrometer
done
clear
C)
Thermometer
done
clear
D)
Lactometer
done
clear
View Answer play_arrow
question_answer 216) Among the following words, choose the word that comes first in the dictionary:
A)
Enter
done
clear
B)
Engagement
done
clear
C)
Election
done
clear
D)
Eloquence
done
clear
View Answer play_arrow
question_answer 217) If in a certain code STUDENT in coded as TSDUNET, then how TEACHER will be coded?
A)
ETCHAER
done
clear
B)
ATECHER
done
clear
C)
ETCAEHR
done
clear
D)
REHACTA
done
clear
View Answer play_arrow
question_answer 218) If INTELLIGENCE is coded as ECNIGELLEINT, then how NATIONALITY will be coded?
A)
YTINOLAITNA
done
clear
B)
YTINALOITNA
done
clear
C)
YTLIANATION
done
clear
D)
YTNIAOLITAN
done
clear
View Answer play_arrow
question_answer 219) If 35698 is coded as 53766, then how 67284 will be coded?
A)
84332
done
clear
B)
86263
done
clear
C)
84353
done
clear
D)
85352
done
clear
View Answer play_arrow
question_answer 220) If 35846 is coded as 63458, then how 24321 will be coded?
A)
12423
done
clear
B)
12234
done
clear
C)
14223
done
clear
D)
12243
done
clear
View Answer play_arrow
question_answer 221) Moon completes its revolution around the earth in how much time?
A)
29.5 days
done
clear
B)
28.5 days
done
clear
C)
27.3 days
done
clear
D)
29 days
done
clear
View Answer play_arrow
question_answer 222) The colour of the paint is .......... from the walls:
A)
falling
done
clear
B)
corroding
done
clear
C)
eroding
done
clear
D)
sticking
done
clear
View Answer play_arrow
question_answer 223) If a match starts between India and England at 10.00 a.m, in London, at what time it would be telecast (live) in India?
A)
6.00 p.m.
done
clear
B)
3.30 p.m.
done
clear
C)
3.30 a.m.
done
clear
D)
10.00 a.m.
done
clear
View Answer play_arrow
question_answer 224)
Insert the missing number: 6 11 16 21 10 14 18 22 5 8 11 14 16 ? 20 22
A)
14
done
clear
B)
26
done
clear
C)
24
done
clear
D)
18
done
clear
View Answer play_arrow
question_answer 225) Two arms of a watch show time of 3.30 in a mirror. What is the actual time?
A)
12.30 a.m.
done
clear
B)
9.30 a.m.
done
clear
C)
3.30 a.m.
done
clear
D)
2.30 a.m.
done
clear
View Answer play_arrow
question_answer 226) Directions: In the following questions, find the number which will come next in the series. 15, 5, 17, 7, 19, 9, 21 ?
A)
23
done
clear
B)
13
done
clear
C)
11
done
clear
D)
25
done
clear
View Answer play_arrow
question_answer 227) Directions: In the following questions, find the number which will come next in the series. 2, 5, 11, 23, ?
A)
36
done
clear
B)
34
done
clear
C)
42
done
clear
D)
47
done
clear
View Answer play_arrow
question_answer 228) Directions: In the following questions, find the number which will come next in the series. 94, 88, 82, 96, 70, 104, ?
A)
52
done
clear
B)
64
done
clear
C)
60
done
clear
D)
58
done
clear
View Answer play_arrow
question_answer 229) Directions: Solve the following problems. \[(0.\overline{6}+0.\overline{7})+0.\overline{8}+0.\overline{3}=\]
A)
\[2\frac{1}{5}\]
done
clear
B)
\[1\]
done
clear
C)
\[2\frac{2}{3}\]
done
clear
D)
\[3\frac{2}{5}\]
done
clear
View Answer play_arrow
question_answer 230) Directions: Solve the following problems. If the ratio of the areas of two squares is\[1:2,\]then ratio of the parameters of these two squares will be:
A)
\[1:\sqrt{2}\]
done
clear
B)
\[2:1\]
done
clear
C)
\[1:2\]
done
clear
D)
\[1:4\]
done
clear
View Answer play_arrow
question_answer 231) Directions: Solve the following problems. If the sum of three consecutive odd numbers is 57, then the middle number is:
A)
23
done
clear
B)
19
done
clear
C)
17
done
clear
D)
21
done
clear
View Answer play_arrow
question_answer 232) Directions: Solve the following problems. If\[\frac{5+2\sqrt{3}}{7+4\sqrt{3}}=a+b\sqrt{3},\]hen values of a and b are:
A)
\[a=-11,b=-6\]
done
clear
B)
\[a=11,b=11\]
done
clear
C)
\[a=11,b=-6\]
done
clear
D)
\[a=11,b=6\]
done
clear
View Answer play_arrow
question_answer 233) Which of the following is the largest stadium in the world?
A)
Lords stadium (England)
done
clear
B)
Eden Garden (Kolkatta)
done
clear
C)
Rose bowel (U.S.A.)
done
clear
D)
Strahov stadium (Czechoslovakia)
done
clear
View Answer play_arrow
question_answer 234) In which Constitutional Amendment of India the words Socialist, Secular and Integrity were added?
A)
78th
done
clear
B)
42nd
done
clear
C)
44th
done
clear
D)
52nd
done
clear
View Answer play_arrow
question_answer 235) The dimensions of a Tennis court for singles are:
A)
\[60\times 48\text{ }feet\]
done
clear
B)
\[78\times 28\text{ }feet\]
done
clear
C)
\[85\times 46\text{ }feet\]
done
clear
D)
\[17\times 44\text{ }feet\]
done
clear
View Answer play_arrow
question_answer 236) In which year UNESCO was established?
A)
1945
done
clear
B)
1950
done
clear
C)
1960
done
clear
D)
1946
done
clear
View Answer play_arrow
question_answer 237) YMCA stands for:
A)
Young Members of Council Academy
done
clear
B)
Young Mens Christian Association
done
clear
C)
Yound Mens Cancer Academy
done
clear
D)
Young Members of Cricket Academy
done
clear
View Answer play_arrow
question_answer 238) Who gave the slogan Inquilab Zindabad?
A)
Lok Manya Tilak
done
clear
B)
Mahatma Gandhi
done
clear
C)
Shaheed Bhagat Singh
done
clear
D)
S.C. Bose
done
clear
View Answer play_arrow
question_answer 239) Which period is known as the Golden Age of India?
A)
English regime
done
clear
B)
Mauryas regime
done
clear
C)
Mughals regime
done
clear
D)
Guptas regime
done
clear
View Answer play_arrow
question_answer 240) First general elections were held in India in:
A)
1951
done
clear
B)
1966
done
clear
C)
1952
done
clear
D)
1956
done
clear
View Answer play_arrow