question_answer 1)
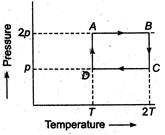
An ideal monoatomic gas is taken thorough the theromodynamic states\[A\to B\to C\to D\]via the paths shown in the figure. If\[{{U}_{A}},{{U}_{B}},{{U}_{C}}\] and\[{{U}_{D}}\]represent the internal energy of the gas in states A, B, C and D respectively, then which of the following is not true?
A)
\[{{U}_{A}}={{U}_{D}}\]
done
clear
B)
\[{{U}_{B}}<{{U}_{A}}\]
done
clear
C)
\[{{U}_{B}}={{U}_{C}}\]
done
clear
D)
\[{{U}_{C}}>{{U}_{D}}\]
done
clear
View Answer play_arrow
question_answer 2) The external diairueter of a 314 m long copper tube is 1.2 cm and the internal diameter is 1 cm. Calculate its resistance if the specific resistance of copper is\[2.2\times {{10}^{-8}}\Omega -m\]
A)
\[5.0\times {{10}^{-2}}\Omega \]
done
clear
B)
\[4.4\times {{10}^{-2}}\Omega \]
done
clear
C)
\[3.14\times {{10}^{-2}}\Omega \]
done
clear
D)
\[2.2\times {{10}^{-2}}\Omega \]
done
clear
E)
None of the above
done
clear
View Answer play_arrow
question_answer 3) A voltmeter of range 2V and resistance\[300\,\Omega \] cannot be converted into ammeter of range
A)
1 A
done
clear
B)
1 mA
done
clear
C)
100 mA
done
clear
D)
10 mA
done
clear
View Answer play_arrow
question_answer 4) If a magnet is suspended at an angle\[30{}^\circ \]to the magnetic meridian, the dip needle makes angle of\[45{}^\circ \]with the horizontal. The real dip is
A)
\[{{\tan }^{-1}}(\sqrt{3}/2)\]
done
clear
B)
\[{{\tan }^{-1}}(\sqrt{3})\]
done
clear
C)
\[{{\tan }^{-1}}(3/\sqrt{2})\]
done
clear
D)
\[{{\tan }^{-1}}(2/\sqrt{3})\]
done
clear
View Answer play_arrow
question_answer 5) Which quantity is increased in step-down transformer?
A)
Current
done
clear
B)
Voltage
done
clear
C)
Power
done
clear
D)
Frequency
done
clear
View Answer play_arrow
question_answer 6) Beyond which frequency, the ionosphere bends any incident electromagnetic radiation but do not reflect it back towards the earth?
A)
50MHz
done
clear
B)
40MHz
done
clear
C)
30MHz
done
clear
D)
20MHz
done
clear
View Answer play_arrow
question_answer 7) In intrinsic semiconductor at room temperature number of electrons and holes are
A)
equal
done
clear
B)
zero
done
clear
C)
unequal
done
clear
D)
infinite
done
clear
View Answer play_arrow
question_answer 8) What should be the velocity of an electron so that its momentum becomes equal to that of a photon of wavelength\[5200\text{ }\overset{o}{\mathop{\text{A}}}\,\]?
A)
700 m/s
done
clear
B)
1000 m/s
done
clear
C)
1400 m/s
done
clear
D)
2800 m/s
done
clear
View Answer play_arrow
question_answer 9) A radioactive element has half-life period of 600 yr. After 3000 yr, what amount will remain?
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{16}\]
done
clear
C)
\[\frac{1}{8}\]
done
clear
D)
\[\frac{1}{32}\]
done
clear
View Answer play_arrow
question_answer 10) An electric motor runs on DC source of emf 200 V and draws a current of 10 A. If the efficiency be 40%, then the resistance of armature is
A)
\[2\,\Omega \]
done
clear
B)
\[8\,\Omega \]
done
clear
C)
\[12\,\Omega \]
done
clear
D)
\[16\,\Omega \]
done
clear
View Answer play_arrow
question_answer 11) A capacitor having capacity of 2.0 p F is charged to 200 V and then the plates of the capacitor are connected to a resistance wire. The heat produced in joule will be
A)
\[2\times {{10}^{-2}}\]
done
clear
B)
\[4\times {{10}^{-2}}\]
done
clear
C)
\[4\times {{10}^{4}}\]
done
clear
D)
\[4\times {{10}^{10}}\]
done
clear
View Answer play_arrow
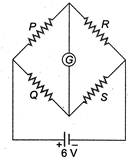
question_answer 12)
In the Wheatstones network given,\[P=10\,\Omega ,\] \[Q=20\text{ }\Omega ,\text{ }R=15\text{ }\Omega ,\text{ S}=30\text{ }\Omega ,\]the current passing through the battery (of negligible internal resistance) is
A)
0.36 A
done
clear
B)
zero
done
clear
C)
0.18 A
done
clear
D)
0.72 A
done
clear
View Answer play_arrow
question_answer 13) Two tangent galvanometers A and B have coils of radii 8 cm and 16 cm respectively and resistance\[8\,\Omega \], each. They are connected in parallel with a cell of emf 4 V and negligible internal ressistance. The deflections produced in the tangent galvanometers A and B are\[30{}^\circ \] and\[60{}^\circ \]respectively. If A has 2 turns, then B must have
A)
18 turns
done
clear
B)
12 turns
done
clear
C)
6 turns
done
clear
D)
2 turns
done
clear
View Answer play_arrow
question_answer 14) A current of 5 A is passing through a metallic wire of cross-sectional area\[4\times {{10}^{-6}}{{m}^{2}}\]. If the density of charge carriers of the wire is\[5\times {{10}^{26}}\] \[{{m}^{-3}},\]the drift velocity of the electrons will be
A)
\[1\times {{10}^{2}}m{{s}^{-1}}\]
done
clear
B)
\[1.56\times {{10}^{-2}}m{{s}^{-1}}\]
done
clear
C)
\[1.56\times {{10}^{-3}}m{{s}^{-1}}\]
done
clear
D)
\[1\times {{10}^{-2}}m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 15) A single slit Fraunhofer diffraction pattern is formed with white light. For what wavelength of light, the third secondary maximum in the diffraction pattern coincides with the second secondary maximum in the pattern for red light of wavelength\[6500\text{ }\overset{o}{\mathop{\text{A}}}\,\]?
A)
\[4400\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[4100\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[4642.8\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[9100\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 16) The potential of a large liquid drop when eight liquid drops are combined is 20 V. Then, the potential of each single drop was
A)
10 V
done
clear
B)
7.5V
done
clear
C)
5V
done
clear
D)
2.5V
done
clear
View Answer play_arrow
question_answer 17) Water rises in plant fibres due to
A)
capillarity
done
clear
B)
viscosity
done
clear
C)
fluid pressure
done
clear
D)
osmosis
done
clear
View Answer play_arrow
question_answer 18) During an adiabatic process, the cube of the pressure is found to be inversely proportional to the fourth power of the volume. Then, the ratio of specific heats is
A)
1
done
clear
B)
1.33
done
clear
C)
1.67
done
clear
D)
1.4
done
clear
View Answer play_arrow
question_answer 19) The volume of a nucleus is directly proportional to
A)
\[A\]
done
clear
B)
\[{{A}^{3}}\]
done
clear
C)
\[\sqrt{A}\]
done
clear
D)
\[{{A}^{1/3}}\]
done
clear
View Answer play_arrow
question_answer 20) A Carnots engine has an efficiency of 50% at sink temperature \[50{}^\circ C\]. Calculate the temperature of source.
A)
\[133{}^\circ C\]
done
clear
B)
\[143{}^\circ C\]
done
clear
C)
\[100{}^\circ C\]
done
clear
D)
\[373{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 21) What is the Q-value of the reaction? \[P{{+}^{7}}Li{{\xrightarrow[{}]{{}}}^{4}}He{{+}^{4}}He\] The atomic masses of\[^{1}H{{,}^{4}}He\]and\[^{7}Li\]are \[1.0078254u,4.0026034u\]and\[7.016004u\] respectively
A)
17.35 MeV
done
clear
B)
18.06 MeV
done
clear
C)
177.35 MeV
done
clear
D)
170.35 MeV
done
clear
View Answer play_arrow
question_answer 22) The water of volume 4 m3 at the height 20 m is pressed by\[2\times {{10}^{5}}N\]pressure. The work done by motor is
A)
\[8\times {{10}^{5}}J\]
done
clear
B)
\[16\times {{10}^{5}}J\]
done
clear
C)
\[12\times {{10}^{5}}J\]
done
clear
D)
\[32\times {{10}^{5}}J\]
done
clear
View Answer play_arrow
question_answer 23) The wavelength of\[{{K}_{\alpha }}\]line in copper is\[1.5\overset{o}{\mathop{\text{A}}}\,\]. The ionisation energy of K electron in copper is
A)
\[11.2\times {{10}^{-18}}J\]
done
clear
B)
\[12.9\times {{10}^{-16}}J\]
done
clear
C)
\[1.7\times {{10}^{-15}}J\]
done
clear
D)
\[10\times {{10}^{-16}}J\]
done
clear
View Answer play_arrow
question_answer 24) A proton is moving in a uniform magiletic field B in a circular path of radius a in a direction perpendicular to Z axis along which field B exists. Calculate the angular momentum. If the radius is a and charge on proton is e.
A)
\[\frac{Be}{{{a}^{2}}}\]
done
clear
B)
\[e{{B}^{2}}a\]
done
clear
C)
\[{{a}^{2}}eB\]
done
clear
D)
\[aeB\]
done
clear
View Answer play_arrow
question_answer 25) Curie-Weiss law is obeyed by iron
A)
at curie temperature only
done
clear
B)
at all temperatures
done
clear
C)
below curie temperature
done
clear
D)
above curie temperature
done
clear
View Answer play_arrow
question_answer 26) When light is incidence on a diffraction grating, then the zero order maximum will be
A)
spectrum of the colours
done
clear
B)
white
done
clear
C)
one of the component colours
done
clear
D)
absent
done
clear
View Answer play_arrow
question_answer 27) The wavelength of first line of Balmer series is\[6563\overset{o}{\mathop{\text{A}}}\,\]. The wavelength of first line of Lymen series will be
A)
\[1215.4\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[2500\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[7500\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[600\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 28) The reactance of inductance at\[{{10}^{4}}Hz\]is\[10{{\,}^{4}}\Omega \]. Its reactance at\[2\times {{10}^{4}}Hz\]will be
A)
\[{{10}^{4}}\,\Omega \]
done
clear
B)
\[2\times {{10}^{4}}\,\Omega \]
done
clear
C)
\[3\times {{10}^{4}}\,\Omega \]
done
clear
D)
\[4\times {{10}^{4}}\,\Omega \]
done
clear
View Answer play_arrow
question_answer 29) In adiabatic process, the work done by system is 50 J, then
A)
the temperature of the system will be increase 50 J
done
clear
B)
the temperature of the system will be constant
done
clear
C)
the internal energy of the system will be increase 50 J
done
clear
D)
the internal energy of the system will be decrease 50 J
done
clear
View Answer play_arrow
question_answer 30) The ozone layer is important because
A)
it prevents the cooling of each at night
done
clear
B)
it prevents the IR rays coming from the space
done
clear
C)
it prevents UV rays from the meteors coming from the space
done
clear
D)
it prevents the UV rays and micro rays the coming from the space
done
clear
View Answer play_arrow
question_answer 31) If resonant frequency is/and capacity become 4 times, then resonant frequency will be
A)
\[\frac{f}{2}\]
done
clear
B)
\[2f\]
done
clear
C)
\[f\]
done
clear
D)
\[\frac{f}{4}\]
done
clear
View Answer play_arrow
question_answer 32) If two soap bubbles of different radii. Are connected by a tube, then
A)
air flow from bigger bubble to the smaller bubble till sizes become equal
done
clear
B)
air flow from bigger bubble to the smaller bubble till sizes are interchanged
done
clear
C)
air flow from smaller bubble to bigger
done
clear
D)
there is no flow of air
done
clear
View Answer play_arrow
question_answer 33) The hardness of X-rays by coolidge tube depends upon
A)
filament current
done
clear
B)
air pressure in tube
done
clear
C)
material of target
done
clear
D)
potential between target and cathode
done
clear
View Answer play_arrow
question_answer 34) A launching vehicle carrying an artificial satellite of mass m is set for launch on the surface of the earth of mass M and radius R. If the satellite is intended to move in a circular orbit of radius 7R, the minimum energy required to be spent by the launching vehicle on the satellite is (Gravitational constant = G)
A)
\[\frac{GMm}{R}\]
done
clear
B)
\[-\frac{13GMm}{14R}\]
done
clear
C)
\[\frac{GMm}{7R}\]
done
clear
D)
\[\frac{GMm}{14R}\]
done
clear
View Answer play_arrow
question_answer 35) The displacements of two particles of same mass executing SHM are represented by the equations \[{{x}_{1}}=4\sin \left( 10t+\frac{\pi }{6} \right)\]and \[{{x}_{2}}=5\cos (\omega t)\] The value of co for which the energies of both the particles remain same is
A)
16 unit
done
clear
B)
6 unit
done
clear
C)
4 unit
done
clear
D)
8 unit
done
clear
View Answer play_arrow
question_answer 36) The excess pressure inside a spherical soap bubble of radius 1 cm is balanced by a column of oil (specific gravity =0.8), 2 mm high, the surface tension of the bubble is
A)
3.92 N/m
done
clear
B)
0.0392 N/m
done
clear
C)
0.392 N/m
done
clear
D)
0.00392 N/m
done
clear
View Answer play_arrow
question_answer 37) Water from a tap emerges vertically downwards with initial velocity\[4\text{ }m{{s}^{-1}}\]. The cross-sectional area of the tap is A. The flow is steady and pressure is constant throughout the stream of water. The distance h vertically below the tap, where the cross-sectional area of the stream becomes\[\left( \frac{2}{3} \right)A,\]is\[(g=10\text{ }m/{{s}^{2}})\]
A)
0.5 m
done
clear
B)
1 m
done
clear
C)
1.5 m
done
clear
D)
2.2 m
done
clear
View Answer play_arrow
question_answer 38) The diameter of objective of a telescope is 1m. Its resolving limit for the light of wavelength \[4538\overset{o}{\mathop{\text{A}}}\,,\] will be
A)
\[5.54\times {{10}^{-7}}rad\]
done
clear
B)
\[2.54\times {{10}^{-4}}rad\]
done
clear
C)
\[6.54\times {{10}^{-7}}rad\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 39) The potential difference between two parallel plates is 104 V. If the plates are separated by 0.5 cm, the force on an electron between the plates is
A)
\[32\times {{10}^{-13}}N\]
done
clear
B)
\[0.32\times {{10}^{-13}}N\]
done
clear
C)
\[0.032\times {{10}^{-13}}N\]
done
clear
D)
\[3.2\times {{10}^{-13}}N\]
done
clear
View Answer play_arrow
question_answer 40) A body of mass\[{{m}_{1}}=4\]kg moves at\[5\hat{i}\text{ }m/s\]and another body of mass\[{{m}_{2}}=2\]kg moves at\[10\hat{i}\] m/s. The kinetic energy of centre of mass is
A)
\[\frac{200}{3}J\]
done
clear
B)
\[\frac{500}{3}J\]
done
clear
C)
\[\frac{400}{3}J\]
done
clear
D)
\[\frac{800}{3}J\]
done
clear
View Answer play_arrow
question_answer 41) Two block of masses of 1 kg and 2 kg are connected by a metal wire going over a smooth pulley. The breaking stress of metal is\[\frac{40}{3\pi }\times {{10}^{6}}N/{{m}^{2}}\]. What should be the minimum radius of wire used if it should not break? \[(g=10m/{{s}^{2}})\].
A)
0.5mm
done
clear
B)
1 mm
done
clear
C)
1.5 mm
done
clear
D)
2 mm
done
clear
View Answer play_arrow
question_answer 42) In the Youngs double slit experiment, intensities of black and bright fringes are 1 and 4 respectively, the ratio of amplitudes of sources will be
A)
\[1:1\]
done
clear
B)
\[1:2\]
done
clear
C)
\[3:1\]
done
clear
D)
\[1:4\]
done
clear
View Answer play_arrow
question_answer 43) If inductance and resistance of chocke coil are \[{{X}_{L}}\]and R respectively, then
A)
\[{{X}_{L}}=R\]
done
clear
B)
\[{{X}_{L}}>>R\]
done
clear
C)
\[{{X}_{L}}<<R\]
done
clear
D)
\[{{X}_{L}}=\infty \]
done
clear
View Answer play_arrow
question_answer 44) The path difference, time difference and phase difference between two successive zones will be
A)
\[\frac{\lambda }{2},\frac{T}{2}\]and \[\pi \]
done
clear
B)
\[\lambda ,T\]and \[\pi \]
done
clear
C)
\[\frac{\lambda }{2},\frac{T}{2}\]and \[\frac{\pi }{2}\]
done
clear
D)
\[\frac{\lambda }{2},\frac{T}{2}\]and \[2\pi \]
done
clear
View Answer play_arrow
question_answer 45) At what speed should a source of sound move so that stationary observer finds the apparent frequency equal to half of the original frequency?
A)
\[\frac{v}{2}\]
done
clear
B)
\[2v\]
done
clear
C)
\[\frac{v}{4}\]
done
clear
D)
\[v\]
done
clear
View Answer play_arrow
question_answer 46) At inductance 1 H is connected in series with an AC source of 220 V and 50 Hz. The inductive resistance (in ohm) is
A)
\[2\pi \]
done
clear
B)
\[50\pi \]
done
clear
C)
\[100\pi \]
done
clear
D)
\[1000\pi \]
done
clear
View Answer play_arrow
question_answer 47) If the pressure of an ideal gas contained in a closed vessel is increased by 0.4%, the increases in temperature is\[1{}^\circ C\]. The initial temperature of the gas is
A)
\[25{}^\circ C\]
done
clear
B)
\[250{}^\circ C\]
done
clear
C)
\[250\,K\]
done
clear
D)
\[2500{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 48) If a van der Waals gas expands freely, then final temperature is
A)
less than the initial temperature
done
clear
B)
equal to the initial temperature
done
clear
C)
more than the initial temperature
done
clear
D)
less or more than the initial temperature depending on the nature of the gas
done
clear
View Answer play_arrow
question_answer 49) The de-Broglie wavelength of a proton (charge\[=1.6\times {{10}^{-19}}C\].mass\[=1.6\times {{10}^{-27}}kg\]) accelerated through a potential difference of 1 kV is
A)
\[600\,\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.9\times {{10}^{-12}}m\]
done
clear
C)
\[7\,\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
0.9 nm
done
clear
View Answer play_arrow
question_answer 50) A radioactive element forms its own isotope after 3 consecutive disintegrations. The particles emitted as
A)
\[3\beta -\]particles
done
clear
B)
\[2\beta -\]particles and 1\[\alpha -\]particle
done
clear
C)
\[2\beta -\]particles and 1\[\gamma -\]particle
done
clear
D)
\[2\beta -\]particles and 1\[\beta -\]particle
done
clear
View Answer play_arrow
question_answer 51) A step-down transformer reduces the voltage of a transmission line from 2200 V to 220 V. The power delivered by it is 880 W and its efficiency is 88%. The input current is
A)
4.65 mA
done
clear
B)
0.045 A
done
clear
C)
0.45 A
done
clear
D)
4.65 A
done
clear
View Answer play_arrow
question_answer 52) If\[{{\lambda }_{1}}\]and\[{{\lambda }_{2}}\]are the wavelengths of the first members of the Lyman and Paschen series respectively, then\[{{\lambda }_{1}}:{{\lambda }_{2}}\]is
A)
\[1:3\]
done
clear
B)
\[1:30\]
done
clear
C)
\[7:50\]
done
clear
D)
\[7:108\]
done
clear
View Answer play_arrow
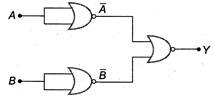
question_answer 53)
Identify the operation performed by the circuit given below
A)
NOT
done
clear
B)
AND
done
clear
C)
OR
done
clear
D)
NAND
done
clear
View Answer play_arrow
question_answer 54) In a. transistor, the collector current is always less than the emitter current because
A)
collector side is reverse biased and the emitter side is forward biased
done
clear
B)
a few electrons are lost in the base and only remaining ones reach the collector
done
clear
C)
collector being reverse biased, attracts less electrons
done
clear
D)
collector side is forward biased and emitter side is reverse biased
done
clear
View Answer play_arrow
question_answer 55) A rectangular vessel when full of water, takes 10 min to be emptied through an orifice in its bottom. How much time will it take to be emptied when half filled with water?
A)
9 min
done
clear
B)
7 min
done
clear
C)
5 min
done
clear
D)
3 min
done
clear
View Answer play_arrow
question_answer 56) The term liquid crystal refers to a state that is intermediate between
A)
crystalline solid and amorphous liquid
done
clear
B)
crystalline solid and vapour
done
clear
C)
amorphous liquid and its vapour
done
clear
D)
a crystal immersed in a liquid
done
clear
View Answer play_arrow
question_answer 57) A wave travelling in air falls on a glass plate. It is partly reflected and partly refracted. The phase difference between the reflected and refracted waves is
A)
zero
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\pi \]
done
clear
D)
\[2\pi \]
done
clear
View Answer play_arrow
question_answer 58) Two coherent monochromatic light beams of intensities\[I\]and\[4I\]are superposed. The maximum and minimum possible resulting intensities are
A)
\[5I\]and O
done
clear
B)
\[5I\] and\[3I\]
done
clear
C)
\[9I\] and I
done
clear
D)
\[9I\] and\[3I\]
done
clear
View Answer play_arrow
question_answer 59) The light of wavelength\[5000\overset{o}{\mathop{\text{A}}}\,\]falls on a photosensitive plate of work function 1.9 eV. The kinetic energy of the emitted photoelectrons is
A)
0.58 eV
done
clear
B)
2.48 eV
done
clear
C)
1.24 eV
done
clear
D)
1.18 eV
done
clear
View Answer play_arrow
question_answer 60) A galvanometer can be converted into a voltmeter by connecting
A)
low resistance in parallel
done
clear
B)
low resistance in series
done
clear
C)
high resistance in parallel
done
clear
D)
high resistance in series
done
clear
View Answer play_arrow
question_answer 61) Benzaldehyde condenses with N,N-dimethyl aniline in the presence of anhydrous\[ZnC{{l}_{2}}\]to give
A)
azodye
done
clear
B)
malachite green
done
clear
C)
buffer yellow
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 62) The general formula\[{{C}_{n}}{{H}_{2n}}{{O}_{2}}\]could be for open chain
A)
diketories
done
clear
B)
carboxylic acids
done
clear
C)
diols
done
clear
D)
dialdehydes
done
clear
View Answer play_arrow
question_answer 63) Which of the following is used widely in the manufacture of lead storage battery?
A)
Arsenic
done
clear
B)
Lithium
done
clear
C)
Bismuth
done
clear
D)
Antimony
done
clear
View Answer play_arrow
question_answer 64) On passing 3A of electricity for 50 min 1.8 g metal deposits. The equivalent mass of metal is
A)
9.3
done
clear
B)
19.3
done
clear
C)
38.3
done
clear
D)
39.5
done
clear
View Answer play_arrow
question_answer 65) Saturated solution of\[KN{{O}_{3}}\]is used to make salt bridge because
A)
velocity of\[{{K}^{+}}\]is greater than that of\[N{{O}_{3}}\]
done
clear
B)
velocity of\[N{{O}_{3}}\]is greater than that of\[{{K}^{+}}\]
done
clear
C)
velocity of both\[{{K}^{+}}\]and\[N{{O}_{3}}\]are nearly same
done
clear
D)
\[KN{{O}_{3}}\]is soluble in water
done
clear
View Answer play_arrow
question_answer 66) For which order half-life period is independent of initial concentration?
A)
Zero
done
clear
B)
First
done
clear
C)
Second
done
clear
D)
Third
done
clear
View Answer play_arrow
question_answer 67) Sodium phenoxide reacts with\[C{{O}_{2}}\]at 400 K and 4-7 atm pressure to give
A)
cathechol
done
clear
B)
salicylaldehyde
done
clear
C)
salicylic acid
done
clear
D)
sodium salicylate
done
clear
View Answer play_arrow
question_answer 68) The rate constant for the first order reaction is\[60\text{ }{{s}^{-1}}\]. How much time will it take to reduce the concentration of the reactant to\[\frac{1}{16}\]th value?
A)
\[4.6\times {{10}^{-2}}s\]
done
clear
B)
\[4.6\times {{10}^{4}}s\]
done
clear
C)
\[4.6\times {{10}^{2}}s\]
done
clear
D)
\[4.6\times {{10}^{-4}}s\]
done
clear
View Answer play_arrow
question_answer 69) The rate of reaction \[CC{{l}_{3}}CHO+NO\xrightarrow[{}]{{}}CHC{{l}_{3}}+NO+CO\] Is equal to rate\[=k[CC{{l}_{3}}CHO][NO]\]. If concentration is expressed in\[mol\text{ }{{L}^{-1}},\]the unit of\[k\]is
A)
\[L\,mo{{l}^{-1}}{{s}^{-1}}\]
done
clear
B)
\[mol\text{ }{{L}^{-1}}{{s}^{-1}}\]
done
clear
C)
\[{{L}^{2}}mo{{l}^{-2}}{{s}^{-1}}\]
done
clear
D)
\[{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 70) When phenolic ether is heated with HI it yields
A)
alkyi halide\[+\]aryl halide\[+\]water
done
clear
B)
alkyrhalide\[+\]phenol
done
clear
C)
alcohol\[+\]aryl halide
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 71) Which of the following is not correct?
A)
Milk is naturally occurring emulsion
done
clear
B)
Gold sol is a lyophilic sol
done
clear
C)
Physical adsorption decreases with rise in temperature
done
clear
D)
Chemical adsorption is unilayered.
done
clear
View Answer play_arrow
question_answer 72) The number of sodium atoms in 2 moles of sodium ferrocynanide is
A)
\[12\times {{10}^{23}}\]
done
clear
B)
\[26\times {{10}^{23}}\]
done
clear
C)
\[34\times {{10}^{23}}\]
done
clear
D)
\[48\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 73) Which of the following statement in relation to the hydrogen atom is correct?
A)
\[3s,3p\]and 3d orbitals all have the same energy.
done
clear
B)
2s and 3p-orbitals are of lower energy than 3d-orbital.
done
clear
C)
3p-orbital is lower in energy than 3d-orbital.
done
clear
D)
3s-orbital is lower in energy than 3p-orbital.
done
clear
View Answer play_arrow
question_answer 74) Which of the following is not tetrahedral?
A)
\[BF_{4}^{-}\]
done
clear
B)
\[NH_{4}^{+}\]
done
clear
C)
\[CO_{3}^{2-}\]
done
clear
D)
\[SO_{4}^{2-}\]
done
clear
View Answer play_arrow
question_answer 75) Considering x-axis as the intemuclear axis, which out of the following will not form a sigma bond.
A)
1s and 1s
done
clear
B)
1s and \[2{{p}_{x}}\]
done
clear
C)
\[2{{p}_{y}}\]and\[2{{p}_{y}}\]
done
clear
D)
1s and 2s
done
clear
View Answer play_arrow
question_answer 76) The chlorination of methane is an example of
A)
addition
done
clear
B)
elimination
done
clear
C)
substitution
done
clear
D)
chain reaction
done
clear
View Answer play_arrow
question_answer 77) Reactivity towards nucleophilic addition reaction of (I) HCHO (II)\[C{{H}_{3}}CHO\](III) \[C{{H}_{3}}COC{{H}_{3}}\]is
A)
\[II>III>I\]
done
clear
B)
\[III>II>I\]
done
clear
C)
\[I>II>III\]
done
clear
D)
\[I>II<III\]
done
clear
View Answer play_arrow
question_answer 78) Which of the following is active species in sulphonation of benzene?
A)
\[S{{O}_{3}}\]
done
clear
B)
\[SO_{3}^{+}\]
done
clear
C)
\[SO_{3}^{-}\]
done
clear
D)
\[HSO_{4}^{+}\]
done
clear
View Answer play_arrow
question_answer 79) Which of the following cant be used in Friedel-Crafts reaction?
A)
\[FeC{{l}_{3}}\]
done
clear
B)
\[FeB{{r}_{2}}\]
done
clear
C)
\[AlC{{l}_{3}}\]
done
clear
D)
\[NaCl\]
done
clear
View Answer play_arrow
question_answer 80) The order of reactivities of methyl halide in the formation of Grignard reagent is
A)
\[C{{H}_{3}}Cl>C{{H}_{3}}Br>C{{H}_{3}}I,\]
done
clear
B)
\[C{{H}_{3}}I>C{{H}_{3}}Br>C{{H}_{3}}Cl\]
done
clear
C)
\[C{{H}_{3}}Br>C{{H}_{3}}I>C{{H}_{3}}Cl\]
done
clear
D)
\[C{{H}_{3}}Br>C{{H}_{3}}Cl>C{{H}_{3}}I\]
done
clear
View Answer play_arrow
question_answer 81) The product formed when toluene is heated in light with\[C{{l}_{2}}\]and in absence of halogen carrier is
A)
chlorobenzene
done
clear
B)
gammexene
done
clear
C)
benzotrichloride
done
clear
D)
DDT
done
clear
View Answer play_arrow
question_answer 82) It the pressure on a\[NaCl\]structure is increased, then its coordination number will
A)
increase
done
clear
B)
decrease
done
clear
C)
either [a] or [b]
done
clear
D)
remain the same
done
clear
View Answer play_arrow
question_answer 83) If the bond dissociation energies of\[XY,{{X}_{2}}\]and \[{{Y}_{2}}\] (all diatomic molecules) are in the ratio of \[1:1:0.5\] and\[\Delta {{H}_{f}}\]for the formation of XY is\[-200\text{ }kJ\text{ }mo{{l}^{-1}},\]the bond dissociation energy of\[{{X}_{2}}\]will be .
A)
\[400\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
B)
\[300\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
C)
\[200\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
D)
\[800\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 84) The enthalpies of all the elements in their standard states are
A)
unity
done
clear
B)
zero
done
clear
C)
\[<0\]
done
clear
D)
different for each element
done
clear
View Answer play_arrow
question_answer 85) A reaction,\[A+B\xrightarrow[{}]{{}}C+D+q\]is found to have a positive entropy change. The reaction will be
A)
possible at high temperature
done
clear
B)
possible only at low temperature
done
clear
C)
not possible at any temperature
done
clear
D)
possible at any temperature
done
clear
View Answer play_arrow
question_answer 86) Enthalpy of combustion of carbon to\[C{{O}_{2}}\]is\[-393.5\,kJ\,mo{{l}^{-1}}.\]The heat released upon formation of 35.2 g of\[C{{O}_{2}}\]from carbon and dioxygen gas is (molar mass of \[C{{O}_{2}}=44\,g\,mo{{l}^{-1}}\])
A)
3.148 kJ
done
clear
B)
31.48 kJ
done
clear
C)
314.8 kJ
done
clear
D)
3148 kJ
done
clear
View Answer play_arrow
question_answer 87) Work done during isothermal expansion of one mole of an ideal gas from 10 atm to 1 atm at 300 K is
A)
4938.8 J
done
clear
B)
4138.8 J
done
clear
C)
5744.1 J
done
clear
D)
6257.2 J
done
clear
View Answer play_arrow
question_answer 88) Which of the following is not a conjugate acid-base pair?
A)
\[HPO_{3}^{2-},PO_{3}^{3-}\]
done
clear
B)
\[{{H}_{2}}PO_{4}^{-},HPO_{4}^{2-}\]
done
clear
C)
\[{{H}_{2}}PO_{4}^{-},{{H}_{3}}P{{O}_{4}}\]
done
clear
D)
\[{{H}_{2}}PO_{4}^{-},PO_{3}^{3-}\]
done
clear
View Answer play_arrow
question_answer 89) Which of the following will not function as a buffer solution? [a]\[NaCl\]and\[NaOH\] [b]\[NaOH\]and\[N{{H}_{4}}OH\] [c]\[C{{H}_{3}}COON{{H}_{4}}\]and\[HCl\] [d] Borax and boric acid
A)
(i),(ii),(iii)
done
clear
B)
(ii), (iii),,(iv)
done
clear
C)
(i), (iii), (iv)
done
clear
D)
(i), (ii), (iii), (iv)
done
clear
View Answer play_arrow
question_answer 90) A litre of solution is saturated with\[AgCl\]. To this solution if\[1.0\times {{10}^{-4}}\]of solid\[NaCl\]is added, what will be the\[[A{{g}^{+}}]\]assuming no volume change?
A)
More
done
clear
B)
Less
done
clear
C)
Equal
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 91) Aqueous solution of sodium cyanide is
A)
acidic
done
clear
B)
amphoteric
done
clear
C)
basic
done
clear
D)
neutral
done
clear
View Answer play_arrow
question_answer 92) The equilibrium constant for the reaction \[S{{O}_{3}}(g)S{{O}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\] is\[{{K}_{c}}=4.9\times {{10}^{-2}}\]. The value of\[{{K}_{c}}\]or the reaction \[2S{{O}_{2}}(g)+{{O}_{2}}(g)2S{{O}_{3}}(g)\] will be
A)
\[416\]
done
clear
B)
\[2.40\times {{10}^{-3}}\]
done
clear
C)
\[9.8\times {{10}^{-2}}\]
done
clear
D)
\[4.9\times {{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 93) In the reaction, \[A{{g}_{2}}O+{{H}_{2}}{{O}_{2}}2Ag+{{H}_{2}}O+{{O}_{2}}\] \[{{H}_{2}}{{O}_{2}}\]acts as
A)
a reducing agent
done
clear
B)
oxidising agent
done
clear
C)
bleaching agent
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 94) When two ice cubes are pressed over each other they unites to form one cube. Which of the following forces are responsible to hold them together?
A)
Ionic interaction
done
clear
B)
van der Waals forces
done
clear
C)
Covalent interaction
done
clear
D)
Hydrogen bond formation
done
clear
View Answer play_arrow
question_answer 95) Red lead is
A)
\[PbC{{O}_{3}}\]
done
clear
B)
\[P{{b}_{3}}{{O}_{4}}\]
done
clear
C)
\[Pb{{O}_{2}}\]
done
clear
D)
\[PbS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 96) Which gas is liberated when\[A{{l}_{4}}{{C}_{3}}\]is hydrolysed?
A)
\[C{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 97) When zeolite which is hydrated sodium aluminium silicate is treated with hard water, the sodium ions are exchanged with
A)
\[{{H}^{+}}\]ions
done
clear
B)
\[M{{g}^{2+}}\]ions
done
clear
C)
\[C{{a}^{2+}}\]ions
done
clear
D)
both\[C{{a}^{2+}}\]and\[M{{g}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 98) A codon has a sequence of A and specifies a particular B that is to be incorporated in to C what are A, B, C?
A)
A B C 3 bases Amino acid Carbohydrate
done
clear
B)
A B C 3 acids Carbohydrate Protein
done
clear
C)
A B C 3 bases Protein Amino acid
done
clear
D)
A B C 3 bases Amino acid Protein
done
clear
View Answer play_arrow
question_answer 99) Proteins when heated with cone.\[HN{{O}_{3}}\]give a yellow colour. This is
A)
Hoppes test
done
clear
B)
acid base test
done
clear
C)
Bmrettest
done
clear
D)
xanthoprotic test
done
clear
View Answer play_arrow
question_answer 100) Codon is present in
A)
\[t-RNA\]
done
clear
B)
\[m-RNA\]
done
clear
C)
fats
done
clear
D)
\[r-RNA\]
done
clear
View Answer play_arrow
question_answer 101) Amylopectin is a polymer of
A)
\[\alpha -D\]glucose
done
clear
B)
\[\alpha -D\]fructose
done
clear
C)
lactose
done
clear
D)
amylose
done
clear
View Answer play_arrow
question_answer 102) Which of the following statements is
A)
The repeating monomer units in bakelite are phenol and formaldehyde
done
clear
B)
Low density polythene is an example of branched chain polymers
done
clear
C)
Homopolymer contains a single type of monomer,
done
clear
D)
Vulcanization is the process of heating natural rubber with carbon
done
clear
View Answer play_arrow
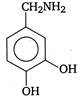
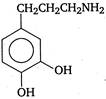
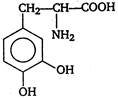
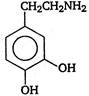
question_answer 103) Parkinsons disease is linked to abnormalities in the levels of dopamine in the body The structure of dopamine is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 104) Which of the following is a natural dye?
A)
Alizarin
done
clear
B)
Malachite green
done
clear
C)
Phenolphthalein
done
clear
D)
Martius yellow
done
clear
View Answer play_arrow
question_answer 105) \[M{{n}^{2+}}\] cab be converted in\[M{{n}^{7+}}\]by reacting
A)
\[Pb{{O}_{2}}\]
done
clear
B)
\[SnC{{l}_{2}}\]
done
clear
C)
\[O{{H}^{-}}\]
done
clear
D)
\[C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 106) Which of the following compounds show optical isomerism?
A)
\[{{[Co{{(CN)}_{6}}]}^{3-}}\]
done
clear
B)
\[{{[Cr{{({{C}_{2}}{{O}_{4}})}_{3}}]}^{3-}}\]
done
clear
C)
\[{{[ZnC{{l}_{4}}]}^{2-}}\]
done
clear
D)
\[{{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 107) Which of the following represents a chelating ligand?
A)
\[{{H}_{2}}O\]
done
clear
B)
\[C{{l}^{-}}\]
done
clear
C)
\[O{{H}^{-}}\]
done
clear
D)
\[DMG\]
done
clear
View Answer play_arrow
question_answer 108) When alkyl halide is heated with dry\[A{{g}_{2}}O,\]it produces
A)
ester
done
clear
B)
ether
done
clear
C)
ketone
done
clear
D)
alcohol
done
clear
View Answer play_arrow
question_answer 109) The best reagent to convert pent-3-en-2-ol into pent-3-en-2-one is
A)
pyridinium chlorochromate
done
clear
B)
chromic anhydride m glacial acetic add
done
clear
C)
acidic dichromate
done
clear
D)
acidic permanganate
done
clear
View Answer play_arrow
question_answer 110) During the process of electrolytic refining of copper, some metals present as impurity settle as anode mud. These are
A)
Fe and Ni
done
clear
B)
Ag and Au
done
clear
C)
Pb and Zn
done
clear
D)
Se and Ag
done
clear
View Answer play_arrow
question_answer 111) Which of the following is not a peroxy acid?
A)
Perphosphoric acid
done
clear
B)
Pemitric acid
done
clear
C)
Perdisulphuric acid
done
clear
D)
Perchloric acid
done
clear
View Answer play_arrow
question_answer 112) Chlorine is liberated when we heat
A)
\[KMn{{O}_{4}}+NaCl\]
done
clear
B)
\[P{{b}_{2}}{{(N{{O}_{3}})}_{4}}+Mn{{O}_{2}}\]
done
clear
C)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}+Mn{{O}_{2}}\]
done
clear
D)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}+HCl\]
done
clear
View Answer play_arrow
question_answer 113) In which case Raoults law is not applicable?
A)
\[1\text{ }M\text{ }NaCl\]
done
clear
B)
1 M urea
done
clear
C)
1M glucose
done
clear
D)
1 M sucrose
done
clear
View Answer play_arrow
question_answer 114) Increasing the temperature of, an aqueous solution will cause
A)
decrease in molarity
done
clear
B)
decrease in molality
done
clear
C)
decrease in mole fraction
done
clear
D)
decrease in % w/w
done
clear
View Answer play_arrow
question_answer 115) To neutralize completely 20 mL of 0.1 M aqueous solution of phosphorus acid\[({{H}_{3}}P{{O}_{3}}),\] the volume of 0.1 M aqueous KOH solution required is
A)
20 mL
done
clear
B)
30 mL
done
clear
C)
40 mL
done
clear
D)
50 mL
done
clear
View Answer play_arrow
question_answer 116) 138 g of ethyl alcohol is mixed with 72 g of water. The ratio of mole fraction of alcohol to water is
A)
\[3:4\]
done
clear
B)
\[1:2\]
done
clear
C)
\[1:4\]
done
clear
D)
\[1:1\]
done
clear
View Answer play_arrow
question_answer 117) \[C{{H}_{3}}COOH\xrightarrow[{}]{LiAl{{H}_{4}}}A+C{{H}_{3}}COOH\]\[\xrightarrow[{}]{{{H}_{3}}{{O}^{+}}}B+{{H}_{2}}O\] In the above reactions A and B respectively are
A)
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}},{{C}_{2}}{{H}_{5}}PH\]
done
clear
B)
\[C{{H}_{3}}CHO,{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}PH,C{{H}_{3}}CHO\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH,C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 118) Acetyl bromide reacts with excess of\[C{{H}_{3}}MgI\] followed by treatment with a saturated solution of\[N{{H}_{4}}Cl\]gives
A)
acetone
done
clear
B)
acetamide
done
clear
C)
2-methyl-2-propanol
done
clear
D)
acetyl iodide
done
clear
View Answer play_arrow
question_answer 119) Reaction of aniline with acetyl chloride in the presence of\[NaOH\]gives
A)
acetanilide
done
clear
B)
\[p-\]chloroaniline
done
clear
C)
red dye
done
clear
D)
aniline hydrochloride
done
clear
View Answer play_arrow
question_answer 120) Which of the following is a biodegradable polymer?
A)
Polythene
done
clear
B)
Bakelite
done
clear
C)
PHBV
done
clear
D)
PVC
done
clear
View Answer play_arrow
question_answer 121) Striped muscles are
A)
syncytial
done
clear
B)
uninucleate
done
clear
C)
binucleate
done
clear
D)
anucleate
done
clear
View Answer play_arrow
question_answer 122) Regarding blood circulation, it may be said that in Pheretima the dorsal vessel is a
A)
collecting vessel in first two segments and distributing vessel in other
done
clear
B)
distributing vessel in the first five segments and collecting vessel in other
done
clear
C)
collecting vessel in thirteen segments and distributing vessel in intestinal region
done
clear
D)
distributing vessel in first thirteen segments and collecting vessel in intestinal region
done
clear
View Answer play_arrow
question_answer 123) Flatworms excrete through
A)
kidney
done
clear
B)
nephridia
done
clear
C)
protonephridia
done
clear
D)
Malpighian tubules
done
clear
View Answer play_arrow
question_answer 124) Which of the following structure is related with vision in rabbits?
A)
Hippocampus
done
clear
B)
Corpus albicans
done
clear
C)
Corpus callosum
done
clear
D)
Corpora quadrigemina
done
clear
View Answer play_arrow
question_answer 125) Which of the following cells are useful for feeding in sponges?
A)
Thesocytes
done
clear
B)
Collar cells
done
clear
C)
Pinacocytes
done
clear
D)
Sclerocytes
done
clear
View Answer play_arrow
question_answer 126) The methods of dispersal in Amoeba is
A)
locomotion
done
clear
B)
sporulation
done
clear
C)
encystment
done
clear
D)
binary fission
done
clear
View Answer play_arrow
question_answer 127) Which of the following factors has little effect on blood flow in arteries?
A)
Heart beat
done
clear
B)
Blood pressure
done
clear
C)
Skeletal muscle contraction
done
clear
D)
Total cross sectional area of vessel
done
clear
View Answer play_arrow
question_answer 128) Telomerase is an enzyme, which is a
A)
repetitive DNA
done
clear
B)
RNA
done
clear
C)
simple protein
done
clear
D)
ribonucleoprotein
done
clear
View Answer play_arrow
question_answer 129) Synapsis occur between
A)
a male and female gamete
done
clear
B)
mRNA and ribosomes
done
clear
C)
spindle fibres and centromere
done
clear
D)
two homologous chromosomes
done
clear
View Answer play_arrow
question_answer 130) What is not true for genetic code?
A)
A codon in mRNA is read in a non-contiguous fashion
done
clear
B)
It is nearly universal
done
clear
C)
It is degenerate
done
clear
D)
It is non-ambiguous
done
clear
View Answer play_arrow
question_answer 131) A sequential expression of set of human gene occurs when steroid molecule binds to the
A)
transfer RNA
done
clear
B)
Messenger RNA
done
clear
C)
DNA sequence
done
clear
D)
ribosome
done
clear
View Answer play_arrow
question_answer 132) Sickle cell anaemia is
A)
an autosomal linked dominant trait
done
clear
B)
caused by substitution of valine by glutamic acid in the\[\beta -\]globin chain of haemoglobin
done
clear
C)
caused by a change in base pair of DNA
done
clear
D)
characterized by elongated sickle like RBCs with a nucleus
done
clear
View Answer play_arrow
question_answer 133) A common test to find the genotype of a hybrid is
A)
crossing of one\[{{F}_{2}}\]progeny with male parent
done
clear
B)
crossing of one\[{{F}_{2}}\]progeny with female parent
done
clear
C)
studying the sexual behaviour of\[{{F}_{1}}\]progenies
done
clear
D)
crossing of one\[{{F}_{1}}\]progeny with recessive parent
done
clear
View Answer play_arrow
question_answer 134) Phenetic classification is based, on
A)
sexual characteristics
done
clear
B)
the ancestral lineage of existing organisms
done
clear
C)
observable characteristics of existing organisms
done
clear
D)
dendogram based on DNA characteristics
done
clear
View Answer play_arrow
question_answer 135) What is true for individuals of same species?
A)
Live m same niche
done
clear
B)
Live in same habitat
done
clear
C)
Interbreeding
done
clear
D)
Live in different habitat
done
clear
View Answer play_arrow
question_answer 136) For retting of jute the fermenting microbe used is
A)
methophillic bacteria
done
clear
B)
butyric acid bacteria
done
clear
C)
Helicobacter pylori
done
clear
D)
Streptococcus lactis
done
clear
View Answer play_arrow
question_answer 137) Which antibiotic inhibits interaction between \[tRNA\]and\[mRNA\]during bacterial protein synthesis?
A)
Neomycin
done
clear
B)
Streptomycin
done
clear
C)
Tetracycline
done
clear
D)
Erythromycin
done
clear
View Answer play_arrow
question_answer 138) Earthworms have no skeleton but during burrowing, the anterior end becomes turgid and acts as a hydraulic skeleton. It is due to
A)
coelomic fluid
done
clear
B)
blood
done
clear
C)
gut peristalsis
done
clear
D)
setae
done
clear
View Answer play_arrow
question_answer 139) Senescence as an active developmental cellular process in the growth and functioning of a flowering plant, is indicated in
A)
leaf abscission
done
clear
B)
annual plants
done
clear
C)
floral parts
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 140) Glycolate induces opening of stomata in
A)
presence of oxygen
done
clear
B)
low\[C{{O}_{2}}\]concentration
done
clear
C)
high\[C{{O}_{2}}\]concentration
done
clear
D)
absence of\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 141) Which one occurs both during cyclic and non-cyclic modes of photophosphorylation?
A)
Involvement of both PS-I and PS-II
done
clear
B)
Formation of ATP
done
clear
C)
Release of\[{{O}_{2}}\]
done
clear
D)
Formation of NADPH
done
clear
View Answer play_arrow
question_answer 142) The translocation of organic solutes in sieve, tube members is supported by
A)
P-proteins
done
clear
B)
mass flow involving a carrier and ATP
done
clear
C)
cytoplasmic streaming
done
clear
D)
root pressure and transpiration pull
done
clear
View Answer play_arrow
question_answer 143) The overall goal of glycolysis, Krebs cycle and the electron transport system is the formation of
A)
ATP in small stepwise units
done
clear
B)
ATP in one large oxidation reaction
done
clear
C)
sugars
done
clear
D)
nucleic acids
done
clear
View Answer play_arrow
question_answer 144) Tendons or ligaments are
A)
connective tissue
done
clear
B)
vascular tissue
done
clear
C)
epithelial tissue
done
clear
D)
skeletal tissue
done
clear
View Answer play_arrow
question_answer 145) Darwins finches represent
A)
morphological variation
done
clear
B)
geographical isolation
done
clear
C)
climatic variation
done
clear
D)
reproductive isolation
done
clear
View Answer play_arrow
question_answer 146) Territoriality occurs as a result of
A)
predation
done
clear
B)
parasitism
done
clear
C)
symbiotism
done
clear
D)
competition
done
clear
View Answer play_arrow
question_answer 147) The type of joint between humerus and radius bone is called
A)
gliding joint
done
clear
B)
saddle joint
done
clear
C)
pivot joint
done
clear
D)
hinge joint
done
clear
View Answer play_arrow
question_answer 148) The nucleus is separated from surrounding cytoplasm by a nuclear membrane, which is
A)
single layered with pores
done
clear
B)
single layered without pores
done
clear
C)
double layered with pores
done
clear
D)
double layered without pores
done
clear
View Answer play_arrow
question_answer 149) In mammals, histamine is secreted by
A)
fibroblasts
done
clear
B)
histiocytes
done
clear
C)
lymphocytes
done
clear
D)
mast cells
done
clear
View Answer play_arrow
question_answer 150) Species is a
A)
group immediately below a phylum
done
clear
B)
closely related interbreeding population
done
clear
C)
taxonomic division of similar genera
done
clear
D)
closely related non-breeding population
done
clear
View Answer play_arrow
question_answer 151) The use of DDT is banned now-a-days because it is
A)
very costly
done
clear
B)
not available
done
clear
C)
inflammable
done
clear
D)
not degraded easily
done
clear
View Answer play_arrow
question_answer 152) Which of the following represents Klinefekers syndrome?
A)
XX
done
clear
B)
XO
done
clear
C)
XY
done
clear
D)
XXY
done
clear
View Answer play_arrow
question_answer 153) In which of the following there is no defect in. the sex chromosome?
A)
Turners syndrome
done
clear
B)
Downs syndrome
done
clear
C)
Colour blindness
done
clear
D)
Klinefelters syndrome
done
clear
View Answer play_arrow
question_answer 154) When a cell is fully turgid, which of the following will be zero?
A)
Wall pressure
done
clear
B)
Osmotic pressure
done
clear
C)
Turgor pressure
done
clear
D)
Water potential
done
clear
View Answer play_arrow
question_answer 155) Embryo development from nucellus and integument is known as
A)
apospory
done
clear
B)
apogamy
done
clear
C)
apomixis
done
clear
D)
adventive embryony
done
clear
View Answer play_arrow
question_answer 156) Acid rain is due to increase in atmospheric concentration of
A)
Ozone
done
clear
B)
\[C{{O}_{2}}\]and\[CO\]
done
clear
C)
\[S{{O}_{3}}\]and\[CO\]
done
clear
D)
\[S{{O}_{2}}\]and nitrogen oxide
done
clear
View Answer play_arrow
question_answer 157) Which of the given is a long day plant?
A)
Glycine max
done
clear
B)
Spinach
done
clear
C)
Chrysanthemum
done
clear
D)
Tobacco
done
clear
View Answer play_arrow
question_answer 158) Life span of Ascaris is
A)
3 to 6 months
done
clear
B)
9 to 12 months
done
clear
C)
1 year
done
clear
D)
2 to 5 months
done
clear
View Answer play_arrow
question_answer 159) Which of the following is false fruit?
A)
Mango
done
clear
B)
Apple
done
clear
C)
Banana
done
clear
D)
Jack fruit
done
clear
View Answer play_arrow
question_answer 160) FAD acts as an\[{{e}^{-}}\]acceptor in between
A)
fumaric and malic acid
done
clear
B)
succinic and fumaric acid
done
clear
C)
malic and oxaloacetic acid
done
clear
D)
citric and isocitric acid
done
clear
View Answer play_arrow
question_answer 161) Pigment iodopsin is contained in
A)
rod cells
done
clear
B)
cone cells
done
clear
C)
anacrine cells
done
clear
D)
horizontal cells
done
clear
View Answer play_arrow
question_answer 162) Sexual dimorphism is absent in
A)
Ascomycetes
done
clear
B)
Deuteromycetes
done
clear
C)
Basidiomycetes
done
clear
D)
Phycomycetes
done
clear
View Answer play_arrow
question_answer 163) Botanical name of peat moss is
A)
Sphagnum
done
clear
B)
Funaria
done
clear
C)
Anthoceros
done
clear
D)
Polytrichum
done
clear
View Answer play_arrow
question_answer 164) The flowers of Oxalis open during the day and close at night. Such type of movement is.
A)
photonasty
done
clear
B)
nyctinasty
done
clear
C)
phototropic
done
clear
D)
seismonastic
done
clear
View Answer play_arrow
question_answer 165) Silent valley is tropical evergreen forest located in
A)
Kerala
done
clear
B)
Kamataka
done
clear
C)
Maharashtra
done
clear
D)
Odisha
done
clear
View Answer play_arrow
question_answer 166) Vitamin-C was first vitamin to be produced by fermentation process using
A)
Penicillium
done
clear
B)
E. coli
done
clear
C)
Yersenia pestis
done
clear
D)
Acetobactor
done
clear
View Answer play_arrow
question_answer 167) The functional unit of DNA molecule that codes for particular gene product is
A)
cistron
done
clear
B)
exon
done
clear
C)
intron
done
clear
D)
gene
done
clear
View Answer play_arrow
question_answer 168) Total number of biodiversity hot spots in the world are
A)
24
done
clear
B)
12
done
clear
C)
34
done
clear
D)
52
done
clear
View Answer play_arrow
question_answer 169) Which of the following is exotic species?
A)
Parthenium
done
clear
B)
Lantana
done
clear
C)
Eichhornia
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 170) In transgenics, expression of transgene in target tissue is determined by
A)
enhancer
done
clear
B)
transgene
done
clear
C)
promotor
done
clear
D)
reporter gene
done
clear
View Answer play_arrow
question_answer 171) When\[C{{O}_{2}}\]concentration in blood increases, breathing becomes
A)
shallower and slow
done
clear
B)
there is no net effect on breathing
done
clear
C)
slow and deep
done
clear
D)
faster and deeper
done
clear
View Answer play_arrow
question_answer 172) Cancer cells can easily be damaged by radiation than normal cells because they are
A)
starved on mutation
done
clear
B)
undergoing rapid division
done
clear
C)
different in structure
done
clear
D)
non-dividing
done
clear
View Answer play_arrow
question_answer 173) Which of the following is not correctly matched?
A)
Glossina palpalis - Sleeping sickness
done
clear
B)
Culex - Filariasis
done
clear
C)
Aedes aegypti - Yellow fever
done
clear
D)
Anopheles culifacies - Leishmaniasis
done
clear
View Answer play_arrow
question_answer 174) A free living nitrogen fixing cyanobacterium which can also form symbiotic association with the aquatic fern Azolla is
A)
Tolypothnx
done
clear
B)
Chlorella
done
clear
C)
Nostoc
done
clear
D)
Anabaena
done
clear
View Answer play_arrow
question_answer 175) Which of the following hormones is not a secretion product of human placenta?
A)
Human choriohic gonadotropin
done
clear
B)
Prolactin
done
clear
C)
Oestrogen
done
clear
D)
Progesterone
done
clear
View Answer play_arrow
question_answer 176) The fangs of python which pulled out can
A)
not come again
done
clear
B)
come again with half the length
done
clear
C)
come again with same length
done
clear
D)
come again with double length
done
clear
View Answer play_arrow
question_answer 177) The wings of bat, locust and pigeon are the examples of
A)
vestigial organs
done
clear
B)
analogous organs
done
clear
C)
homologous organs
done
clear
D)
exoskeletal structures
done
clear
View Answer play_arrow
question_answer 178) Heart lacks sinus venosus in
A)
fishes
done
clear
B)
mammals
done
clear
C)
amphibians
done
clear
D)
echinoderms
done
clear
View Answer play_arrow
question_answer 179) Krebs cycle takes place in
A)
cytoplasm
done
clear
B)
chloroplast
done
clear
C)
nucleus
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 180) In which of the following plants, leaf apex changes into tendril?
A)
Gloriosa
done
clear
B)
smilax
done
clear
C)
Pisum sativum
done
clear
D)
Australian Acacia
done
clear
View Answer play_arrow
question_answer 181) The plants having vascular tissue but lacking seeds are placed under
A)
algae
done
clear
B)
bryophytes
done
clear
C)
pteridophytes
done
clear
D)
gymnosperms
done
clear
View Answer play_arrow
question_answer 182) The rate of transpiration in plants is dependent upon
A)
temperature and soil
done
clear
B)
light and temperature
done
clear
C)
wind, temperature and light
done
clear
D)
light, temperature, atmospheric humidity and wind
done
clear
View Answer play_arrow
question_answer 183) A plant hormone used for inducing morphogenesis in plant tissue culture is
A)
ethylene
done
clear
B)
gibberellin
done
clear
C)
cytokinin
done
clear
D)
abscisic acid
done
clear
View Answer play_arrow
question_answer 184) Which of the following minerals helps in\[{{N}_{2}}\]fixation?
A)
Copper
done
clear
B)
Zinc
done
clear
C)
Manganese
done
clear
D)
Molybdenum
done
clear
View Answer play_arrow
question_answer 185) What is the similarity between fly, mosquitoes and cockroach?
A)
All have 13 chambered heart
done
clear
B)
All have 4 pairs of legs
done
clear
C)
All have closed circulatory system
done
clear
D)
All belong to class-Insecta
done
clear
View Answer play_arrow
question_answer 186) Protein present in cartilage is
A)
chondrin
done
clear
B)
oesein
done
clear
C)
cartilagin
done
clear
D)
ossein
done
clear
View Answer play_arrow
question_answer 187) Insectivorous plants usually survive in
A)
water rich soil
done
clear
B)
\[{{N}_{2}}\]deficient soil
done
clear
C)
\[{{N}_{3}}\]rich soil
done
clear
D)
sugar deficient medium
done
clear
View Answer play_arrow
question_answer 188) Spore of Funaria, on germination gives rise to
A)
protonema
done
clear
B)
embryo
done
clear
C)
antheridia
done
clear
D)
archegonia
done
clear
View Answer play_arrow
question_answer 189) The function of a vessel is
A)
conduction of food
done
clear
B)
conduction of water and minerals
done
clear
C)
conduction of hormones
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 190) Multiplication of DNA is called
A)
translation
done
clear
B)
replication
done
clear
C)
duplication
done
clear
D)
transcription
done
clear
View Answer play_arrow
question_answer 191) The pigment sensitive for red and far red light is
A)
chlorophyll
done
clear
B)
phytochrome
done
clear
C)
cytochrome
done
clear
D)
carotene
done
clear
View Answer play_arrow
question_answer 192) Lemon is sour due to the presence of
A)
acetic acid
done
clear
B)
carbonic acid
done
clear
C)
citric acid
done
clear
D)
sulphuric acid
done
clear
View Answer play_arrow
question_answer 193) The type of vertebrae in the sub order Ophidia is
A)
acoelous
done
clear
B)
procoelous
done
clear
C)
heterocoelous
done
clear
D)
amphicoelous
done
clear
View Answer play_arrow
question_answer 194) Removal of anthers at the time of plant breeding is called
A)
emasculation
done
clear
B)
anthems
done
clear
C)
pollination
done
clear
D)
fertilization
done
clear
View Answer play_arrow
question_answer 195) Haemoglobin is a
A)
protein
done
clear
B)
fibrous protein
done
clear
C)
globular protein
done
clear
D)
enzyme
done
clear
View Answer play_arrow
question_answer 196) LPP-1 is a
A)
bacterium
done
clear
B)
fungus
done
clear
C)
bacteriophage
done
clear
D)
cyanophage
done
clear
View Answer play_arrow
question_answer 197) Hormogonia are vegetative reproductive structures of
A)
Ulothrix
done
clear
B)
Oscillatoria
done
clear
C)
Spirogyra
done
clear
D)
Chara
done
clear
View Answer play_arrow
question_answer 198) Evolutionary history of an organisms is known as
A)
Phylogeny
done
clear
B)
Ancestry
done
clear
C)
Palaeontology
done
clear
D)
Ontogeny
done
clear
View Answer play_arrow
question_answer 199) Sertoli cells, are regulated by the pituitary hormone known as
A)
FSH
done
clear
B)
GH
done
clear
C)
Prolactin
done
clear
D)
LH
done
clear
View Answer play_arrow
question_answer 200) In which of the following fruits, the edible part is the aril?
A)
Apple
done
clear
B)
Pomegranate
done
clear
C)
Orange
done
clear
D)
Litchi
done
clear
View Answer play_arrow
question_answer 201) Last night I dream
A)
Last night I dream
done
clear
B)
/I was a Sheikh on the 169td floor
done
clear
C)
/of Burj Khalifa.
done
clear
D)
/No error,
done
clear
View Answer play_arrow
question_answer 202) As soon as
A)
As soon as
done
clear
B)
/the lion saw the deer
done
clear
C)
/he began to run after it.
done
clear
D)
/No error,
done
clear
View Answer play_arrow
question_answer 203) The police asked us
A)
The police asked us
done
clear
B)
/about our movements
done
clear
C)
/on a night of the crime.
done
clear
D)
/No error,
done
clear
View Answer play_arrow
question_answer 204) Did he tell you
A)
Did he tell you
done
clear
B)
/why he hasnt
done
clear
C)
/come yesterday?
done
clear
D)
/No error,
done
clear
View Answer play_arrow
question_answer 205) It was a pleasant
A)
It was a pleasant
done
clear
B)
/four hours drive
done
clear
C)
/from Pune to Nakhik.
done
clear
D)
/No error,
done
clear
View Answer play_arrow
question_answer 206) He travelled all ...... the world when he was eighty years old.
A)
in
done
clear
B)
over
done
clear
C)
with
done
clear
D)
of
done
clear
View Answer play_arrow
question_answer 207) Dr. Sharma concluded his speech ...... stressing on Buddhas teachings of the importance of charity.
A)
by
done
clear
B)
with
done
clear
C)
at
done
clear
D)
in
done
clear
View Answer play_arrow
question_answer 208) Shivaji Maharaj fought ...... every kind of aggression.
A)
against
done
clear
B)
to
done
clear
C)
with
done
clear
D)
at
done
clear
View Answer play_arrow
question_answer 209) Dont depend ..... others; you must stand on your own feet.
A)
upon
done
clear
B)
on
done
clear
C)
to
done
clear
D)
for
done
clear
View Answer play_arrow
question_answer 210) Our life promises a lot ...... pleasure and we must learn to enjoy it.
A)
with
done
clear
B)
for
done
clear
C)
of
done
clear
D)
at
done
clear
View Answer play_arrow
question_answer 211) Benevolent
A)
beneficial
done
clear
B)
kind
done
clear
C)
helpful
done
clear
D)
supportive
done
clear
View Answer play_arrow
question_answer 212) Ancestors
A)
extinct tribes
done
clear
B)
relatives
done
clear
C)
forefathers
done
clear
D)
old people
done
clear
View Answer play_arrow
question_answer 213) Embrace
A)
impress
done
clear
B)
except
done
clear
C)
embarrass
done
clear
D)
accept
done
clear
View Answer play_arrow
question_answer 214) Meek
A)
light hearted
done
clear
B)
serious
done
clear
C)
submissive
done
clear
D)
begins
done
clear
View Answer play_arrow
question_answer 215) Sufficient
A)
full
done
clear
B)
complete
done
clear
C)
enough
done
clear
D)
less
done
clear
View Answer play_arrow
question_answer 216) Gloomy
A)
radiant
done
clear
B)
fragrant
done
clear
C)
melodious
done
clear
D)
illusory
done
clear
View Answer play_arrow
question_answer 217) Bleasing
A)
dull
done
clear
B)
curse
done
clear
C)
hurt
done
clear
D)
harsh
done
clear
View Answer play_arrow
question_answer 218) Accomplish
A)
fail
done
clear
B)
improper.
done
clear
C)
disagreeable
done
clear
D)
scatter
done
clear
View Answer play_arrow
question_answer 219) Famous
A)
obscure
done
clear
B)
eminent
done
clear
C)
lenient
done
clear
D)
fabulous
done
clear
View Answer play_arrow
question_answer 220) Orderly
A)
unclear
done
clear
B)
valueless
done
clear
C)
chaotic
done
clear
D)
incomplete
done
clear
View Answer play_arrow
question_answer 221) Helena was over head and ears in love with Demetrius.
A)
carefully
done
clear
B)
completely
done
clear
C)
brilliantly
done
clear
D)
cautiously
done
clear
View Answer play_arrow
question_answer 222) Gopi work by fits and starts.
A)
consistently
done
clear
B)
irregularly
done
clear
C)
in high spirits
done
clear
D)
enthusiastically
done
clear
View Answer play_arrow
question_answer 223) Naresh Goyal had to stand on his feet very early in his life.
A)
to be physically strong
done
clear
B)
to be independent
done
clear
C)
to stand erect
done
clear
D)
to be successful
done
clear
View Answer play_arrow
question_answer 224) The possession of Jerusalem is a bone of contention between Israrel and Palestine.
A)
a subject of peace
done
clear
B)
a subject of trade
done
clear
C)
a subject of dispute
done
clear
D)
a subject of exports
done
clear
View Answer play_arrow
question_answer 225) My friend turned a deafear to my tale of loss and refused to help me.
A)
paid no heed
done
clear
B)
went for away
done
clear
C)
listened carefully
done
clear
D)
turned his ear away
done
clear
View Answer play_arrow
question_answer 226) The strong breeze blew his hat away.
A)
The strong air
done
clear
B)
The strong breath
done
clear
C)
The strong wind
done
clear
D)
No improvement
done
clear
View Answer play_arrow
question_answer 227) The Japanese are hardly working people.
A)
a hard working people
done
clear
B)
a hardly working people
done
clear
C)
hard working people
done
clear
D)
No improvement
done
clear
View Answer play_arrow
question_answer 228) The monkey was seated at the foot of a tree.
A)
bottom
done
clear
B)
end
done
clear
C)
root
done
clear
D)
No improvement
done
clear
View Answer play_arrow
question_answer 229) My father lives on Delhi.
A)
in Delhi
done
clear
B)
at Delhi
done
clear
C)
inside Delhi
done
clear
D)
No improvement
done
clear
View Answer play_arrow
question_answer 230) He will come instantaneously.
A)
just now
done
clear
B)
presently
done
clear
C)
instantly
done
clear
D)
No improvement
done
clear
View Answer play_arrow
question_answer 231) Instruments to measure atmospheric pressure
A)
metronome
done
clear
B)
compass
done
clear
C)
pedometer
done
clear
D)
barometer
done
clear
View Answer play_arrow
question_answer 232) One who tends to take a hopeful view of life
A)
magnate
done
clear
B)
creator
done
clear
C)
pacifist
done
clear
D)
optimist
done
clear
View Answer play_arrow
question_answer 233) Belonging to all parts of the world
A)
common
done
clear
B)
universal
done
clear
C)
worldly
done
clear
D)
international
done
clear
View Answer play_arrow
question_answer 234) To be known for bad acts
A)
famous
done
clear
B)
notorious
done
clear
C)
criminal
done
clear
D)
terrorist
done
clear
View Answer play_arrow
question_answer 235) Words of similar meaning
A)
homonyms
done
clear
B)
pseudonyms
done
clear
C)
antonyms
done
clear
D)
synonyms
done
clear
View Answer play_arrow
question_answer 236) In the following questions of four words are given. In each group one word is correctly spelt. Find the correctly spelt word.
A)
envelope
done
clear
B)
envelope
done
clear
C)
envalope
done
clear
D)
envelop
done
clear
View Answer play_arrow
question_answer 237) In the following questions of four words are given. In each group one word is correctly spelt. Find the correctly spelt word.
A)
character
done
clear
B)
charecter
done
clear
C)
character
done
clear
D)
chaerecter
done
clear
View Answer play_arrow
question_answer 238) In the following questions of four words are given. In each group one word is correctly spelt. Find the correctly spelt word.
A)
drunkeness
done
clear
B)
drunkenness
done
clear
C)
dumkness
done
clear
D)
diunkennes
done
clear
View Answer play_arrow
question_answer 239) In the following questions of four words are given. In each group one word is correctly spelt. Find the correctly spelt word.
A)
surprise
done
clear
B)
supprise
done
clear
C)
suprise
done
clear
D)
surprise
done
clear
View Answer play_arrow
question_answer 240) In the following questions of four words are given. In each group one word is correctly spelt. Find the correctly spelt word.
A)
comitee
done
clear
B)
committee
done
clear
C)
committie
done
clear
D)
committee
done
clear
View Answer play_arrow