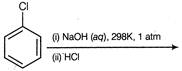
question_answer 1) A body freely falling from the rest has a velocity v after his falls through a height h. The distance, it has to fall down further for its velocity becomes double, is
A)
\[4\text{ }h\]
done
clear
B)
\[2\text{ }s\]
done
clear
C)
\[1/\sqrt{2}s\]
done
clear
D)
\[\sqrt{2}s\]
done
clear
View Answer play_arrow
question_answer 2) A 600 kg rocket is set for vertical firing the exhaust speed is 800 m/s to given an initial upward acceleration of\[20\text{ }m/{{s}^{2}},\]the amount of gas ejected per second to supply. The needed thrust will be
A)
\[137.5kg/s\]
done
clear
B)
\[185.5kg/s\]
done
clear
C)
\[187.5kg/s\]
done
clear
D)
\[127.5kg/s\]
done
clear
View Answer play_arrow
question_answer 3) A running man has half the kinetic energy of that of a boy of half of his mass. The man speeds up by 1 m/s. So as to have same kinetic energy as that of the boy. The original speed of the man is
A)
\[\sqrt{2}m/s\]
done
clear
B)
\[(\sqrt{2}-1)m/s\]
done
clear
C)
\[\frac{1}{\sqrt{2}}m/s\]
done
clear
D)
\[\frac{1}{\sqrt{2}-1}m/s\]
done
clear
View Answer play_arrow
question_answer 4) If a cycle wheel of radius 4m completes one revolution in two seconds. Then acceleration of the cycle is
A)
\[4{{\pi }^{2}}m/{{s}^{2}}\]
done
clear
B)
\[2{{\pi }^{2}}\,m/{{s}^{2}}\]
done
clear
C)
\[{{\pi }^{2}}\,m/{{s}^{2}}\]
done
clear
D)
\[4\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 5) 5g of ice at\[0{}^\circ C\]is mixed with 5 g of steam at \[100{}^\circ C\], what is the final temperature?
A)
\[100{}^\circ C\]
done
clear
B)
\[50{}^\circ C\]
done
clear
C)
\[0{}^\circ C\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 6)
Each capacitor shown in figure is\[2\mu F\]. Then the equivalent capacitance between A and B is
A)
\[2\mu F\]
done
clear
B)
\[4\mu F\]
done
clear
C)
\[6\mu F\]
done
clear
D)
\[8\mu F\]
done
clear
View Answer play_arrow
question_answer 7) What is the refractive index of a prism whose angle\[A=60{}^\circ \]and angle of minimum deviation \[{{d}_{m}}=30{}^\circ \]?
A)
\[\sqrt{2}\]
done
clear
B)
\[{{\sin }^{-1}}(\sqrt{3})\]
done
clear
C)
\[{{\tan }^{-1}}(\sqrt{2})\]
done
clear
D)
\[{{\tan }^{-1}}(\sqrt{3})\]
done
clear
View Answer play_arrow
question_answer 8) A cell of constant emf first connected to a resistance\[{{R}_{1}}\]and then connected to a resistance\[{{R}_{2}}\]. If power delivered in both cases is same, then the internal resistance of the cell is
A)
\[\sqrt{{{R}_{1}}{{R}_{2}}}\]
done
clear
B)
\[\sqrt{\frac{{{R}_{1}}}{{{R}_{2}}}}\]
done
clear
C)
\[\frac{{{R}_{1}}-{{R}_{2}}}{2}\]
done
clear
D)
\[\frac{{{R}_{1}}+{{R}_{2}}}{2}\]
done
clear
View Answer play_arrow
question_answer 9) A gas is compressed at constant pressure 50 \[N/{{m}^{2}}\]from a volume of\[10{{m}^{3}}\]to a volume\[4{{m}^{3}}\]. Energy 100 J is then added to the gas by heating. Its internal energy is
A)
increased by 100 J
done
clear
B)
increased by 200 J
done
clear
C)
decreased by 200 J
done
clear
D)
increased by 400 J
done
clear
View Answer play_arrow
question_answer 10) A gas is compressed adiabatically till its temperature is doubled. The ratio of his final volume to its initial volume will be
A)
between\[\frac{3}{2}\]and 2
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
more than\[\frac{1}{2}\]
done
clear
D)
less than\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 11) Energy is being emitted from the surface of a black body at\[127{}^\circ C\]at the rate of \[(1.0\times {{10}^{6}})/s{{m}^{2}}\]. The temperature of a black body at which the rate of energy emission is \[(16.0\times {{10}^{6}})/s{{m}^{2}}\]will be
A)
\[727{}^\circ C\]
done
clear
B)
\[527{}^\circ C\]
done
clear
C)
\[508{}^\circ C\]
done
clear
D)
\[254{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 12) Two plates of a parallel plate capacitor of capacity\[50\mu F\]are charged by a battery to a potential of 100 V. The battery remains connected and the plates are separated from each other so that the distance between them is doubled. The energy spent by the battery in doing so, will be
A)
\[12.5\times {{10}^{-2}}J\]
done
clear
B)
\[-25\times {{10}^{-2}}J\]
done
clear
C)
\[25\times {{10}^{-2}}J\]
done
clear
D)
\[-12.5\times {{10}^{-1}}J\]
done
clear
View Answer play_arrow
question_answer 13) An electron moves in a circle of radius 1.0 cm with a constant speed of\[4.0\times {{10}^{6}}m/s\], the electric current at a point on the circle will be\[(e=1.6\times {{10}^{-19}}C)\]
A)
\[1\times {{10}^{-11}}\Omega \]
done
clear
B)
\[1.1\times {{10}^{-7}}\Omega \]
done
clear
C)
\[5.1\times {{10}^{-7}}\Omega \]
done
clear
D)
\[2.1\times {{10}^{-7}}\Omega \]
done
clear
View Answer play_arrow
question_answer 14) A light ray is incident normally on a plane mirror. The angle of reflection will be
A)
135
done
clear
B)
90
done
clear
C)
45
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 15) In an artificial satellite, a space traveller tries to fill ink in a pen by dipping it in ink. The amount of ink filled in the pen as compared to the quantity of ink filled on the earths surface will be
A)
less
done
clear
B)
more
done
clear
C)
same
done
clear
D)
nil
done
clear
View Answer play_arrow
question_answer 16) The earth revolves round the sun in one year. If the distance between them becomes doubles, then the new period of the revolution will be
A)
1/2 yr
done
clear
B)
\[2\sqrt{2}yr\]
done
clear
C)
4 yr
done
clear
D)
8 yr
done
clear
View Answer play_arrow
question_answer 17)
A block of mass 2 kg is kept on the floor. The coefficient of static friction is 0.4. If a force of 2.5 N is applied on the block as shown in the figure, the frictional force between the block and the floor will be
A)
2.5 N
done
clear
B)
5 N
done
clear
C)
7.84 N
done
clear
D)
10 N
done
clear
View Answer play_arrow
question_answer 18)
A string of length (L) and uniform cross-section is spread on a smooth plane. One of its ends is pulled by a force F. Find the tension in it at a distance I from this end
A)
\[\frac{1}{2}F\]
done
clear
B)
\[\frac{L}{l}F\]
done
clear
C)
\[\left( 1-\frac{l}{L} \right)F\]
done
clear
D)
\[\left( 1+\frac{l}{L} \right)F\]
done
clear
View Answer play_arrow
question_answer 19) A fighter plane is moving in a vertical circle of radius V. Its minimum velocity at the highest point A of the circle will be
A)
\[\sqrt{3gr}\]
done
clear
B)
\[\sqrt{2gr}\]
done
clear
C)
\[\sqrt{gr}\]
done
clear
D)
\[\sqrt{gr/2}\]
done
clear
View Answer play_arrow
question_answer 20) Pressure inside two soap bubbles are 1.01 atm and 1.03 atm. Ratio between their volumes is
A)
\[27:1\]
done
clear
B)
\[3:1\]
done
clear
C)
\[127:101\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 21) A stationary object at\[4{}^\circ C\]and weighing 3.5 kg falls from a height 2000 m on a snow mountain at \[0{}^\circ C\]. If the temperature of the object just before hitting the snow is \[0{}^\circ C\] and the object before comes to rest immediately\[(g=10\text{ }m/{{s}^{2}})\]and latent heat of ice \[=3.5\times {{10}^{5}}\]J/s), then the object
A)
2kg of ice
done
clear
B)
200 g of ice
done
clear
C)
20g ice
done
clear
D)
2g ice
done
clear
View Answer play_arrow
question_answer 22) A sphere at temperature 600 K is placed in environment of temperature 200 K, its cooling rate is H. If the temperature is reduced to 400 K, the cooloing is same environment will be
A)
\[\frac{H}{16}\]
done
clear
B)
\[\left( \frac{9}{27} \right)H\]
done
clear
C)
\[\left( \frac{16}{3} \right)H\]
done
clear
D)
\[\left( \frac{3}{16} \right)H\]
done
clear
View Answer play_arrow
question_answer 23) The acceleration due to gravity on the planet A is 9 times the acceleration due to gravity on the planet B. A man jumps to a height of 2m on the surface of A. What is the height of jump by the same person on the planet B?
A)
6m
done
clear
B)
2/9 m
done
clear
C)
2/3m
done
clear
D)
18m
done
clear
View Answer play_arrow
question_answer 24) When a long spring is stretched by 2 cm, its potential energy is U. If the spring is stretched by 10 cm, the potential energy in it will be
A)
\[2U\]
done
clear
B)
\[25U\]
done
clear
C)
\[U/5\]
done
clear
D)
\[5U\]
done
clear
View Answer play_arrow
question_answer 25) An observer moves towards a stationary source of sound with a speed\[1/{{5}^{th}}\]of the speed of sound. The wavelength and frequency of the source emitted are\[\lambda \]and\[f\]respectively. The apparent frequency and wavelength recorded by the observer are respectively
A)
\[f,1.2\lambda \]
done
clear
B)
\[0.8f,0.8\lambda \]
done
clear
C)
\[1.2f,1.2\lambda \]
done
clear
D)
\[1,f2\,\lambda \]
done
clear
View Answer play_arrow
question_answer 26)
An equiconvex lens is cut into two halves a long (i) XOX and (ii) YOY as shown in the figure let \[f.f,f\]be the focal lengths of the complete lens of each half in case (i) and of each half in case (ii) respectively.
A)
\[f=f.f=f\]
done
clear
B)
\[f=2f,f=2f\]
done
clear
C)
\[f=f,f=2f\]
done
clear
D)
\[f=2f,f=f\]
done
clear
View Answer play_arrow
question_answer 27) A diamagnetic material in a magnetic field moves
A)
perpendicular to the field
done
clear
B)
from weaker to the stronger parts of the field
done
clear
C)
from stronger to the weaker parts of the field
done
clear
D)
in none of the above directions
done
clear
View Answer play_arrow
question_answer 28) A coil in the shape of an equilateral triangles of side\[l\]is suspended between the pole pieces of a permanent magnet such that B is in plane of the coil. If due to a current\[i\]in the triangle, a torque\[\tau \]acts on it, the side\[l\]of the triangle is
A)
\[\frac{2}{\sqrt{3}}{{\left( \frac{\tau }{Bi} \right)}^{1/2}}\]
done
clear
B)
\[\frac{2}{3}\left( \frac{\tau }{Bi} \right)\]
done
clear
C)
\[{{\left( \frac{\tau }{\sqrt{3}Bi} \right)}^{1/2}}\]
done
clear
D)
\[\frac{1}{\sqrt{3}}\frac{\tau }{Bi}\]
done
clear
View Answer play_arrow
question_answer 29) A certain electrical conductor has a square cross-section, 2.0 mm on a side and is 12 m long. The resistance between its ends is\[0.072\,\Omega \]. The resistivity of its material is equal to
A)
\[2.4\times {{10}^{-6}}\Omega m\]
done
clear
B)
\[1.2\times {{10}^{-6}}\Omega m\]
done
clear
C)
\[1.2\times {{10}^{-8}}\Omega m\]
done
clear
D)
\[2.4\times {{10}^{-8}}\Omega m\]
done
clear
View Answer play_arrow
question_answer 30)
Figure shows three points A, B and C in a region of uniform electric field E. The line AB is perpendicular and BC is parallel to the field lines. Then which of the following holds good?
A)
\[{{V}_{A}}={{V}_{B}}={{V}_{C}}\]
done
clear
B)
\[{{V}_{A}}={{V}_{B}}>{{V}_{C}}\]
done
clear
C)
\[{{V}_{A}}={{V}_{B}}<{{V}_{C}}\]
done
clear
D)
\[{{V}_{A}}>{{V}_{B}}={{V}_{C}}\]
done
clear
View Answer play_arrow
question_answer 31) The\[(x,\text{ }y,\text{ }z)\]co-ordinates of two points A and B are given respectively as\[(0,3,-1)\]and\[(-2,6,4)\]. The displacement vector from A to B may be given by
A)
\[-2i+6j+4k\]
done
clear
B)
\[-2i+3j+3k\]
done
clear
C)
\[-2i+3j+5k\]
done
clear
D)
\[2i-3j-5k\]
done
clear
View Answer play_arrow
question_answer 32) In the first second of its flight, rocket ejects 1/60 of its mass with a velocity of 2400 m/s. The acceleration of the rocket is
A)
\[19.6\text{ }m/{{s}^{2}}\]
done
clear
B)
\[30.2\text{ }m/{{s}^{2}}\]
done
clear
C)
\[40\text{ }m/{{s}^{2}}\]
done
clear
D)
\[49.8\text{ }m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 33) When temperature of an ideal gas is increased from\[27{}^\circ C\]to\[277{}^\circ C,\]its rms speed is changed rom 400 m/s to\[{{\upsilon }_{s}}\].The Vg is
A)
516m/s
done
clear
B)
450 m/s
done
clear
C)
310 m/s
done
clear
D)
746 m/s
done
clear
View Answer play_arrow
question_answer 34) A bar magnet of magnetic moment M is placed in the magnetic field B. The torque acting on the magnet is.
A)
\[M\times B\]
done
clear
B)
\[M-B\]
done
clear
C)
\[\frac{1}{2}M\times B\]
done
clear
D)
\[M+B\]
done
clear
View Answer play_arrow
question_answer 35) For the production of characteristic\[kr-x-\]ray the electron transition is
A)
\[n=4\]to \[n=1\]
done
clear
B)
\[n=3\]to\[n=1\]
done
clear
C)
\[n=3\]to \[n=2\]
done
clear
D)
\[n=2\]to\[n=1\]
done
clear
View Answer play_arrow
question_answer 36) The current passing through an inductor coil of 5H is decreasing at the rate of 2A/s, the emf developed across the coil is
A)
\[+10.0V\]
done
clear
B)
\[-10.0V\]
done
clear
C)
\[+2.5V\]
done
clear
D)
\[-2.5V\]
done
clear
View Answer play_arrow
question_answer 37) A capacitor of\[10\text{ }\mu F\]charged upto 250 V is connected in parallel with another capacitor of 5aF charged upto 100 V. The common potential is
A)
200 V
done
clear
B)
300 V
done
clear
C)
400 V
done
clear
D)
500 V
done
clear
View Answer play_arrow
question_answer 38) A body of mass m slides down on a rough plane inclination a. If\[\mu \]is the coefficient of friction, the acceleration of the body will be
A)
\[g(\cos \alpha -\mu \sin \alpha )\]
done
clear
B)
\[g(\sin \alpha -\mu cos\alpha )\]
done
clear
C)
\[\mu cos\alpha \]
done
clear
D)
\[g\sin \alpha \]
done
clear
View Answer play_arrow
question_answer 39) Half-life of radium is 1600 yr. If the initial mass is 1 kg, what is the amount of radium left after 4800 yr?
A)
Zero
done
clear
B)
0.125 kg
done
clear
C)
0.5 kg
done
clear
D)
0.25 kg
done
clear
View Answer play_arrow
question_answer 40) Light of wavelength K = 4000 A and intensity \[100\text{ }W/{{m}^{2}}\]is incident On a plate of threshold frequency\[5.5\times {{10}^{14}}\]Hz. Find the number of photons incident \[{{m}^{2}}\]per sec.
A)
\[{{10}^{21}}\]
done
clear
B)
\[3.0\times {{10}^{19}}\]
done
clear
C)
\[2.02\times {{10}^{20}}\]
done
clear
D)
\[2.02\times {{10}^{21}}\]
done
clear
View Answer play_arrow
question_answer 41) When electron is accelerated between 500 keV, what is the percentage increase in mass?
A)
82.35%
done
clear
B)
97.85%
done
clear
C)
42.35%
done
clear
D)
59.45%
done
clear
View Answer play_arrow
question_answer 42) A hypothetical experiment conducted to determine Youngs formula\[Y=\frac{\cos \theta {{T}^{x}}.\tau }{{{l}^{3}}}\]. If Y = Youngs modulus, T = time period, t = Torque and\[l=\]length, then find the value of \[x\].
A)
zero
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 43) The speed of sound in hydrogen at NTP is 1270 m/s. Then, the speed in a mixture of hydrogen and oxygen in the ratio 4:1 by volume will be
A)
317 m/s
done
clear
B)
635 m/s
done
clear
C)
830 m/s
done
clear
D)
950 m/s
done
clear
View Answer play_arrow
question_answer 44) The displacement of a particle executing periodic motion is given by \[y=4{{\cos }^{2}}\left( \frac{t}{2} \right)\sin 1000\,\omega t.\] This expression may be considered to be a result of superposition of
A)
two waves
done
clear
B)
three waves
done
clear
C)
four waves
done
clear
D)
five waves
done
clear
View Answer play_arrow
question_answer 45) Assuming that about 20 MeV of energy is released per fusion reaction, \[_{1}{{H}^{2}}{{+}_{1}}{{H}^{3}}{{\xrightarrow[{}]{{}}}_{0}}{{n}^{1}}{{+}_{2}}H{{e}^{4}}\] then the mass of\[_{1}{{H}^{1}}\]consumed per day in a fusion reactor of power 1 MW will approximately be
A)
0.001 g
done
clear
B)
0.1 g
done
clear
C)
10.0g
done
clear
D)
100g
done
clear
View Answer play_arrow
question_answer 46) The work function of a substance is 4.0 eV. The longest wavelength of light that can cause photoelectron emission from this substance is approximately
A)
540nm
done
clear
B)
400 nm
done
clear
C)
310nm
done
clear
D)
220 nm
done
clear
View Answer play_arrow
question_answer 47) When light wave suffers reflection at the interference between air and glass, the change of phase of reflected wave is equal to
A)
zero
done
clear
B)
\[n/2\]
done
clear
C)
\[n\]
done
clear
D)
\[2n\]
done
clear
View Answer play_arrow
question_answer 48) A convex lens of focal length 1.0 m and a concave lens of focal length 0.25 m are 0.75 m apart. A parallel beam of light is incident in the convex lens. The beam emerging after refraction from both lenses is
A)
parallel to principal axis
done
clear
B)
convergence
done
clear
C)
divergence
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 49) Two instruments having stretched strikes are being played in unison. When the tension in one of the instrument is increased by 1%, 3 beats, are produced in 2s. The initial frequency of vibration of each wire is
A)
600 Hz
done
clear
B)
300 Hz
done
clear
C)
200 Hz
done
clear
D)
150 Hz
done
clear
View Answer play_arrow
question_answer 50) Find the inductance L of a solenoid of length\[l\] whose windings are made of material of density D and resistivity\[\rho \]. The winding resistance is R
A)
\[\frac{{{\mu }_{0}}}{4\pi l}.\frac{{{R}_{m}}}{\rho D}\]
done
clear
B)
\[\frac{{{\mu }_{0}}}{4\pi R}.\frac{{{l}_{m}}}{\rho D}\]
done
clear
C)
\[\frac{{{\mu }_{0}}}{4\pi l}.\frac{{{R}^{2}}m}{\rho D}\]
done
clear
D)
\[\frac{{{\mu }_{0}}}{2\pi R}.\frac{{{l}_{m}}}{\rho D}\]
done
clear
View Answer play_arrow
question_answer 51) A long straight wire carrying a current of 30 A is placed in an external uniform magnetic field. of induction\[4\times {{10}^{-4}}\]T. The magnetic field is acting parallel to the direction of current. The magnitude of the resultant magnetic induction in tesia at a point 2.0 cm away from the wire is \[({{\mu }_{0}}=4\pi \times {{10}^{-7}}H/m)\]
A)
\[{{10}^{-4}}\]
done
clear
B)
\[3\times {{10}^{-4}}\]
done
clear
C)
\[5\times {{10}^{-4}}\]
done
clear
D)
\[6\times {{10}^{-4}}\]
done
clear
View Answer play_arrow
question_answer 52) A block of mass 10 kg is moving in\[x-\]direction with a constant speed of 10 m/s. It is subjected to a retarding force\[F=-0.1\text{ }x\text{ }J/m\]during its travel from\[x=20m\]to\[x=30m\]. Its final kinetic energy will be
A)
475 J
done
clear
B)
450 J
done
clear
C)
275 J
done
clear
D)
250 J
done
clear
View Answer play_arrow
question_answer 53) What is the effect of increasing the intensity of light that falls on the emitter in a photoelectric effect apparatus?
A)
Cut-off frequency decrease
done
clear
B)
Stopping potential decrease
done
clear
C)
Time delay for emission of photoetectron decrease
done
clear
D)
Saturation photocurrent increases
done
clear
View Answer play_arrow
question_answer 54) A body falls from rest. In the last second of its fall it covers half of the total distance. If g is \[9.8\text{ }m/{{s}^{2}},\]then the total time of its falls is (in second)
A)
2
done
clear
B)
\[2+\sqrt{2}\]
done
clear
C)
\[4-\sqrt{2}\]
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 55) If\[|A\times B|=\sqrt{3}A.B,\]then the value of\[|A+B|\]is
A)
\[{{({{A}^{2}}+{{B}^{2}}+AB)}^{1/2}}\]
done
clear
B)
\[{{\left( {{A}^{2}}+{{B}^{2}}+\frac{AB}{\sqrt{3}} \right)}^{1/2}}\]
done
clear
C)
\[A+B\]
done
clear
D)
\[{{({{A}^{2}}+{{B}^{2}}+\sqrt{3}AB)}^{1/2}}\]
done
clear
View Answer play_arrow
question_answer 56) The distance between the two successive nodes is
A)
\[\frac{\lambda }{4}\]
done
clear
B)
\[\frac{\lambda }{2}\]
done
clear
C)
\[\lambda \]
done
clear
D)
\[2\lambda \]
done
clear
View Answer play_arrow
question_answer 57) Dopplers effect in sound takes place when source and observer are
A)
stationary
done
clear
B)
moving with same velocity
done
clear
C)
in relative motion
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 58) The current and voltage in AC circuit are given by \[I=5\sin \left( 100t-\frac{\pi }{2} \right)A\]and \[V=200\sin (100t)\]volt. The power dissipated in the circuit will be
A)
20 W
done
clear
B)
40 W
done
clear
C)
1000 W
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 59) Copper has facecentered cubic (fee) lattice with interatomic spacing equal to\[2.54\text{ }\overset{o}{\mathop{\text{A}}}\,\], the value of lattice constant for this lattice is
A)
\[1.27\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[5.08\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[2.54\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[3.57\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 60) The wavelength of a radiowave of frequency 1 MHz is
A)
400 m
done
clear
B)
300 m
done
clear
C)
350 m
done
clear
D)
200 m
done
clear
View Answer play_arrow
question_answer 61) Which of the following compounds can exist in enantiomeric (i.e., D and L) forms?
A)
3-methyl butanoic acid
done
clear
B)
cis-2-butene
done
clear
C)
isopropyi amine
done
clear
D)
1-methyl butanamine
done
clear
View Answer play_arrow
question_answer 62) Among the following molecules or ions, which has the longest\[C-N\]bond?
A)
\[{{H}_{2}}CNC{{H}_{3}}\]
done
clear
B)
\[{{H}_{3}}CCN\]
done
clear
C)
\[{{({{H}_{3}}C)}_{4}}{{N}^{+}}\]
done
clear
D)
\[{{({{H}_{2}}C)}_{2}}{{N}^{+}}\]
done
clear
View Answer play_arrow
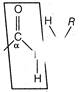
question_answer 63)
Which statement best explains why A is more acidic than B?
A)
The larger size of ketone group helps to stabilize the conjugate base
done
clear
B)
The ketone group exert a large inductive effect in conjugate base of A
done
clear
C)
The ketone group allows for resonance delocalisation of the charge in conjugate base
done
clear
D)
The OH oxygen in A is more electronegative than the OH oxygen in B
done
clear
View Answer play_arrow
question_answer 64) Choose the reagent to carry out the reaction. \[C{{H}_{3}}C\equiv CC{{H}_{3}}\xrightarrow[{}]{{}}\underset{cis\text{ }form}{\mathop{C{{H}_{3}}CH=CHC{{H}_{3}}}}\,\]
A)
\[{{H}_{2}}+\]Lindlar reagent
done
clear
B)
\[Li+N{{H}_{3}}\]
done
clear
C)
\[Conc.\,{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[{{H}_{2}}O+{{H}^{+}}\]
done
clear
View Answer play_arrow
question_answer 65) Which of the following would not form upon electrolysis of aqueous solution of potassium propanoate?
A)
Butane
done
clear
B)
Ethyl ethanoate
done
clear
C)
Ethyl propanoate
done
clear
D)
Ethene
done
clear
View Answer play_arrow
question_answer 66) \[Ca{{(HC{{O}_{3}})}_{2}}(s)\]decomposes as \[Ca{{(HC{{O}_{3}})}_{2}}(s)\xrightarrow[{}]{{}}CaC{{O}_{3}}(s)+{{H}_{2}}O(g)\] \[+C{{O}_{2}}(g)\]Total pressure at equilibrium is found to be 0.12 bar. Thus,\[{{K}_{p}}\]is
A)
0.24
done
clear
B)
0.06
done
clear
C)
0.0036
done
clear
D)
0.0144
done
clear
View Answer play_arrow
question_answer 67) Super cooled water is liquid water that has been cooled below its normal freezing point.This state is thermodynamically
A)
unstable and tends to freeze into ice spontaneously
done
clear
B)
stable and tends to freeze into ice spontaneously
done
clear
C)
stable and tends to fuse into liquid spontaneously
done
clear
D)
unstable and tends to fuse into liquid spontaneously
done
clear
View Answer play_arrow
question_answer 68)
For the reactions, I. \[C{{H}_{4}}(g)\xrightarrow[{}]{{}}C(g)+4H(g);\] \[\Delta H={{x}_{1}}\] II. \[{{C}_{2}}{{H}_{6}}(g)\xrightarrow[{}]{{}}2C(g)+6H(g);\] \[\Delta H={{x}_{2}}\] from I and II, bond energy of\[C-C\]bond is
A)
\[{{x}_{1}}-{{x}_{2}}\]
done
clear
B)
\[{{x}_{2}}-{{x}_{1}}\]
done
clear
C)
\[{{x}_{2}}+1.5{{x}_{1}}\]
done
clear
D)
\[{{x}_{2}}-1.5{{x}_{1}}\]
done
clear
View Answer play_arrow
question_answer 69) The heat of formation of\[C{{O}_{2}}\]is\[-95\text{ }kcal\]. The amount of carbon which on burning will evolve 1000 kcal is
A)
12.63 g
done
clear
B)
17.95 g
done
clear
C)
126.3 g
done
clear
D)
179.5 g
done
clear
View Answer play_arrow
question_answer 70) % ionisatipn of a weak acid is 1% at 1M, hence % ionisation at 4M will be
A)
0.2%
done
clear
B)
0.5%
done
clear
C)
4%
done
clear
D)
5%
done
clear
View Answer play_arrow
question_answer 71)
A)
0.5
done
clear
B)
1.0
done
clear
C)
0.8
done
clear
D)
2.0
done
clear
View Answer play_arrow
question_answer 72) When the following five anions are arranged in order of decreasing ionic radius, the correct sequence is
A)
\[S{{e}^{2-}},{{I}^{-}},B{{r}^{-}},{{O}^{2-}},{{F}^{-}}\]
done
clear
B)
\[{{I}^{-}},S{{e}^{2-}},B{{r}^{-}},{{O}^{2-}},{{F}^{-}}\]
done
clear
C)
\[{{I}^{-}},S{{e}^{2-}},{{O}^{2-}},B{{r}^{-}},{{F}^{-}}\]
done
clear
D)
\[S{{e}^{2-}},{{I}^{-}},B{{r}^{-}},{{F}^{-}},{{O}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 73) Which one of the following statement is incorrect?
A)
\[T{{i}^{3+}}\]salts are better oxidising agents
done
clear
B)
\[G{{a}^{+}}\]salts are better reducing agents
done
clear
C)
\[P{{b}^{4+}}\]salts are better oxidising agents
done
clear
D)
\[A{{s}^{5+}}\]salts are better oxidising agents
done
clear
View Answer play_arrow
question_answer 74) Hydrogen is produced by the reaction
A)
\[N{{a}_{2}}{{O}_{2}}+2HCl\]
done
clear
B)
\[Mg+{{H}_{2}}O\]
done
clear
C)
\[Ba{{O}_{2}}+HCl\]
done
clear
D)
\[{{H}_{2}}{{S}_{4}}{{O}_{8}}+{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 75) There is loss in weight when mixture of\[L{{i}_{2}}C{{O}_{3}}\]and\[N{{a}_{2}}C{{O}_{3}}.10{{H}_{2}}O\]is heated strongly. This loss is due to
A)
\[L{{i}_{2}}C{{O}_{3}}\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}.10{{H}_{2}}O\]
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 76) The absorption of UV radiation by\[{{O}_{3}}\]
A)
protects the inhabitants of our planet from injurious radiation
done
clear
B)
maintains an equilibrium between the concentrations of\[{{O}_{2}}\]and\[{{O}_{3}}\]
done
clear
C)
makes both the function effective
done
clear
D)
makes no function effective
done
clear
View Answer play_arrow
question_answer 77) Pyrolusite in\[Mn{{O}_{2}}\]is used to prepare\[KMn{{O}_{4}}\]. Steps are \[Mn{{O}_{2}}\xrightarrow[{}]{I}MnO_{4}^{2-}\xrightarrow[{}]{II}MnO_{4}^{-}\] I and II are
A)
fuse with KOH/air, electrolytic oxidation
done
clear
B)
fuse with KOH/air, electrolytic reduction
done
clear
C)
fuse with cone HN03/air, electrolytic reduction
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 78) The ability of d-block elements to form complexes is due to
A)
small and highly charged ions
done
clear
B)
vacant low energy orbitals to accept lone pair of electrons from ligands
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 79) Which one of the following species has maximum conductance in their aqueous solutions?
A)
\[{{K}_{2}}PtC{{l}_{6}}\]
done
clear
B)
\[PtC{{l}_{4}}.2N{{H}_{3}}\]
done
clear
C)
\[PtC{{l}_{4}}.3N{{H}_{3}}\]
done
clear
D)
\[PtC{{l}_{4}}.5N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 80) The coordination of Pt in the complex ion \[{{[Pt{{(en)}_{2}}C{{l}_{2}}]}^{2+}}\]is
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 81) The ratio between the root mean square velocity of\[{{H}_{2}}\]at 50K and that of\[{{O}_{2}}\]at 800 K is
A)
0.25
done
clear
B)
1
done
clear
C)
2
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 82)
Consider the following statements I. Repulsive forces are significant when the molecules are close together on average. II. Attractive intermolecular forces are important when the molecules are fairly close together but not necessarily touching. III. Attractive forces are ineffective when the molecules are for apart.
Select correct statements.
A)
I, II and III
done
clear
B)
I and III
done
clear
C)
II and III
done
clear
D)
I and II
done
clear
View Answer play_arrow
question_answer 83) Energy equivalent to\[10.00\text{ }c{{m}^{-1}}\]is
A)
\[2.0\times {{10}^{-22}}J\] peratom
done
clear
B)
\[28.6\times {{10}^{-3}}kcal\text{ }mo{{l}^{-1}}\]photon
done
clear
C)
\[12.0\times {{10}^{-2}}kJ\text{ }mo{{l}^{-1}}\]photon
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 84) The radius of hydrogen atom in the ground state is\[0.53\overset{o}{\mathop{\text{A}}}\,\]. The radius of\[L{{i}^{2+}}\]ion (atomic number = 3) in a similar state is
A)
\[0.17\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.265\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[0.53\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[1.06\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 85) How many mL of\[0.125\text{ }M\text{ }C{{r}^{3+}}\]must be reacted with 12.0 mL of\[0.200\text{ }M\text{ }MnO_{4}^{-}\] if the redox products are\[C{{r}_{2}}O_{7}^{2-}\]and\[M{{n}^{2+}}\]?
A)
32 mL
done
clear
B)
24 mL
done
clear
C)
16 mL
done
clear
D)
8 mL
done
clear
View Answer play_arrow
question_answer 86) \[10\,g\]of hydrogen and 64 g of oxygen were filled in a steel vessel and exploded. Amount of water produced in this reaction will be
A)
3 mol
done
clear
B)
4 mol
done
clear
C)
1 mol
done
clear
D)
2 mol
done
clear
View Answer play_arrow
question_answer 87) A group of 13 element if added in small amounts to Ge, then the type of semiconductor formed is
A)
n-type semiconductor
done
clear
B)
p-type semiconductor
done
clear
C)
super semiconductor
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 88) The density of solid argon is 1.65 g per cc at\[-233{}^\circ C\]. If the argon atom is assumed to be a sphere of radius\[1.54\times {{10}^{-8}}cm,\]the percentage of empty space in solid argon is
A)
32%
done
clear
B)
54%
done
clear
C)
68%
done
clear
D)
62%
done
clear
View Answer play_arrow
question_answer 89) What is not true regarding free radical polymerisation of propene?
A)
Without proper control, atactic polypropylene is formed
done
clear
B)
Use of Ziegler-Natta catalyst results in isotactic polypropylene
done
clear
C)
During polymerisation, a linear unbranched. crystalline polymer is usually obtained
done
clear
D)
During polymerisation, a secondary free radical Is produced in every step
done
clear
View Answer play_arrow
question_answer 90) The incorrect statement regarding cellulose is
A)
it is a polymer of D-glucose
done
clear
B)
it has\[\beta -1,\]4-glycosidic linkage
done
clear
C)
it is used for making rayon fibre
done
clear
D)
it can be obtained by polymerisation of D-glucose
done
clear
View Answer play_arrow
question_answer 91) A glycoside is the carbohydrate form of an
A)
ether
done
clear
B)
acetal
done
clear
C)
aglycone
done
clear
D)
alcohol
done
clear
View Answer play_arrow
question_answer 92) When a carbohydrate reacts with\[NaB{{H}_{4}},\]the product is an
A)
alditol
done
clear
B)
aldaric acid
done
clear
C)
aldonic acid
done
clear
D)
aglycone
done
clear
View Answer play_arrow
question_answer 93)
In the figure below, the plane drawn behind the peptide bond indicates the
A)
absence of rotation around\[C-N\]bond because of its partial double bond character
done
clear
B)
plane of rotation around the\[C-N\]bond
done
clear
C)
region of steric hindrance determined by the large\[C=O\]group
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 94) Which statement about aspirin is not true?
A)
Aspirin belongs to narcotic analgesics
done
clear
B)
It is effective in relieving pain
done
clear
C)
It has antiblood clotting action
done
clear
D)
It is a neurologically active drug
done
clear
View Answer play_arrow
question_answer 95) The correct decreasing order of basic strength of the following species is
A)
\[O{{H}^{-}}>NH_{2}^{-}>{{H}_{2}}O>N{{H}_{3}}\]
done
clear
B)
\[NH_{2}^{-}>O{{H}^{-}}>N{{H}_{3}}>{{H}_{2}}O\]
done
clear
C)
\[N{{H}_{3}}>{{H}_{2}}O>NH_{2}^{-}>O{{H}^{-}}\]
done
clear
D)
\[{{H}_{2}}O>N{{H}_{3}}>O{{H}^{-}}>NH_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 96) \[C{{H}_{3}}CHO\xrightarrow[{}]{dil.\,NaOH}A\xrightarrow[{}]{\Delta ,\,dehydration}B\] \[\xrightarrow[{}]{Ni/{{H}_{2}}}C\xrightarrow[{}]{Ni/{{H}_{2}}}D\] In the above sequence of reactions, compound D is
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CHO\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{CH}}\,CHO\]
done
clear
View Answer play_arrow
question_answer 97) Which one of the following test is used to distinguish between benzoic acid and ethyl benzoate?
A)
\[NaHC{{O}_{3}}\]test
done
clear
B)
lodoform test
done
clear
C)
\[FeC{{l}_{3}}\]test
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 98)
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic shbstitution.
A)
\[I<II<III\]
done
clear
B)
\[II<I<III\]
done
clear
C)
\[III<II<I\]
done
clear
D)
\[I<III<II\]
done
clear
View Answer play_arrow
question_answer 99) Which one of the following reagents can not be used to oxidise primary alcohols to aldehydes?
A)
\[Cr{{O}_{3}}\]in anhydrous medium
done
clear
B)
\[KMn{{O}_{4}}\]in acidic medium
done
clear
C)
Pyridinium chlorochromate
done
clear
D)
Heat in presence of Cu at 573K
done
clear
View Answer play_arrow
question_answer 100) Which of the following reactions will not yield phenol?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 101) In qualitative analysis, when\[{{H}_{2}}S\]is passed through an aqueous solution of salt acidified with dil.\[HCl,\]a black precipitate is obtained. On boiling the precipitate with dil\[HN{{O}_{3}},\]it forms a solution of blue colour. Addition of excess of aqueous solution of ammonia to this solution gives
A)
deep blue precipitate of\[Cu{{(OH)}_{2}}\]
done
clear
B)
deep blue solution of \[{{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}}\]
done
clear
C)
deep blue solution of \[Cu{{(N{{O}_{3}})}_{2}}\]
done
clear
D)
deep blue solution of \[Cu{{(OH)}_{2}}.Cu{{(N{{O}_{3}})}_{2}}\]
done
clear
View Answer play_arrow
question_answer 102) Which of the following acids forms three series of salts?
A)
\[{{H}_{3}}P{{O}_{2}}\]
done
clear
B)
\[{{H}_{3}}B{{O}_{3}}\]
done
clear
C)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
D)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 103) Which one of the following statements is incorrect?
A)
All halogens have weaker\[X-X\]bond than \[X-X\]bond in interhalogens (except\[F-F\]bond in fluorine)
done
clear
B)
Radius ratio between iodine and fluorine (among halogens) is maximum
done
clear
C)
Interhalogen compounds are more reactive than halogen compounds
done
clear
D)
Among interhalogen compounds, maximum number of atoms are present in iodine fluoride
done
clear
View Answer play_arrow
question_answer 104) Stability of lyophilic colloids is due to
A)
same charge on all the colloidal particles
done
clear
B)
solvation of the colloidal particles
done
clear
C)
the fact that they are organic substances
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 105) Hardening of leather in tanning industry is based on
A)
electrophoresis
done
clear
B)
electro-osmosis
done
clear
C)
mutual coagulation
done
clear
D)
persistent dialysis
done
clear
View Answer play_arrow
question_answer 106) Oxidation states of the metal in the minerals haematite and magnetite, respectively are
A)
III in haematite and II and III in magnetite
done
clear
B)
II and III in haematite and II in magnetite
done
clear
C)
II and III in haematite and III in magnetite
done
clear
D)
II in haematite and III in magnetite
done
clear
View Answer play_arrow
question_answer 107) Bauxite ore is treated with cone.\[NaOH\] solution at 500 K and 35 bar pressure for few hours and filtered hot. In the fittrate, the species present are,
A)
\[NaAl{{(OH)}_{4}}\]and\[N{{a}_{2}}Si{{O}_{3}}\]
done
clear
B)
\[NaAl{{(OH)}_{4}}\]only
done
clear
C)
\[N{{a}_{2}}Si{{O}_{3}}\]and\[N{{a}_{2}}Ti{{(OH)}_{6}}\]
done
clear
D)
\[N{{a}_{2}}Si{{O}_{3}}\]only
done
clear
View Answer play_arrow
question_answer 108) The unit of rate constant for zero order reaction is
A)
\[{{s}^{-1}}\]
done
clear
B)
\[mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
C)
\[L\,mo{{l}^{-1}}{{s}^{-1}}\]
done
clear
D)
\[{{L}^{2}}\,mo{{l}^{-2}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 109) Collision theory is applicable to
A)
first order reactions
done
clear
B)
zero order reactions
done
clear
C)
bimolecular reactions
done
clear
D)
intramolecular reactions
done
clear
View Answer play_arrow
question_answer 110) The rate constant for an isomerization reaction,\[A\xrightarrow[{}]{{}}B\]is\[4.5\times {{10}^{-3}}mi{{n}^{-1}}\]. If the initial concentration of A is 1M, calculate the rate of reaction after 1 h.
A)
\[3.44\times {{10}^{-3}}mol\,{{L}^{-1}}{{\min }^{-1}}\]
done
clear
B)
\[3.44\times {{10}^{3}}mol\,{{L}^{-1}}{{\min }^{-1}}\]
done
clear
C)
\[1.86\times {{10}^{-3}}mol\,{{L}^{-1}}{{\min }^{-1}}\]
done
clear
D)
\[1.86\times {{10}^{3}}mol\,{{L}^{-1}}{{\min }^{-1}}\]
done
clear
View Answer play_arrow
question_answer 111) When 1 mole of\[N{{a}_{2}}C{{O}_{3}}\]is heated,\[C{{O}_{2}}\]lost is
A)
zero mole
done
clear
B)
one mole
done
clear
C)
twomoes
done
clear
D)
four moles
done
clear
View Answer play_arrow
question_answer 112) Which one of the following is paramagnetic?
A)
\[{{N}_{2}}\]
done
clear
B)
\[NO\]
done
clear
C)
\[CO\]
done
clear
D)
\[{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 113) In\[TeC{{l}_{4}},\]the central atom tellurium involves
A)
\[s{{p}^{3}}\]hybridisation
done
clear
B)
\[s{{p}^{3}}d\]hybridization
done
clear
C)
\[ds{{p}^{2}}\]hybridisation
done
clear
D)
\[s{{p}^{3}}{{d}^{2}}\]hybridization
done
clear
View Answer play_arrow
question_answer 114) Which of the following salts of silver is insoluble in water?
A)
\[AgCl{{O}_{4}}\]
done
clear
B)
\[A{{g}_{2}}S{{O}_{4}}\]
done
clear
C)
\[AgF\]
done
clear
D)
\[AgN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 115) 25.6 g of sulphur in 100 g benzene shows depression in freezing point of\[5.12{}^\circ \]. Ks for benzene is\[5.12{}^\circ kg\text{ }mo{{l}^{-1}}\]. Molecular formula of sulphur in benzene is
A)
\[{{S}_{2}}\]
done
clear
B)
\[{{S}_{6}}\]
done
clear
C)
\[{{S}_{8}}\]
done
clear
D)
\[{{S}_{12}}\]
done
clear
View Answer play_arrow
question_answer 116) \[\Delta {{T}_{f}}/{{K}_{f}}\] is expressed in the unit of
A)
degree
done
clear
B)
degree \[mo{{l}^{-1}}kg\]
done
clear
C)
degree \[mol\text{ }k{{g}^{-1}}\]
done
clear
D)
\[mol\text{ }k{{g}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 117) Two liquids X and Y form an ideal solution at 300K, vapour pressure of the solution containing 1 mole of X and 3 moles of Y is 550 mm Hg. At the same temperature, if 1 mole of V is further added to this solution, vapour pressure of the solution increases by 10 mm Hg. Vapour pressure (in mm Hg) of X and V in their pure states will be respectively
A)
200 and 300
done
clear
B)
300 and 400
done
clear
C)
400 and 600
done
clear
D)
500 and 600
done
clear
View Answer play_arrow
question_answer 118) For the following half cell reactions,\[E{}^\circ \]values are also given \[M{{n}^{2+}}+2{{H}_{2}}O\xrightarrow[{}]{{}}Mn{{O}_{2}}+4{{H}^{+}}+2{{e}^{-}};\] \[E{}^\circ =-1.23V\] \[MnO_{4}^{-}+4{{H}^{+}}+3{{e}^{-}}\xrightarrow[{}]{{}}Mn{{O}_{2}}+2{{H}_{2}}O;\] \[E{}^\circ =+1.70V\] Select correct statements
A)
\[M{{n}^{2+}}\]reacts with\[MnO_{4}^{-}\]in acid solution to form\[Mn{{O}_{2}}\]
done
clear
B)
\[Mn{{(Mn{{O}_{4}})}_{2}}\]is stable in acid solution
done
clear
C)
\[Mn{{O}_{2}}\]disproporttbnates to\[M{{n}^{2+}}\]and \[MnO_{4}^{-}\] in acid solution
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 119) For the fuel cell reaction, \[2{{H}_{2}}+{{O}_{2}}\xrightarrow[{}]{{}}2{{H}_{2}}O\] \[\Delta G=-475\,kJ,\] Hence,\[E_{cell}^{o}\]is
A)
1.23 V
done
clear
B)
2.46 V
done
clear
C)
0.615 V
done
clear
D)
0.31 V
done
clear
View Answer play_arrow
question_answer 120) 1 mole each of\[AgN{{O}_{3}},CuS{{O}_{4}}\]and\[AlC{{l}_{3}}\]is electrolysed. Number offaradays required are in the ratio of
A)
\[1:1:1\]
done
clear
B)
\[1:2:3\]
done
clear
C)
\[3:2:1\]
done
clear
D)
\[1:3:1\]
done
clear
View Answer play_arrow
question_answer 121) Which term does not apply to human heart?
A)
Pacemaker
done
clear
B)
Four chambered
done
clear
C)
Mitral value
done
clear
D)
Neurogenic
done
clear
View Answer play_arrow
question_answer 122) Specific redioactive identification of ribosomal RNA can be achieved by using\[^{14}C\]labelled
A)
guanine
done
clear
B)
uracil
done
clear
C)
thyonine
done
clear
D)
cytosine
done
clear
View Answer play_arrow
question_answer 123) How many mitotic divisions must occur in a cell of root tip to form 256 cells.
A)
256
done
clear
B)
8
done
clear
C)
128
done
clear
D)
64
done
clear
View Answer play_arrow
question_answer 124) An operon unit consist of
A)
regulator, operator and recessive gene
done
clear
B)
regulator, structural and operator gene
done
clear
C)
regulator, structural, operator and promoter gene
done
clear
D)
regulator, structural and promoter gene
done
clear
View Answer play_arrow
question_answer 125) Site of formation of ribosomal precursor or ribosomal sub-units in a cell
A)
nucleus
done
clear
B)
nucleolus
done
clear
C)
nucleus body
done
clear
D)
stroma
done
clear
View Answer play_arrow
question_answer 126) Alzheimers disease affects
A)
child
done
clear
B)
youth (adolescence)
done
clear
C)
adult
done
clear
D)
old (elderly)
done
clear
View Answer play_arrow
question_answer 127) Lack of independent assortment of two genes A and B in fruit fly Dorsophila is due to
A)
linkage
done
clear
B)
repulsion
done
clear
C)
crossing over
done
clear
D)
recombination
done
clear
View Answer play_arrow
question_answer 128) Which one pair/ set exhibit uricotelism?
A)
Bird, land reptiles and insect
done
clear
B)
Fish, birds and amphibians
done
clear
C)
Mammals, birds, and reptiles
done
clear
D)
Amphibians, mammal and reptiles
done
clear
View Answer play_arrow
question_answer 129) Karyotaxonomy is the modern branch of classification which is bassed on
A)
number of chromosomes
done
clear
B)
bands found on chromosomes
done
clear
C)
organic evolution
done
clear
D)
trinomial nomenclature
done
clear
View Answer play_arrow
question_answer 130) Periodic appearence of malaria symptoms occurs due to periodic
A)
entry of merozoites into erythrocytes
done
clear
B)
attack of liver cells by merozoites
done
clear
C)
formation of signet ring
done
clear
D)
release of pyrogen in blood
done
clear
View Answer play_arrow
question_answer 131) Which antibiotic act on the cell wall of bacteria?
A)
p-lactum group
done
clear
B)
Tetracycline
done
clear
C)
Neomycine
done
clear
D)
Streptomycin
done
clear
View Answer play_arrow
question_answer 132) The group amniota includes
A)
birds and reptiles
done
clear
B)
birds and mammals
done
clear
C)
reptiles and mammals
done
clear
D)
reptiles, birds and mammals
done
clear
View Answer play_arrow
question_answer 133) Edible part of tomato is
A)
epicarp
done
clear
B)
pericarp and plecenta
done
clear
C)
mesocarp
done
clear
D)
thalamus
done
clear
View Answer play_arrow
question_answer 134) Interfascicular cambium is
A)
apical meristem
done
clear
B)
secondary meristem
done
clear
C)
primary meristem
done
clear
D)
abnormal meristem
done
clear
View Answer play_arrow
question_answer 135) The first event in photosynthesis is
A)
synthesis of ATP
done
clear
B)
photoexcitation of chlorophyll and ejection of electron
done
clear
C)
photolysis of water
done
clear
D)
release of oxygen
done
clear
View Answer play_arrow
question_answer 136) In water logged soil, plants generally are killed because of
A)
deficiency of minerals
done
clear
B)
excessive absorption of water
done
clear
C)
absense of air in the soil
done
clear
D)
starvation
done
clear
View Answer play_arrow
question_answer 137) R.Q. for glucose (carbohydrates) is
A)
1
done
clear
B)
0.5
done
clear
C)
2
done
clear
D)
0.05
done
clear
View Answer play_arrow
question_answer 138) Number of bones of face is
A)
12
done
clear
B)
30
done
clear
C)
40
done
clear
D)
14
done
clear
View Answer play_arrow
question_answer 139) Reason of diversity in living being is
A)
mutation
done
clear
B)
long term evolution
done
clear
C)
gradual changes
done
clear
D)
short term evolutionary changes
done
clear
View Answer play_arrow
question_answer 140) Constituent of gasonal is
A)
90% petrol of + 10% alcohol
done
clear
B)
80% petrol of + 20% etnanol
done
clear
C)
60% petrol of + 40% etnanol
done
clear
D)
50% petrol of + 50% etnanol
done
clear
View Answer play_arrow
question_answer 141) The type of joint at atlanto axiial joint
A)
pivot joint
done
clear
B)
glinding joint
done
clear
C)
saddle joint
done
clear
D)
hinge joint
done
clear
View Answer play_arrow
question_answer 142) Pure fractions of cellular component can be isolated by
A)
chromatography
done
clear
B)
scanning electron microscopy
done
clear
C)
X-ray
done
clear
D)
differential configuration
done
clear
View Answer play_arrow
question_answer 143) Lymph (nodes) glands form
A)
hormones
done
clear
B)
lymphs
done
clear
C)
antigen
done
clear
D)
antibodies
done
clear
View Answer play_arrow
question_answer 144) Who has written the book Genera Plantarum?
A)
Hutchinson
done
clear
B)
Engler and Prantal
done
clear
C)
Eichler
done
clear
D)
Bentham and Hooker
done
clear
View Answer play_arrow
question_answer 145) Choanocytes are found in
A)
Sycon
done
clear
B)
Proterospongia
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 146) Albinism is an
A)
autosomal recessive
done
clear
B)
autosomal dominant
done
clear
C)
X-linked disease
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 147) According to Wilhelm Stern IQ (Intelligence Quotient) is
A)
\[IQ=\frac{Mental\text{ }age}{Actual\text{ }age}\times 100\]
done
clear
B)
\[IQ=\frac{Actual\text{ }age}{Mental\text{ }age}\times 100\]
done
clear
C)
\[IQ=\frac{100}{Actual\text{ }age\times Mental\text{ }age}\]
done
clear
D)
\[IQ=\frac{Actual\text{ }age\times Mental\text{ }age}{100}\]
done
clear
View Answer play_arrow
question_answer 148) A thin film of water, held by the soil particles under the influence of internal attractive force is called which of the following water
A)
capillary
done
clear
B)
combined
done
clear
C)
hygroscopic
done
clear
D)
gravitational
done
clear
View Answer play_arrow
question_answer 149) Number of meiotic divisions necessary to produce 100 seeds in cyperus is
A)
100
done
clear
B)
200
done
clear
C)
300
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 150) In blood carboxyhaemoglobin forms by
A)
inhalation of\[CO\]
done
clear
B)
inhalation of \[C{{O}_{2}}\]
done
clear
C)
inhalation of \[S{{O}_{2}}\]
done
clear
D)
inhalation of ozone
done
clear
View Answer play_arrow
question_answer 151) In which of the following groups would you place a plant which produces spores and embryos but lack seeds and vascular tissues?
A)
Fungi
done
clear
B)
Bryophytes
done
clear
C)
Pteridophytes
done
clear
D)
Gymnosperm
done
clear
View Answer play_arrow
question_answer 152) In a colony of honey bee, male drones are onginated by
A)
diptoid parthenogenesis
done
clear
B)
cyclic parthenogenesis
done
clear
C)
arrhenotoky
done
clear
D)
thelotoky
done
clear
View Answer play_arrow
question_answer 153) If the ovary is inferior, the outermost layer of fruit produced by this ovary will be formed by
A)
epicarp
done
clear
B)
mesocarp
done
clear
C)
pericarp
done
clear
D)
thalamus
done
clear
View Answer play_arrow
question_answer 154) ATP synthesis occurs on the
A)
outer membrane of mitochondria
done
clear
B)
inner membrane of mitochondria
done
clear
C)
matrix
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 155) Repressible enzyme are formed
A)
in the absence of corepressor
done
clear
B)
in the presence of corepressor
done
clear
C)
in the pressence of apressor
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 156) A mature ligul having a prominent basal protion is called
A)
trrichocyst
done
clear
B)
heterocyst
done
clear
C)
rhizophore
done
clear
D)
glossopodium
done
clear
View Answer play_arrow
question_answer 157) Number of criteria used as classifying organisms in five-kingdom classification is
A)
5
done
clear
B)
4
done
clear
C)
3
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 158) Slit roots are reported from
A)
pandanus
done
clear
B)
radish
done
clear
C)
mango ginger
done
clear
D)
Bryophy/fum
done
clear
View Answer play_arrow
question_answer 159) Astela comprises consists
A)
xylem, phloem and pith
done
clear
B)
endodermis, xylemand phloem
done
clear
C)
vascular tissue, pericycle and pith
done
clear
D)
vascular tissue, endodermis and pith
done
clear
View Answer play_arrow
question_answer 160) Which of the following is also called as root ripe?
A)
Umbiicaria escufenta
done
clear
B)
Cetraria islandica
done
clear
C)
Cladonia rangiferina
done
clear
D)
Rocello
done
clear
View Answer play_arrow
question_answer 161) Protein infectious particles better known as Trions are also called as
A)
incomplete virus
done
clear
B)
slow virus
done
clear
C)
gemini viruses
done
clear
D)
satellite virus
done
clear
View Answer play_arrow
question_answer 162) Agar-Agar is obtained from
A)
Gigartina
done
clear
B)
Gefidium
done
clear
C)
Gracillaria
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 163) Life cycle of Funaria is not completed without water. Choose the correct statement.
A)
As Funaria is a bryophyte plant
done
clear
B)
As branches will not develop
done
clear
C)
As fertilization takes place in presence of water only
done
clear
D)
As plant is delicate and will become dry and die without water
done
clear
View Answer play_arrow
question_answer 164) Which of the following is the pribnow box?
A)
\[5\,TATAAT\,3\]
done
clear
B)
\[5\,TAATTA\,3\]
done
clear
C)
\[5AATAAT\,3\]
done
clear
D)
\[5ATATTA\,3\]
done
clear
View Answer play_arrow
question_answer 165) In human beings, lungs are divided into
A)
3. right and 2 left lobes
done
clear
B)
2 right and 3 left lobes
done
clear
C)
2 right and 2 left lobes
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 166) Extranuclear chromosomes are found in
A)
peroxysomes and mitochondria
done
clear
B)
chloroplast and mitochondria
done
clear
C)
mitochondria and ribosomes
done
clear
D)
chloroplast and ribosome
done
clear
View Answer play_arrow
question_answer 167) Contractile vacuole of Amoeba
A)
burst
done
clear
B)
disappear
done
clear
C)
enlarge
done
clear
D)
multiply
done
clear
View Answer play_arrow
question_answer 168) A genetically engineered microbe utilised for cleaning oil spiel is
A)
Bacillus subtilis
done
clear
B)
Escherichia coil
done
clear
C)
Pseudomonas putida
done
clear
D)
Agrobacterium tumefaciens
done
clear
View Answer play_arrow
question_answer 169) Enzyme hyaluronidase is synthesised in
A)
tail of sperm
done
clear
B)
head of sperm
done
clear
C)
golgi body of acrosome
done
clear
D)
mitochondria of acrosome
done
clear
View Answer play_arrow
question_answer 170) Which one is not a correct match?
A)
Hirudinea - Hirudo
done
clear
B)
Oligochaeta - Pheretima
done
clear
C)
Nematoda - Ascans
done
clear
D)
Pofychaeta - Lumbncus
done
clear
View Answer play_arrow
question_answer 171) Which of the following is the most primitive ancestor of man?
A)
Ramapithecus
done
clear
B)
Homohabilis
done
clear
C)
Australopithecus
done
clear
D)
Homo sapiens neanderthalencis
done
clear
View Answer play_arrow
question_answer 172) The only mammal, other than man which suffer from leprosy
A)
Dasypus
done
clear
B)
Desmodus
done
clear
C)
Rhinolopus
done
clear
D)
Mus
done
clear
View Answer play_arrow
question_answer 173) Protein is used as respiratory substrate only when
A)
carbohydrates are absent
done
clear
B)
fats are absent
done
clear
C)
both exhausted
done
clear
D)
fats and carbohydrates are abundant
done
clear
View Answer play_arrow
question_answer 174) An example of vestigial organ is
A)
ear of cow
done
clear
B)
hair of bear
done
clear
C)
nail of monkey
done
clear
D)
nictitating membrane of man
done
clear
View Answer play_arrow
question_answer 175) Sexual reproduction in which cells of two different Spirogyra filamants conjugate is known as
A)
lateral conjugation
done
clear
B)
scalariform conjugation
done
clear
C)
parthenocarpy
done
clear
D)
azygospory
done
clear
View Answer play_arrow
question_answer 176) The condition under which transpiration would be most rapid
A)
high humidity
done
clear
B)
excess of water in soil
done
clear
C)
low humidity, high temperature, guard cells are turgid (open) and moist soil
done
clear
D)
low velocity of wind
done
clear
View Answer play_arrow
question_answer 177) A rootless aquatic in which a portion of leaf is modified to from a bladder for catching small aquatic animal is
A)
Dionaea
done
clear
B)
Drosera
done
clear
C)
Utricularia
done
clear
D)
Nepenthese
done
clear
View Answer play_arrow
question_answer 178) Electron from excited chlorophyll molecule of photosystem. II are accepted first by
A)
ferredoxin
done
clear
B)
cytochrome-o
done
clear
C)
cytochrome-f
done
clear
D)
quinone
done
clear
View Answer play_arrow
question_answer 179) Sponges have evolved from
A)
ciliates
done
clear
B)
flagellates
done
clear
C)
protozoans
done
clear
D)
choanoflagellates
done
clear
View Answer play_arrow
question_answer 180) Sella turcica is a
A)
covering of kidney
done
clear
B)
covering of testis
done
clear
C)
depression in brain
done
clear
D)
depression is skull which lodges the pituitary body
done
clear
View Answer play_arrow
question_answer 181) Majority of the orchids are
A)
epizois
done
clear
B)
epiphytes
done
clear
C)
saprophytes
done
clear
D)
parasites
done
clear
View Answer play_arrow
question_answer 182) Intra Ovarian Fertilisation (IOF) means fertilisation
A)
outside ovule
done
clear
B)
outside embryo sac
done
clear
C)
by putting pollens directly into ovary-wall
done
clear
D)
between male gamete and synergids
done
clear
View Answer play_arrow
question_answer 183) Which of the following carries gases as well as unused products in the human body?
A)
Blood
done
clear
B)
Lymph
done
clear
C)
Blood and lymph
done
clear
D)
Haemocynin
done
clear
View Answer play_arrow
question_answer 184) The great barrier reef along the eastern coastal region of Australia can be categorised as
A)
ecosystem
done
clear
B)
biome
done
clear
C)
community
done
clear
D)
population
done
clear
View Answer play_arrow
question_answer 185) The complementary synthetic and random DNA are used as
A)
transposons
done
clear
B)
passenger DNA
done
clear
C)
cloning vectors
done
clear
D)
recombinant DNA
done
clear
View Answer play_arrow
question_answer 186) Mosses are indicator of
A)
air pollution
done
clear
B)
water pollution
done
clear
C)
radiation pollution
done
clear
D)
soil pollution
done
clear
View Answer play_arrow
question_answer 187) Milk glands are characteristic of
A)
all vertebrates
done
clear
B)
all mammals
done
clear
C)
only placental mammals
done
clear
D)
only primates and ruminants
done
clear
View Answer play_arrow
question_answer 188) Cybrids carry
A)
two similar genomes
done
clear
B)
only one genomes
done
clear
C)
several genomes
done
clear
D)
only genomes and two plasmone
done
clear
View Answer play_arrow
question_answer 189) If heart of a mammal is injected with 2%\[CaC{{l}_{2}}\] solution, then
A)
heart beat will increase
done
clear
B)
heart beat will decrease
done
clear
C)
heart beat will stop
done
clear
D)
no effect
done
clear
View Answer play_arrow
question_answer 190) Organism without any specific shape are
A)
mycoplasm
done
clear
B)
bacteria
done
clear
C)
viruses
done
clear
D)
cyanobacteria
done
clear
View Answer play_arrow
question_answer 191) Homo habilis habilis refers to
A)
tool maker
done
clear
B)
modern man
done
clear
C)
ancient man
done
clear
D)
wandering species
done
clear
View Answer play_arrow
question_answer 192) Graafian follicles posses
A)
theca externa
done
clear
B)
granulosa
done
clear
C)
theca interna
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 193) The pH of acid rain water is
A)
1.2
done
clear
B)
5.7
done
clear
C)
3.1
done
clear
D)
6.0
done
clear
View Answer play_arrow
question_answer 194) Light loving plants are known as
A)
heliophytes
done
clear
B)
xerophytes
done
clear
C)
lithophytes
done
clear
D)
sosiophytes
done
clear
View Answer play_arrow
question_answer 195) The thread-like tendons of papillary muscles inserted upon the flaps of tricuspid and bicuspid value are
A)
chordae tendinae
done
clear
B)
yellow elastin fibre
done
clear
C)
reticulate fibre
done
clear
D)
collagen fibre
done
clear
View Answer play_arrow
question_answer 196) The peculiar pungent smell of cockroach is produced by the secretions of
A)
pheromones
done
clear
B)
flame cells
done
clear
C)
abdominal glands
done
clear
D)
cervical glands
done
clear
View Answer play_arrow
question_answer 197) The main function of clitellum is
A)
coccon formation
done
clear
B)
locomotion
done
clear
C)
excretion
done
clear
D)
copulation
done
clear
View Answer play_arrow
question_answer 198) Diaphragm is found in
A)
crocodile
done
clear
B)
kangaroo
done
clear
C)
ostrich
done
clear
D)
snake
done
clear
View Answer play_arrow
question_answer 199) Last stable community in succession which depends on climate is
A)
serai community
done
clear
B)
climax community
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 200) Which one is spindle-shaped mobile with microtubules?
A)
Sporont
done
clear
B)
Ookinete
done
clear
C)
Cryptozoite
done
clear
D)
Sporozoite
done
clear
View Answer play_arrow
question_answer 201) Directions: In the following questions; choose the word opposite in meaning to the given word. INDIGENOUS
A)
Native
done
clear
B)
Cheap
done
clear
C)
Foreign
done
clear
D)
Inferior
done
clear
View Answer play_arrow
question_answer 202) Directions: In the following questions; choose the word opposite in meaning to the given word. FRAIL
A)
Vigorous
done
clear
B)
Sturdy
done
clear
C)
Hardy
done
clear
D)
Strong
done
clear
View Answer play_arrow
question_answer 203) Directions: In the following questions; choose the word opposite in meaning to the given word. WITHIN
A)
Without
done
clear
B)
Past
done
clear
C)
Over
done
clear
D)
Beyond
done
clear
View Answer play_arrow
question_answer 204) Directions: In the following questions; choose the word opposite in meaning to the given word. BRAZEN
A)
Respectful
done
clear
B)
Innocent
done
clear
C)
Delicious
done
clear
D)
Helpful
done
clear
View Answer play_arrow
question_answer 205) Directions: In the following questions; choose the word opposite in meaning to the given word. ADVERSITY
A)
Diversity
done
clear
B)
Affliction
done
clear
C)
Prosperity
done
clear
D)
Catastrophe
done
clear
View Answer play_arrow
question_answer 206) She was compear sated .......... the loss of her belongings.
A)
over
done
clear
B)
for
done
clear
C)
against
done
clear
D)
at
done
clear
View Answer play_arrow
question_answer 207) He has bean entrusted .......... this work.
A)
to
done
clear
B)
on
done
clear
C)
with
done
clear
D)
by
done
clear
View Answer play_arrow
question_answer 208) Lets make ....... our quarrel and be friends again.
A)
off
done
clear
B)
up
done
clear
C)
out
done
clear
D)
with
done
clear
View Answer play_arrow
question_answer 209) She yelled.......... him and he hostily retreated.
A)
to
done
clear
B)
towards
done
clear
C)
on
done
clear
D)
at
done
clear
View Answer play_arrow
question_answer 210) I devote much of my time .......... writing.
A)
to
done
clear
B)
on
done
clear
C)
at
done
clear
D)
in
done
clear
View Answer play_arrow
question_answer 211) The Ahiyas
A)
The Ahiyas
done
clear
B)
/are living in this colony
done
clear
C)
/for the last eight years.
done
clear
D)
/No error
done
clear
View Answer play_arrow
question_answer 212) You will come
A)
You will come
done
clear
B)
/to my party tomorrow
done
clear
C)
/ is ait it.
done
clear
D)
/No error
done
clear
View Answer play_arrow
question_answer 213) This room would look much better
A)
This room would look much better
done
clear
B)
/if you put a furniture
done
clear
C)
/in that corner.
done
clear
D)
/ No error
done
clear
View Answer play_arrow
question_answer 214) If I will have enough
A)
If I will have enough
done
clear
B)
/time tomorrow
done
clear
C)
/I will come and see you.
done
clear
D)
/No error
done
clear
View Answer play_arrow
question_answer 215) My father is
A)
My father is
done
clear
B)
/appreciated by his friends
done
clear
C)
/for his honestness.
done
clear
D)
/No error,
done
clear
View Answer play_arrow
question_answer 216) FILTHY
A)
Healthy
done
clear
B)
Ugly
done
clear
C)
Dirty
done
clear
D)
Angry
done
clear
View Answer play_arrow
question_answer 217) NOSTALGIC
A)
Soothing
done
clear
B)
Homesick
done
clear
C)
Diseased
done
clear
D)
Indolent
done
clear
View Answer play_arrow
question_answer 218) COMBAT
A)
Quarrel
done
clear
B)
Fight
done
clear
C)
Conflict
done
clear
D)
Feud
done
clear
View Answer play_arrow
question_answer 219) SUBSIDE
A)
Submit
done
clear
B)
Oppress
done
clear
C)
Subdue
done
clear
D)
Surrender
done
clear
View Answer play_arrow
question_answer 220) ABSCOND
A)
Turn
done
clear
B)
Flee
done
clear
C)
Manage
done
clear
D)
Avoid
done
clear
View Answer play_arrow
question_answer 221) I wish I was with him.
A)
have been
done
clear
B)
were
done
clear
C)
am
done
clear
D)
No improvement
done
clear
View Answer play_arrow
question_answer 222) Upto the time the last vote was recorded it was difficult to decide whether victory lay with the ruling party or the opposition.
A)
To
done
clear
B)
Until
done
clear
C)
Till
done
clear
D)
No improvement
done
clear
View Answer play_arrow
question_answer 223) Having had in the Foreign Service for a long time, Mr Verma has met many prominent personalities.
A)
Having
done
clear
B)
He has been
done
clear
C)
Having been
done
clear
D)
Had he been
done
clear
View Answer play_arrow
question_answer 224) Who does not know that this was broadcasted ten days ago?
A)
Had broadcast
done
clear
B)
Was broadcast
done
clear
C)
Was broadcasting
done
clear
D)
No improvement
done
clear
View Answer play_arrow
question_answer 225) Since 1986, there is no earthquakes here.
A)
were being
done
clear
B)
have been
done
clear
C)
are
done
clear
D)
No improvement
done
clear
View Answer play_arrow
question_answer 226) One who speaks or understands many languages?
A)
Scholar
done
clear
B)
Grammarian
done
clear
C)
Linguist
done
clear
D)
Polyglot
done
clear
View Answer play_arrow
question_answer 227) To talk without respect of something sacred or holy
A)
blasphemy
done
clear
B)
obscenity
done
clear
C)
rudeness
done
clear
D)
vulgarity
done
clear
View Answer play_arrow
question_answer 228) Land so surrounded by water as to be almost an island
A)
archipelago
done
clear
B)
isthmus
done
clear
C)
peninsula
done
clear
D)
lagoon
done
clear
View Answer play_arrow
question_answer 229) A place adjoining kitchen, for washing dishes etc.
A)
Cellar
done
clear
B)
Wardrobe
done
clear
C)
Scullery
done
clear
D)
Pantry
done
clear
View Answer play_arrow
question_answer 230) Incapable of being wounded
A)
Invulnerable
done
clear
B)
Invincible
done
clear
C)
Infallible
done
clear
D)
Impregnable
done
clear
View Answer play_arrow
question_answer 231) In the following questions, groups of four words are given. In each group, one word is correctly spelt. Find the correctly spelt word.
A)
Posesion
done
clear
B)
Possesion
done
clear
C)
Possession
done
clear
D)
Posession
done
clear
View Answer play_arrow
question_answer 232) In the following questions, groups of four words are given. In each group, one word is correctly spelt. Find the correctly spelt word.
A)
Liesure
done
clear
B)
Leisure
done
clear
C)
Leasure
done
clear
D)
Lesiure
done
clear
View Answer play_arrow
question_answer 233) In the following questions, groups of four words are given. In each group, one word is correctly spelt. Find the correctly spelt word.
A)
Bouquete
done
clear
B)
Boquet
done
clear
C)
Bouquet
done
clear
D)
Bouquette
done
clear
View Answer play_arrow
question_answer 234) In the following questions, groups of four words are given. In each group, one word is correctly spelt. Find the correctly spelt word.
A)
Pitiaeble
done
clear
B)
Pitiable
done
clear
C)
Pitiaeble
done
clear
D)
Pitiabale
done
clear
View Answer play_arrow
question_answer 235) In the following questions, groups of four words are given. In each group, one word is correctly spelt. Find the correctly spelt word.
A)
Ocasion
done
clear
B)
Ocassion
done
clear
C)
Occasion
done
clear
D)
Occasion
done
clear
View Answer play_arrow
question_answer 236) The robbery was committed in the wee hours of the day.
A)
after midnight
done
clear
B)
at dawn
done
clear
C)
at noontime
done
clear
D)
in the evening
done
clear
View Answer play_arrow
question_answer 237) Harassed by repeated acts of injustice, he decided to put his foot down.
A)
resign
done
clear
B)
not to yield
done
clear
C)
withdraw
done
clear
D)
accept the proposal unconditionally
done
clear
View Answer play_arrow
question_answer 238) Mrs Khanna has been in the blues for the last several weeks.
A)
unwell
done
clear
B)
lonely
done
clear
C)
penniless
done
clear
D)
depressed
done
clear
View Answer play_arrow
question_answer 239) The doctor says the patient has turned the comers.
A)
completely recovered
done
clear
B)
become worse
done
clear
C)
passed the crisis
done
clear
D)
died
done
clear
View Answer play_arrow
question_answer 240) He is in the habit of fishing in troubled waters.
A)
putting others in trouble
done
clear
B)
indulging in evil conspirancies
done
clear
C)
aggravating the situation
done
clear
D)
taking advantage of troubled conditions for personal profit
done
clear
View Answer play_arrow
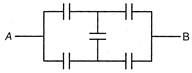
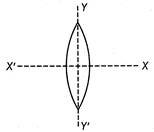 Choose the correct statement from the following
Choose the correct statement from the following
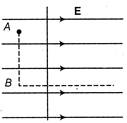
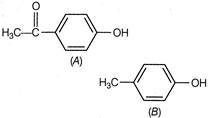
 Aspirin is a pain reliever with\[p{{K}_{a}}=2\]. Two tablets each containing 0.09 g of aspirin are dissolved in 100 mL solution. pH will be
Aspirin is a pain reliever with\[p{{K}_{a}}=2\]. Two tablets each containing 0.09 g of aspirin are dissolved in 100 mL solution. pH will be