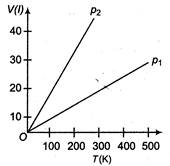
A) \[{{p}_{1}}>{{p}_{2}}\]
B) \[{{p}_{1}}={{p}_{2}}\]
C) \[{{p}_{1}}<{{p}_{2}}\]
D) data is insufficient
Correct Answer: A
Solution :
\[pV=nRT\Rightarrow V=\left( \frac{nR}{p} \right)T\] Slope of the\[V-T\]graph, \[m=\frac{dV}{dT}=\frac{nR}{p}\] \[\Rightarrow \] \[m\propto \frac{1}{p}\] [\[\therefore \]\[nR=\]constant] Or \[p\propto \frac{1}{m}\] Hence, \[\frac{{{p}_{2}}}{{{p}_{1}}}=\frac{{{m}_{1}}}{{{m}_{2}}}<1\] where,\[{{m}_{1}}\]is slope of the graph corresponding of\[{{p}_{1}}\]and similarly\[{{m}_{2}}\]is slope corresponding to \[\Rightarrow \] \[{{p}_{2}}<{{p}_{1}}\,or\,\,{{p}_{1}}>{{p}_{2}}\]You need to login to perform this action.
You will be redirected in
3 sec
