A) \[s{{p}^{3}}\]
B) \[ds{{p}^{2}}\]
C) \[s{{p}^{3}}{{d}^{2}}\]
D) \[{{d}^{3}}s{{p}^{3}}\]
Correct Answer: C
Solution :
In\[Br{{F}_{5}},\]there are seven electrons in the valence shell of the central Br-atom. Five out of these are shared with fluorine atoms. Thus. there are five bond pairs and one lone pair. Hence, the hybridisation is\[s{{p}^{3}}{{d}^{2}}\]and shape is square pyramidal.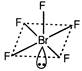
You need to login to perform this action.
You will be redirected in
3 sec
