question_answer 1) Which of the following has centre of mass not situated in the material of body?
A)
A rod bent in the form of a circle
done
clear
B)
Solid sphere
done
clear
C)
Cricket bat
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 2) In a carnival ride the passengers travel in a circle of radius 5.0 m, making one complete circle in 4.0 s. What is the acceleration?
A)
24.6 m/s2
done
clear
B)
12.3 m/s2
done
clear
C)
6.15 m/s2
done
clear
D)
49.2 m/s2
done
clear
View Answer play_arrow
question_answer 3) A road is 8 m wide. Its radius of curvature is 40 m. The outer edge is above the lower edge by a distance of 1.2 m. This road is most suited for a velocity of
A)
5.7 m/s
done
clear
B)
7.7 m/s
done
clear
C)
36.1 m/s
done
clear
D)
9.7 m/s
done
clear
View Answer play_arrow
question_answer 4) A 0.5 kg ball moves in a circle of radius 0.4 m at a velocity of 4 m/s. The centripetal force on the ball is.
A)
10 N
done
clear
B)
20 N
done
clear
C)
40 N
done
clear
D)
80 N
done
clear
View Answer play_arrow
question_answer 5) A rod of length L, whose lower end is sliding along the horizontal plane, starts to topple from the vertical position. The velocity of the upper end when it hits the ground is
A)
\[\sqrt{gL}\]
done
clear
B)
\[\sqrt{3gL}\]
done
clear
C)
\[\sqrt{5gL}\]
done
clear
D)
\[3\sqrt{gL}\]
done
clear
View Answer play_arrow
question_answer 6) Two bodies of moments of inertia \[{{I}_{1}}\] and \[{{I}_{2}}\] \[({{I}_{1}}>{{I}_{2}})\]have equal angular momentum. If \[{{E}_{1,}}\]\[{{E}_{2}}\] are their kinetic energies of rotation, then
A)
\[{{E}_{1}}>{{E}_{2}}\]
done
clear
B)
\[{{E}_{1}}={{E}_{2}}\]
done
clear
C)
\[{{E}_{1}}<{{E}_{2}}\]
done
clear
D)
cannot be said
done
clear
View Answer play_arrow
question_answer 7) An artificial satellite revolves around the earth in a circular orbit with a speed v. If m is the mass of the satellite, its total energy is
A)
\[\frac{1}{2}m{{v}^{2}}\]
done
clear
B)
\[-\frac{1}{2}m{{v}^{2}}\]
done
clear
C)
\[-m{{v}^{2}}\]
done
clear
D)
\[\frac{3}{2}m{{v}^{2}}\]
done
clear
View Answer play_arrow
question_answer 8) In an earth's satellite the astronaut stands on a platform. His weight as indicated by the balance will be
A)
6g
done
clear
B)
2g
done
clear
C)
8 g
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 9) A body of mass m is moved to a height h equal to the radius of the earth. The increase in potential energy is
A)
\[2mgR\]
done
clear
B)
\[mgR\]
done
clear
C)
\[\frac{1}{2}mgR\]
done
clear
D)
\[\frac{1}{4}mgR\]
done
clear
View Answer play_arrow
question_answer 10) At which of the following temperatures, the value of surface tension of water is minimum?
A)
\[4{}^\circ C\]
done
clear
B)
\[25{}^\circ C\]
done
clear
C)
\[50{}^\circ C\]
done
clear
D)
\[75{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 11) Work done in spliting a drop of water of 1 mm radius into 64 droplets is (surface tension of waters \[=72\times {{10}^{-3}}J/{{m}^{2}}\])
A)
\[2.0\times {{10}^{-6}}J\]
done
clear
B)
\[2.7\times {{10}^{-6}}J\]
done
clear
C)
\[4\times {{10}^{-6}}J\]
done
clear
D)
\[5.4\times {{10}^{-6}}J\]
done
clear
View Answer play_arrow
question_answer 12) A black body at 227oC radiates heat at the rate of \[7\text{ }cal/c{{m}^{2}}-s.\] At a temperature of 727°C, the rate of heat radiated in same units will be
A)
80
done
clear
B)
60
done
clear
C)
50
done
clear
D)
112
done
clear
View Answer play_arrow
question_answer 13) The vertical extension in a light spring by a weight of 1 kg suspended from the wire is 9.8 cm. The period of oscillation is
A)
\[20\pi s\]
done
clear
B)
\[2\pi \]
done
clear
C)
\[\frac{2\pi }{10}s\]
done
clear
D)
\[200\pi \,s\]
done
clear
View Answer play_arrow
question_answer 14) A panicle of mass 10 g is describing SHM along a straight line with period of 2 s and amplitude of 10 cm. Its kinetic energy when it is al 5 cm from its equilibrium position is
A)
\[3.75{{\pi }^{2}}\,erg\]
done
clear
B)
\[375{{\pi }^{2}}\,erg\]
done
clear
C)
\[0.375{{\pi }^{2}}\,erg\]
done
clear
D)
\[37.5{{\pi }^{2}}\,erg\]
done
clear
View Answer play_arrow
question_answer 15) The angular amplitude of a simple pendulum is \[{{\theta }_{0.}}\] The maximum tension in its string will be
A)
\[mg(1-{{\theta }_{0}})\]
done
clear
B)
\[mg(1+{{\theta }_{0}})\]
done
clear
C)
\[mg(1-\theta \,_{0}^{2})\]
done
clear
D)
\[mg(1+\theta \,_{0}^{2})\]
done
clear
View Answer play_arrow
question_answer 16) It is possible to recognise a person by hearing his voice even if he is hidden behind a solid wall. This is due to the fact that his voice
A)
has a definite capacity
done
clear
B)
has a definite quality
done
clear
C)
has definite pitch
done
clear
D)
can penetrate the wall
done
clear
View Answer play_arrow
question_answer 17) The velocity of sound in air at NTP is 330 m/s. What will be its value when temperature is doubled and pressure is halved?
A)
330 m/s
done
clear
B)
165 m/s
done
clear
C)
330 \[\sqrt{2}\] m/s
done
clear
D)
\[\frac{330}{\sqrt{2}}\]m/s
done
clear
View Answer play_arrow
question_answer 18) The intensity of sound gets reduced by 10% on passing through a slab. The reduction in intensity on passing through two consecutive slabs would be
A)
20%
done
clear
B)
19%
done
clear
C)
5%
done
clear
D)
50%
done
clear
View Answer play_arrow
question_answer 19) Point charges + 4q, - q and + 4q are kept on the \[x\]-axis at points \[x=0,x-a\] and \[x=2a\] respectively, then
A)
only - q is in stable equilibrium
done
clear
B)
all the charges are in stable equilibrium
done
clear
C)
all the charges are in unstable equilibrium
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 20) If \[{{E}_{a}}\] be the electric field strength of a short dipole at a point on its axial line and \[{{E}_{e}}\] that on equilateral line at the same distance, then
A)
\[E=2{{E}_{a}}\]
done
clear
B)
\[E{{ }_{a}}=2{{E}_{e}}\]
done
clear
C)
\[E{{ }_{a}}={{E}_{e}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 21) If emf E = 4 cos 1000t volt is applied to an L-R circuit of inductance 3 mH and resistance 40\[\Omega \] the amplitude of current in the circuit is
A)
\[\frac{4}{\sqrt{7}}A\]
done
clear
B)
\[1.0A\]
done
clear
C)
\[\frac{4}{7}A\]
done
clear
D)
\[0.8A\]
done
clear
View Answer play_arrow
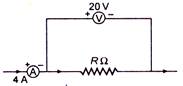
question_answer 22)
A candidate connects a moving coil ammeter A and a moving coil voltmeter V and a resistance R as shown. If the voltmeter reads 20 V and the ammeter reads 4 A, then R is
A)
equal to 5\[\Omega \]
done
clear
B)
greater than 5\[\Omega \]
done
clear
C)
less than 5\[\Omega \]
done
clear
D)
none of these
done
clear
View Answer play_arrow
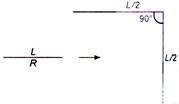
question_answer 23)
A wire of length L has resistance R. It is bent at mid-point such that the two halves make an angle 90° with each other. The new resistance would be
A)
R
done
clear
B)
\[\sqrt{2}R\]
done
clear
C)
\[\frac{R}{\sqrt{2}}\]
done
clear
D)
\[\frac{R}{4}\]
done
clear
View Answer play_arrow
question_answer 24) The ratio of magnetic potentials due magnetic dipole in the end on position to that broad side on position for the same distance from it is
A)
zero
done
clear
B)
infinite
done
clear
C)
1
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 25) In the unmagnetized state magnetic domain of a magnetic substance are oriented at
A)
\[60{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
randomly
done
clear
D)
\[150{}^\circ \]
done
clear
View Answer play_arrow
question_answer 26) When a ferromagnetic material is heated above its Curie temperature it
A)
gets demagnetized
done
clear
B)
becomes diamagnetic
done
clear
C)
behaves like a paramagnetic substance
done
clear
D)
remains unaffected
done
clear
View Answer play_arrow
question_answer 27) Four independent waves are expressed as (i)\[{{y}_{1}}={{a}_{1}}\sin \omega t\] (ii)\[{{y}_{1}}={{a}_{2}}\sin 2\omega t\] (iii)\[{{y}_{3}}={{a}_{3}}\cos \omega t\]and (iv)\[{{y}_{4}}={{a}_{4}}\sin \,\,\left( \omega t+\frac{\pi }{3} \right)\] The interference is possible between
A)
(i) and (iii)
done
clear
B)
(i) and (iv)
done
clear
C)
(ii) and (iv)
done
clear
D)
not possible at all
done
clear
View Answer play_arrow
question_answer 28)
Two gases A and B at same pressure contain number of moles \[{{n}_{1}}\] and \[{{n}_{2}}\] Their volume temperature graphs are shown in figure. Then the ratio \[{{n}_{1}}/{{n}_{2}}\]is
A)
1
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[\frac{1}{3}\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 29) A DC circuit contains 10 \[\Omega \] of resistance in series with 10 H coil. The impedance of the circuit is
A)
10 \[\Omega \]
done
clear
B)
20\[\Omega \]
done
clear
C)
1\[\Omega \]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 30) Two coils of self-inductances \[{{L}_{1}}\] and \[{{L}_{2}}\] are placed so close together that effective flux in one coil is completely linked with the other. If M is the mutual inductance between them, then
A)
\[M={{L}_{1}}{{L}_{2}}\]
done
clear
B)
\[M={{L}_{1}}/{{L}_{2}}\]
done
clear
C)
\[M=L\]
done
clear
D)
\[M=\sqrt{{{L}_{1}}{{L}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 31) Two coherent monochromatic light beams of intensities \[I\] and 4 \[I\] superimpose. The maximum and minimum possible intensities in the resulting beam are respectively
A)
5\[I\] and \[I\]
done
clear
B)
5\[I\] and 3\[I\]
done
clear
C)
3\[I\]and\[I\]
done
clear
D)
9 \[I\]and\[I\]
done
clear
View Answer play_arrow
question_answer 32) Ordinary light incident on a glass slab at the polarising angle suffers a deviation of 220. The value of the angle of refraction in glass in this case is
A)
\[56{}^\circ\]
done
clear
B)
\[68{}^\circ\]
done
clear
C)
\[34{}^\circ\]
done
clear
D)
\[22{}^\circ\]
done
clear
View Answer play_arrow
question_answer 33) A source of sound emits 200 \[\pi \]W power which is uniformly distributed over a sphere of 10 m radius. What is the loudness of sound on the surface of a sphere?
A)
200 dB
done
clear
B)
200\[\pi \] dB
done
clear
C)
120 dB
done
clear
D)
120\[\pi \]dB
done
clear
View Answer play_arrow
question_answer 34) A radio isotope has half-life of 5 yr. The fraction of the atoms of this material that would decay in 15 yr will be
A)
1
done
clear
B)
\[\frac{2}{3}\]
done
clear
C)
\[\frac{7}{8}\]
done
clear
D)
\[\frac{5}{8}\]
done
clear
View Answer play_arrow
question_answer 35) The ground state energy of H-atom is 13.6 eV. The energy needed to ionize H-atom from its second excited state is
A)
1.51 eV
done
clear
B)
3.4 eV
done
clear
C)
13.6 eV
done
clear
D)
12.1 eV
done
clear
View Answer play_arrow
question_answer 36) Forward bias characteristics of a p-n junction diode is used in which of the following devices?
A)
Transistor
done
clear
B)
Tank circuit
done
clear
C)
Rectifier
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 37) The energy gap between conduction band and valence band is of the order of 0.07 eV. It is a/an
A)
insulator
done
clear
B)
conductor
done
clear
C)
semiconductor
done
clear
D)
alloy
done
clear
View Answer play_arrow
question_answer 38) In an L-R circuit, the current increases to three fourth of its maximum value in 4 s, then the S- time constant of the circuit is
A)
\[2{{\log }_{e}}\,2\]
done
clear
B)
\[\frac{4}{{{\log }_{e}}2}\]
done
clear
C)
\[\frac{2}{{{\log }_{e}}2}\]
done
clear
D)
\[\frac{{{\log }_{e\,}}2}{2}\]
done
clear
View Answer play_arrow
question_answer 39) An oscillator is basically an amplifier with gain
A)
less than unity
done
clear
B)
more than unity
done
clear
C)
zero
done
clear
D)
0.5
done
clear
View Answer play_arrow
question_answer 40) A particle of mass m at rest decays into two particles of masses \[{{m}_{1}}\]and \[{{m}_{2}}\] having non-zero velocities. The ratio of de-Broglie wavelengths of the particles\[\frac{{{\lambda }_{1}}}{{{\lambda }_{2}}}\]is
A)
\[\frac{{{m}_{1}}}{{{m}_{2}}}\]
done
clear
B)
\[\frac{{{m}_{2}}}{{{m}_{1}}}\]
done
clear
C)
\[1.0\]
done
clear
D)
\[\sqrt{\frac{{{m}_{2}}}{{{m}_{1}}}}\]
done
clear
View Answer play_arrow
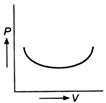
question_answer 41)
Figure shows a P-V diagram. How does the work done in the process change with time ?
A)
First decreases and then increases
done
clear
B)
First increases and then decreases
done
clear
C)
Continuously decreases
done
clear
D)
Continuously increases
done
clear
View Answer play_arrow
question_answer 42) Two identical galvanometers are taken, one is to be converted into an ammeter and other into a milliammeter. Shunt of milliammeter compared to ammeter is
A)
less
done
clear
B)
more
done
clear
C)
zero
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 43) If the vibrations of a string are to be increased by a factor of two, the tension in the string should be made
A)
twice
done
clear
B)
four times
done
clear
C)
eight rimes
done
clear
D)
half
done
clear
View Answer play_arrow
question_answer 44) A triode valve has an amplification factor of 20 and its plate is given a potential of 300 V. The grid voltage to reduce the plate current to zero, is
A)
25V
done
clear
B)
15V
done
clear
C)
12V
done
clear
D)
10V
done
clear
View Answer play_arrow
question_answer 45) An electron of mass m and charge e is accelerated from rest through a potential difference V in vacuum. The final speed will be
A)
\[\frac{2eV}{m}\]
done
clear
B)
\[V\sqrt{\frac{e}{m}}\]
done
clear
C)
\[\sqrt{\frac{3eV}{m}}\]
done
clear
D)
\[\sqrt{\frac{2eV}{m}}\]
done
clear
View Answer play_arrow
question_answer 46) In a circuit, the current lags behind the voltage by a phase difference of \[\pi /2,\]the circuit will contain which of the following?
A)
Only R
done
clear
B)
Only C
done
clear
C)
R and C
done
clear
D)
Only L
done
clear
View Answer play_arrow
question_answer 47) Which one of the following does not support the wave nature of light?
A)
Photoelectric effect
done
clear
B)
Interference
done
clear
C)
Polarization
done
clear
D)
Diffraction
done
clear
View Answer play_arrow
question_answer 48) A siren emitting sound of frequency 800 Hz is going away from a static listener with a speed of 30 m/s. Frequency of sound to be heard by the listener is (velocity of sound = 330 m/s)
A)
286.5 Hz
done
clear
B)
481.2 Hz
done
clear
C)
733.3 Hz
done
clear
D)
644.8 Hz
done
clear
View Answer play_arrow
question_answer 49) A wire fixed at the upper end stretches by length \[\Delta l\] by applying a force F. The work done in stretching is
A)
\[\frac{F}{2\Delta l}\]
done
clear
B)
\[F\Delta l\]
done
clear
C)
\[2F\Delta l\]
done
clear
D)
\[\frac{F\Delta l}{2}\]
done
clear
View Answer play_arrow
question_answer 50) The Young's modulus of a wire is numerically equal to the stress which will
A)
not change the length of the wire
done
clear
B)
double the length of the wire
done
clear
C)
increase the length
done
clear
D)
change the radius of the wire to half
done
clear
View Answer play_arrow
question_answer 51) The musical interval between two tones of frequency 400 Hz and 200 Hz is
A)
2
done
clear
B)
200
done
clear
C)
1
done
clear
D)
0.5
done
clear
View Answer play_arrow
question_answer 52) Which of the following has the minimum resistance?
A)
Voltmeter
done
clear
B)
Millivoltmeter
done
clear
C)
Ammeter
done
clear
D)
Milliammeter
done
clear
View Answer play_arrow
question_answer 53) N identical cells, each of emf E, are connected in parallel. The emf of the combination is
A)
NE
done
clear
B)
E
done
clear
C)
N2E
done
clear
D)
E/N
done
clear
View Answer play_arrow
question_answer 54) According to Newton's law of cooling, the rate of cooling of a body is proportional to \[{{(\Delta \theta )}^{n}},\]where \[\Delta \theta \] is the difference of the temperature of the body and the surroundings and n is equal to
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 55) Which of the following parameters does not characterize the thermodynamic state of matter?
A)
Volume
done
clear
B)
Temperature
done
clear
C)
Pressure
done
clear
D)
Work
done
clear
View Answer play_arrow
question_answer 56) A 30 \[\pi F\] capacitor is charged by a constant current of 30 mA. If the capacitor is initially uncharged, how long does it take for the potential difference to reach 400 V?
A)
0.2s
done
clear
B)
0.4s
done
clear
C)
0.6s
done
clear
D)
0.8s
done
clear
View Answer play_arrow
question_answer 57) A panicle moving with a velocity \[\frac{1}{10}\]the of light will cross a nucleus in about
A)
\[{{10}^{-8}}s\]
done
clear
B)
\[{{10}^{-12}}s\]
done
clear
C)
\[{{10}^{-47}}s\]
done
clear
D)
\[{{10}^{-21}}s\]
done
clear
View Answer play_arrow
question_answer 58) Which of the following nuclei is fissionable but not possible?
A)
\[_{92}{{U}^{235}}\]
done
clear
B)
\[_{92}{{U}^{233}}\]
done
clear
C)
\[_{92}{{U}^{238}}\]
done
clear
D)
\[_{94}{{U}^{239}}\]
done
clear
View Answer play_arrow
question_answer 59) In a common base amplifier, the phase difference between the input signal voltage and output voltage is
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\pi \]
done
clear
C)
zero
done
clear
D)
\[\frac{\pi }{2}\]
done
clear
View Answer play_arrow
question_answer 60) Therm-ions are
A)
photons
done
clear
B)
protons
done
clear
C)
electrons
done
clear
D)
nuclei
done
clear
View Answer play_arrow
question_answer 61) What is the half-life of\[_{6}{{C}^{14}},\]if its disintegration constant is\[2.31\times {{10}^{-4}}y{{r}^{-1}}\]?
A)
\[0.3\times {{10}^{4}}yr\]
done
clear
B)
\[0.3\times {{10}^{3}}yr\]
done
clear
C)
\[0.3\times {{10}^{8}}yr\]
done
clear
D)
\[0.3\times {{10}^{2}}yr\]
done
clear
View Answer play_arrow
question_answer 62) What is the frequency of a X-ray photon whose momentum is\[1.1\times {{10}^{-23}}kg-m{{s}^{-2}}\]?
A)
\[5\times {{10}^{16}}Hz\]
done
clear
B)
\[0.5\times {{10}^{27}}Hz\]
done
clear
C)
\[0.5\times {{10}^{18}}Hz\]
done
clear
D)
\[5\times {{10}^{18}}Hz\]
done
clear
View Answer play_arrow
question_answer 63) Metals are good conductor of electricity because they contain
A)
a network structure
done
clear
B)
ionic bonds
done
clear
C)
very few valence electrons
done
clear
D)
free electrons
done
clear
View Answer play_arrow
question_answer 64) Which of the following is an ester?
A)
Coconut oil
done
clear
B)
Kerosene oil
done
clear
C)
Soap
done
clear
D)
Glycerine
done
clear
View Answer play_arrow
question_answer 65) Identify the ?C? in the reaction \[RN{{H}_{2}}\xrightarrow{HN{{O}_{2}}}A+B+C\uparrow \]
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{O}_{2}}\]
done
clear
C)
\[{{N}_{2}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 66) The magnitude of orbital angular momentum of an electron of azimuthal quantum number 2 is
A)
\[\frac{2h}{2\pi }\]
done
clear
B)
\[\frac{\sqrt{6}h}{2\pi }\]
done
clear
C)
\[\frac{\sqrt{2}h}{2\pi }\]
done
clear
D)
\[\frac{6h}{2\pi }\]
done
clear
View Answer play_arrow
question_answer 67) Hydrolysis of lactose with dilute acid yields
A)
D-glucose + D-glucose
done
clear
B)
D-glucose + D-galactose
done
clear
C)
D-glucose + D-fructose
done
clear
D)
D-galactose + D-fructose
done
clear
View Answer play_arrow
question_answer 68) The pH of a 0.02 M solution of\[HCl\]is
A)
2.2
done
clear
B)
2.0
done
clear
C)
0.3
done
clear
D)
1.7
done
clear
View Answer play_arrow
question_answer 69) The solubility product of\[Mg{{(OH)}_{2}}\]at\[25{}^\circ C\]is \[1.4\times {{10}^{-11}}\]. What is the solubility of\[Mg{{(OH)}^{2}}\]in g/L?
A)
0.0047 g/L
done
clear
B)
0.047 g/L
done
clear
C)
0.0087 g/L
done
clear
D)
0.087 g/L
done
clear
View Answer play_arrow
question_answer 70) \[Ni/N{{i}^{2+}}[1.0M]||A{{u}^{3+}}[1.0M]/Au\]where\[E{}^\circ \] for\[N{{i}^{2+}}/Ni\]is\[-0.25\,V\,{{E}^{o}}\]for\[A{{u}^{3+}}/Au\] is 0.150V) What is the emf of the cell?
A)
+ 0.4V
done
clear
B)
\[-1.75V\]
done
clear
C)
+ 1.25V
done
clear
D)
+ 1.75V
done
clear
View Answer play_arrow
question_answer 71) The heat of formation of\[C{{O}_{2}}\]is\[-393\text{ }kJ\text{ }mo{{l}^{-1}}\] The amount of heat evolved in the formation of 0.156 kg of\[C{{O}_{2}}\]
A)
\[-1393kJ\]
done
clear
B)
\[+1165.5\,kJ\]
done
clear
C)
\[+1275.9kJ\]
done
clear
D)
\[-1165.5\text{ }kJ\]
done
clear
View Answer play_arrow
question_answer 72) Which is used to obtain salicylic acid from phenol?
A)
\[CC{{l}_{4}}\]
done
clear
B)
\[CHC{{l}_{3}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
D)
\[CC{{l}_{2}}{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 73) Dehydration of glycerol produces
A)
propanone
done
clear
B)
allyl alcohol
done
clear
C)
glycol
done
clear
D)
acrolein
done
clear
View Answer play_arrow
question_answer 74) Which is the strongest acid?
A)
\[ClC{{H}_{2}}COOH\]
done
clear
B)
\[C{{H}_{3}}COOH\]
done
clear
C)
\[C{{l}_{2}}CHCOOH\]
done
clear
D)
\[CC{{l}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 75) \[C{{H}_{3}}Br+KCN(alco.)\xrightarrow{{}}X\xrightarrow[Na+{{C}_{2}}{{H}_{5}}OH]{\operatorname{Re}duction}Y\]. What is Y in the series?
A)
\[C{{H}_{3}}CN\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}CN\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 76) When fluorine reacts with hot and cone. \[NaOH,\]it gives
A)
\[N{{a}_{2}}O\]
done
clear
B)
\[Na\]
done
clear
C)
\[{{H}_{2}}\]
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 77) Which of the compound is optically active?
A)
1-butanol
done
clear
B)
Ethanol
done
clear
C)
2-butanol
done
clear
D)
Isopropyl alcohol
done
clear
View Answer play_arrow
question_answer 78) In acidic medium\[MnO_{4}^{-}\]is converted to\[M{{n}^{2+}}\]. The quantity of electricity in faraday required to reduce 0.5 mole of \[MnO_{4}^{-}\]to\[M{{n}^{2+}}\]would be
A)
2.5
done
clear
B)
1
done
clear
C)
5
done
clear
D)
0.5
done
clear
View Answer play_arrow
question_answer 79) During electrolysis of water the volume of oxygen liberated is\[2.24\text{ }d{{m}^{3}}\]. The volume of hydrogen liberated, under same conditions will be
A)
\[2.24\,d{{m}^{3}}\]
done
clear
B)
\[1.12\,d{{m}^{3}}\]
done
clear
C)
\[4.48\,d{{m}^{3}}\]
done
clear
D)
\[0.56\,d{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 80) Crystalline form of silica is called
A)
crystalline silicon
done
clear
B)
quartz
done
clear
C)
rock
done
clear
D)
talc
done
clear
View Answer play_arrow
question_answer 81) The elements commonly used for making transistors are
A)
Ga and In
done
clear
B)
C and Si
done
clear
C)
P and As
done
clear
D)
Si and Ge
done
clear
View Answer play_arrow
question_answer 82) 2-pentanone and 3-pentanone can be distinguished by
A)
cannizaro's reaction
done
clear
B)
iodoform reaction
done
clear
C)
aldol condensation
done
clear
D)
Clemmensen's reduction
done
clear
View Answer play_arrow
question_answer 83) Proteins are
A)
polypeptides with low molecular weights
done
clear
B)
polypeptides with high molecular weights
done
clear
C)
polymers of amides
done
clear
D)
polymers of secondary amines
done
clear
View Answer play_arrow
question_answer 84) End product of the hydrolysis of\[Xe{{F}_{6}}\]is
A)
\[XeO{{F}_{4}}\]
done
clear
B)
\[Xe{{O}_{2}}{{F}_{2}}\]
done
clear
C)
\[Xe{{O}_{3}}\]
done
clear
D)
\[Xe{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 85) Which method is not used for the extraction of\[Al\]?
A)
Van-Arkel
done
clear
B)
Serpeck
done
clear
C)
Baeyer
done
clear
D)
Hall-Heroult
done
clear
View Answer play_arrow
question_answer 86) The radiant energy from the sun is due to
A)
combustion
done
clear
B)
chemical reaction
done
clear
C)
nuclear fusion
done
clear
D)
nuclear fission
done
clear
View Answer play_arrow
question_answer 87) Which will give cannizaro reaction?
A)
\[C{{l}_{3}}C-CHO\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CHO\]
done
clear
C)
\[(C{{H}_{3}}){{ }_{3}}-C-CHO\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 88) Aluminium becomes passive on treatment with
A)
dil.\[HN{{O}_{3}}\]
done
clear
B)
cone.\[HN{{O}_{3}}\]
done
clear
C)
dil. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 89) Which is not a true peroxide?
A)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
B)
Caro's acid
done
clear
C)
Marshall's acid
done
clear
D)
Thiosulphuric acid
done
clear
View Answer play_arrow
question_answer 90)
The two isomers given below are
A)
enantiomers
done
clear
B)
diastereomers
done
clear
C)
mesomers
done
clear
D)
position isomers
done
clear
View Answer play_arrow
question_answer 91) \[C{{H}_{3}}CH{{I}_{2}}\xrightarrow{KCN}\xrightarrow{{{H}_{2}}O,\Delta }?\] Here the end product would be
A)
2-cyano propionic acid
done
clear
B)
ethane-1,1-dicarboxylic acid
done
clear
C)
2-methyl ethanoic acid
done
clear
D)
propionic acid
done
clear
View Answer play_arrow
question_answer 92) A lyophobic colloid is
A)
\[A{{s}_{2}}{{S}_{3}}\]
done
clear
B)
starch
done
clear
C)
agar-agar
done
clear
D)
gelatin
done
clear
View Answer play_arrow
question_answer 93) Which of the following is correct according to Freundlich adsorption isotherm?
A)
\[\frac{x}{m}\propto {{p}^{0}}\]
done
clear
B)
\[\frac{x}{m}\propto p\]
done
clear
C)
\[\frac{x}{m}\propto \frac{1}{p}\]
done
clear
D)
\[\frac{x}{m}\propto {{p}^{1/n}}\]
done
clear
View Answer play_arrow
question_answer 94) The rust of iron is catalysed by which of the following?
A)
\[Fe\]
done
clear
B)
\[{{O}_{2}}\]
done
clear
C)
\[Zn\]
done
clear
D)
\[{{H}^{+}}\]
done
clear
View Answer play_arrow
question_answer 95) An ester linkage is present in
A)
nylon-6
done
clear
B)
nylon-6,6
done
clear
C)
dacron
done
clear
D)
rayon
done
clear
View Answer play_arrow
question_answer 96) Williamson's synthesis involves
A)
\[{{S}_{N}}1\] mechanism
done
clear
B)
nucleophilic addition
done
clear
C)
\[{{S}_{N}}2\]mechanism
done
clear
D)
\[{{S}_{E}}\]mechanism
done
clear
View Answer play_arrow
question_answer 97) HF is not preserved in glass bottle because
A)
it reacts with the visible part of the light
done
clear
B)
it reacts with ^he sodium oxide of the glass
done
clear
C)
it reacts with the aluminium oxide of the glass composition
done
clear
D)
it reacts with\[Si{{O}_{2}}\]of the glass
done
clear
View Answer play_arrow
question_answer 98) Oils can be converted into fats by
A)
hydrogenolysis
done
clear
B)
hydrogenation
done
clear
C)
decarboxylation
done
clear
D)
hydration
done
clear
View Answer play_arrow
question_answer 99) The geometry of methane molecule is
A)
tetrahedral
done
clear
B)
pyramidal
done
clear
C)
octahedral
done
clear
D)
square planar
done
clear
View Answer play_arrow
question_answer 100) Heisenberg's uncertainty principle can be explained as
A)
\[\Delta x\ge \frac{\Delta p\times h}{4\pi }\]
done
clear
B)
\[\Delta x\times \Delta p\ge \frac{h}{4\pi }\]
done
clear
C)
\[\Delta x\times \Delta p\ge \frac{h}{\pi }\]
done
clear
D)
\[\Delta p\ge \frac{\pi h}{\Delta x}\]
done
clear
View Answer play_arrow
question_answer 101) The bond energy is the energy required to
A)
dissociate one mole of the substance
done
clear
B)
dissociate bond in 1 kg of the substance
done
clear
C)
break one mole of similar bonds
done
clear
D)
break bonds in one mole of the substance
done
clear
View Answer play_arrow
question_answer 102) Heat of neutralisation of an acid with a base is 13.7 kcal when
A)
both acid and base are weak
done
clear
B)
acid is weak and base is strong
done
clear
C)
both acid and base are strong
done
clear
D)
acid is strong and base is weak
done
clear
View Answer play_arrow
question_answer 103) Hess's law is based on
A)
law of conservation of mass
done
clear
B)
law of conservation of energy
done
clear
C)
enthalpy is a state function
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 104) In a lead storage battery, reaction at cathode is
A)
\[Pb+SO_{4}^{2-}\xrightarrow{{}}PbS{{O}_{4}}+2{{e}^{-}}\]
done
clear
B)
\[Pb+Pb{{O}_{2}}+{{H}_{2}}S{{O}_{4}}\] \[\xrightarrow{{}}2PbS{{O}_{4}}+2{{H}_{2}}O\]
done
clear
C)
\[PbS{{O}_{4}}+2{{e}^{-}}\xrightarrow[{}]{{}}pb+SO_{4}^{2-}\]
done
clear
D)
\[Pb{{O}_{2}}+4{{H}^{+}}+SO_{4}^{2-}+2{{e}^{-}}\] \[\xrightarrow[{}]{{}}pbS{{O}_{4}}+2{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 105) A buffer solution contains 500 mL of 0.2 M \[C{{H}_{3}}COONa\]and 500 mL of\[0.1\text{ }M\text{ }C{{H}_{3}}COOH\]. 1L of water is added to this buffer, pH before and after dilution will be (\[p{{k}_{a}}\] of\[C{{H}_{3}}COOH=4.74\])
A)
5.04, 3.74
done
clear
B)
5.04, 5.04
done
clear
C)
5.04, 4.89
done
clear
D)
9.56, 9.56
done
clear
View Answer play_arrow
question_answer 106) In coagulating the colloidal solution of\[A{{s}_{2}}{{S}_{3}}\] which has the minimum coagulating value?
A)
\[NaCl\]
done
clear
B)
\[KCl\]
done
clear
C)
\[BaC{{l}_{2}}\]
done
clear
D)
\[AlC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 107) What is binding energy in \[_{5}^{11}\] B nucleus, if mass is 0.08181 amu?
A)
\[1.2\times {{10}^{-4}}erg/nucleus\]
done
clear
B)
\[8.2\times {{10}^{-4}}erg/nucleus\]
done
clear
C)
\[10.2\times {{10}^{-4}}erg/nucleus\]
done
clear
D)
\[1.9\times {{10}^{-4}}erg/nucleus\]
done
clear
View Answer play_arrow
question_answer 108) Which radioactive element is being used in cancer therapy?
A)
\[_{27}^{60}Co\]
done
clear
B)
\[_{28}^{62}Ni\]
done
clear
C)
\[_{56}^{140}Ba\]
done
clear
D)
\[_{15}^{32}P\]
done
clear
View Answer play_arrow
question_answer 109) Among third row elements, the IP value
A)
irregularly changes from left to right across the third row
done
clear
B)
increases from left to right across the third row
done
clear
C)
decrease for\[Na\]to\[Ar\]
done
clear
D)
remains constant
done
clear
View Answer play_arrow
question_answer 110) Hydrofluoric acid shows hydrogen bonding due to
A)
high electron affinity of fluorine
done
clear
B)
high bond dissociation energy of fluorine
done
clear
C)
high electronegativity of fluorine
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 111) Which of the following products are formed when\[Si{{C}_{4}}\]vapour are passed over hot\[Mg\]?
A)
\[SiC{{l}_{2}}+MgC{{l}_{2}}\]
done
clear
B)
\[Si+MgC{{l}_{2}}\]
done
clear
C)
\[M{{g}_{2}}Si+MgC{{l}_{2}}\]
done
clear
D)
\[MgSiC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 112) Chloropicrin is used as an insecticide and has been used as tear war gas. The chloropicrin is
A)
\[C{{H}_{2}}ClCC{{l}_{3}}\]
done
clear
B)
\[CC{{l}_{3}}N{{O}_{2}}\]
done
clear
C)
\[C{{H}_{2}}(C{{H}_{3}})C{{H}_{2}}Cl\]
done
clear
D)
\[CH{{I}_{3}}\]
done
clear
View Answer play_arrow
question_answer 113)
A)
\[C{{H}_{3}}Cl\]
done
clear
B)
\[KOH+{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[KOH+{{C}_{2}}{{H}_{5}}SH\]
done
clear
D)
\[KOH+C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 114) Identify the product Y in the sequence\[C{{H}_{3}}CHO+C{{H}_{3}}MgI\xrightarrow{Ether}X\xrightarrow{{{H}_{2}}O/{{H}^{+}}}Y,\]is
A)
\[C{{H}_{3}}OH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}CHOH\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}COH\]
done
clear
View Answer play_arrow
question_answer 115) Two moles of acetic acid are heated with\[{{P}_{2}}{{O}_{5}}\] The product formed is
A)
2 moles of ethyl alcohol
done
clear
B)
formic anhydride
done
clear
C)
acetic anhydride
done
clear
D)
2 moles of methyl cyanide
done
clear
View Answer play_arrow
question_answer 116) The reaction,\[ROH+{{H}_{2}}C{{N}_{2}}\]in the presence of\[HB{{F}_{4}},\]gives the following product
A)
\[ROC{{H}_{3}}\]
done
clear
B)
\[RC{{H}_{2}}OH\]
done
clear
C)
\[ROHC{{N}_{2}}{{N}_{2}}\]
done
clear
D)
\[RC{{H}_{2}}C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 117) The conjugate base of \[(C{{H}_{3}}){{ }_{2}}\overset{+}{\mathop{N}}\,{{H}_{2}}\]is
A)
\[{{(C{{H}_{3}})}_{3}}N\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}NH\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}{{N}^{-}}\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}{{N}^{+}}\]
done
clear
View Answer play_arrow
question_answer 118) Lecithin is an example of
A)
carbohydrate
done
clear
B)
hormones
done
clear
C)
vitamins
done
clear
D)
phospholipid
done
clear
View Answer play_arrow
question_answer 119) Artificial silk is
A)
nylon-6
done
clear
B)
rayon
done
clear
C)
nylon 66
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 120) Which of the following is common example of fibres?
A)
Bakelite
done
clear
B)
Buna-S
done
clear
C)
Nylon-66
done
clear
D)
PVC
done
clear
View Answer play_arrow
question_answer 121) Curdling of milk in small intestine occurs doe to the action of
A)
trypsin
done
clear
B)
erypsin
done
clear
C)
rennin
done
clear
D)
chymotrypsin
done
clear
View Answer play_arrow
question_answer 122) ADH controls water permeability of
A)
collecting tube
done
clear
B)
distal convoluted tubule
done
clear
C)
proximal convoluted tubule
done
clear
D)
Bowman's capsule
done
clear
View Answer play_arrow
question_answer 123) Biomagnification refers to
A)
rapid growth due to excessive intake of nutrients
done
clear
B)
increase in population size
done
clear
C)
decrease in population size
done
clear
D)
increase in concentration of non-degrabable pollutants as they pass through food chain
done
clear
View Answer play_arrow
question_answer 124) During the conduction of nerve impulse, the action potential is the result of movement of
A)
\[N{{a}^{+}}\]from intracellular fluid to extracellular fluid
done
clear
B)
\[N{{a}^{+}}\] from extracellular fluid to intracellular fluid
done
clear
C)
\[N{{a}^{+}}\] towards both directions
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 125) The AIDS virus spreads through
A)
killer -T cells
done
clear
B)
helper T cells
done
clear
C)
suppressor T cells
done
clear
D)
carrier T cells
done
clear
View Answer play_arrow
question_answer 126) Apis dorsata refers to
A)
rock bee
done
clear
B)
little bee
done
clear
C)
Indian bee
done
clear
D)
European bee
done
clear
View Answer play_arrow
question_answer 127) Which are the longest cells in the body of man?
A)
Muscle cells of legs
done
clear
B)
Bone cells
done
clear
C)
Nerve cells
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 128) CO is more toxic than \[C{{O}_{2}}\]because
A)
it affects the nervous system
done
clear
B)
it damages lungs
done
clear
C)
it reduces the oxygen carrying capacity of haemoglobin
done
clear
D)
it forms acid with water
done
clear
View Answer play_arrow
question_answer 129) Leprosy is also called as
A)
Hansen's disease
done
clear
B)
sarcoma
done
clear
C)
slim disease
done
clear
D)
leukemia
done
clear
View Answer play_arrow
question_answer 130) Which of the following is not a character of Chordata?
A)
Dorsal tubular nerve cord
done
clear
B)
Pharyngeal gill slits
done
clear
C)
Presence of notochord
done
clear
D)
Presence of spinal cord
done
clear
View Answer play_arrow
question_answer 131) Vitamin essential for proper functioning of liver and clotting of blood is
A)
vitamin-K
done
clear
B)
vitamin-A
done
clear
C)
vitamin-E
done
clear
D)
vitamin-\[{{\text{B}}_{\text{12}}}\]
done
clear
View Answer play_arrow
question_answer 132) Cancer causing genes (oncogenes) were discovered by
A)
Watson and Crick
done
clear
B)
Beadle and Tatum
done
clear
C)
Bishop and Varamus
done
clear
D)
Temin and Baltimore
done
clear
View Answer play_arrow
question_answer 133) Beak is toothed in
A)
pelican
done
clear
B)
kiwi
done
clear
C)
ostrich
done
clear
D)
Archaeopteryx
done
clear
View Answer play_arrow
question_answer 134) The sliding filament theory of muscle contraction was proposed by
A)
A.F. Huxley and A.P. Pullman
done
clear
B)
A.F. Huxley and H.E. Huxley
done
clear
C)
B. Pullman and A.P. Huxley
done
clear
D)
A. Pullman and E. Pullman
done
clear
View Answer play_arrow
question_answer 135) Which one is uricotelic ?
A)
Frog and toads
done
clear
B)
Lizards and birds /cockroach
done
clear
C)
Cattle, monkey and man
done
clear
D)
Molluscs and teleost fishes
done
clear
View Answer play_arrow
question_answer 136) Interacting populations are
A)
symbiotic
done
clear
B)
mutualistic
done
clear
C)
parasitic
done
clear
D)
coevolved
done
clear
View Answer play_arrow
question_answer 137) salamandra atra is
A)
ovoviviparous
done
clear
B)
oviparous
done
clear
C)
sexually sterile
done
clear
D)
parthenogenetic
done
clear
View Answer play_arrow
question_answer 138) Flying fish is
A)
Torpedo
done
clear
B)
Scoliodon
done
clear
C)
Anguilla
done
clear
D)
Exocoetus
done
clear
View Answer play_arrow
question_answer 139) Tay Sach's disease is due to
A)
sex linked recessive gene
done
clear
B)
sex linked dominant gene
done
clear
C)
autosomal dominant gene
done
clear
D)
autosomal recessive gene
done
clear
View Answer play_arrow
question_answer 140) The earliest fossil form in phylogeny of horse is
A)
Merychippus
done
clear
B)
Mesohtppus
done
clear
C)
Eohippus
done
clear
D)
Equus
done
clear
View Answer play_arrow
question_answer 141) What is true about vein ?
A)
All veins carry deoxygenated blood
done
clear
B)
All veins cany oxygenated blood
done
clear
C)
They carry blood from organs towards hean
done
clear
D)
They carry blood from heart towards organs
done
clear
View Answer play_arrow
question_answer 142) Autosomes in humans are
A)
11 pairs
done
clear
B)
22 pairs
done
clear
C)
23 pairs
done
clear
D)
43 pairs
done
clear
View Answer play_arrow
question_answer 143) Haeckel's biogenetic law or recapitulation theory states that
A)
life history of an animal reflects evolutionary history of the same
done
clear
B)
progeny resembles parents
done
clear
C)
mutations are acquired characters
done
clear
D)
all organisms begin their life from zygote
done
clear
View Answer play_arrow
question_answer 144) A colour blind mother and normal father would have
A)
colour blind sons and normal/carrier daughters
done
clear
B)
colour blind sons and daughters
done
clear
C)
all colour blind
done
clear
D)
all normal
done
clear
View Answer play_arrow
question_answer 145) Cranial capacity of modem man is
A)
450-650 cc
done
clear
B)
600-1000 cc
done
clear
C)
900-1100 cc
done
clear
D)
1200-1600 cc
done
clear
View Answer play_arrow
question_answer 146) Inheritance of ABO blood groups illustrates
A)
polyploidy
done
clear
B)
multiple allelism
done
clear
C)
euploidy
done
clear
D)
dominance
done
clear
View Answer play_arrow
question_answer 147) The most primitive cell like chemical aggregates capable of growth and division were
A)
chemoautotrophs
done
clear
B)
eobionts
done
clear
C)
prokaryotes
done
clear
D)
microspheres
done
clear
View Answer play_arrow
question_answer 148) Name the ship on which Darwin got opportunity for a voyage of world.
A)
Titanic
done
clear
B)
Vikrant
done
clear
C)
Vectoria
done
clear
D)
Beagle
done
clear
View Answer play_arrow
question_answer 149) Which of the following are most abundant types of antibodies ?
A)
\[{{\text{I}}_{\text{g}}}\text{A}\]
done
clear
B)
\[{{\text{I}}_{\text{g}}}\text{E}\]
done
clear
C)
\[{{\text{I}}_{\text{g}}}\text{G}\]
done
clear
D)
\[{{\text{I}}_{\text{g}}}\text{M}\]
done
clear
View Answer play_arrow
question_answer 150) Acromegaly is due to hyper secretion of
A)
thyroxin
done
clear
B)
prolactin
done
clear
C)
insulin
done
clear
D)
growth hormone
done
clear
View Answer play_arrow
question_answer 151) Ruminate endosperm occurs in
A)
Annonaceae/Areca nut
done
clear
B)
Compositae
done
clear
C)
Cruciferae
done
clear
D)
Euphorbiaceae
done
clear
View Answer play_arrow
question_answer 152) The edible part of apple/pear is
A)
cotyledons
done
clear
B)
thalamus/receptacle
done
clear
C)
mesocarp
done
clear
D)
endocarp
done
clear
View Answer play_arrow
question_answer 153) In synapsis two homologous chromosomes are connected at
A)
centromeres
done
clear
B)
chromomeres
done
clear
C)
telomeres
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 154) Stages in proper sequence of prophase-I are
A)
zygotene, leptotene, pachytene, diakinesis
done
clear
B)
leptotene, zygotene, pachyiene, diplotene and diakinesis
done
clear
C)
leptotene, pachyiene, zygotene, diakinesis and diplotene
done
clear
D)
diplotene, diakinesis, pachytene, zygotene and leptotene
done
clear
View Answer play_arrow
question_answer 155) Plasmid is
A)
small extrachromosomal circular self replicating DNA that can carry genes into host organism
done
clear
B)
bacteriophage
done
clear
C)
DNA found in mitochondria
done
clear
D)
DNA incorporated in bacterial chromosome
done
clear
View Answer play_arrow
question_answer 156) A group of plants or animals with similar traits of any rank is
A)
species
done
clear
B)
genus
done
clear
C)
order
done
clear
D)
taxon
done
clear
View Answer play_arrow
question_answer 157) Fermentation products of yeast are
A)
\[{{H}_{2}}O+C{{O}_{2}}\]
done
clear
B)
Methyl alcohol \[+C{{O}_{2}}\]
done
clear
C)
Methyl alcohol + Water
done
clear
D)
Ethyl alcohol \[+C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 158) Grafting is not possible in monocots because they
A)
lack cambium
done
clear
B)
have scattered vascular bundles
done
clear
C)
have parallel venation
done
clear
D)
are herbaceous
done
clear
View Answer play_arrow
question_answer 159) What is true about a gamo spermous apospory?
A)
Formation of embryo from egg of embryo sac proliferated from a nucellar cell
done
clear
B)
Formation of embryo from egg of embryo sac formed directly from megaspore mother cell
done
clear
C)
Formation of embryo directly from nucellus
done
clear
D)
Formation of embryo directly from integument
done
clear
View Answer play_arrow
question_answer 160) RQ for fatty substance/fat is
A)
unity
done
clear
B)
less than one
done
clear
C)
more than one
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 161) Seed which are able to withstand reduction in moisture and temperature are called
A)
dormant seeds
done
clear
B)
recalcitrant seeds
done
clear
C)
orthodox seeds
done
clear
D)
non-viable seeds
done
clear
View Answer play_arrow
question_answer 162) Enzyme required for transcription is
A)
RNAse
done
clear
B)
Endonuclease
done
clear
C)
RNA polymerase
done
clear
D)
DNA polymerase
done
clear
View Answer play_arrow
question_answer 163) The synthetic hormone used as a weedicide is
A)
Indole 3-acetic acid
done
clear
B)
gibberellic acid
done
clear
C)
2-4 dichlorophenoxyacetic acid
done
clear
D)
maleic hydrazide
done
clear
View Answer play_arrow
question_answer 164) Which is present in monocot flowers ?
A)
Sepals
done
clear
B)
Petals
done
clear
C)
Tepals
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 165) Anthesis means
A)
growth of pollen tube inside the carpel
done
clear
B)
dehiscence of anthers
done
clear
C)
opening of floral bud
done
clear
D)
emergence of anthers
done
clear
View Answer play_arrow
question_answer 166) Which one of the following contain agranal chloropiasts ?
A)
\[{{C}_{3}}\]plants
done
clear
B)
Succulants
done
clear
C)
C4 plants
done
clear
D)
Hydrophytes
done
clear
View Answer play_arrow
question_answer 167) The process of multiplication of DNA from DNA is known as
A)
replication
done
clear
B)
transversion
done
clear
C)
transcription
done
clear
D)
translation
done
clear
View Answer play_arrow
question_answer 168) Phycobilins absorb light of wavelength
A)
670-700 nm
done
clear
B)
610-650 nm
done
clear
C)
500-650 nm
done
clear
D)
420-520 nm
done
clear
View Answer play_arrow
question_answer 169) For synthesis of one hexose sugar, how many times Calvin cycle is turned ?
A)
12
done
clear
B)
2
done
clear
C)
6
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 170) The number of chlorophyll molecules in a quantasomc is
A)
50-100
done
clear
B)
200-250
done
clear
C)
300-400
done
clear
D)
500-600
done
clear
View Answer play_arrow
question_answer 171) A plant having seeds but lacking flowers and fruits belong to
A)
pteridophytes
done
clear
B)
mosses
done
clear
C)
ferns
done
clear
D)
gymnosperms
done
clear
View Answer play_arrow
question_answer 172) Transpiration differ from evaporation in
A)
rate of water loss
done
clear
B)
transpiration is a physiological process whereas evaporation is physical process
done
clear
C)
transpiration is a physical process while evaporation is physiological process
done
clear
D)
frequency of water loss
done
clear
View Answer play_arrow
question_answer 173) Pigments common to all algae are
A)
chlorophyll-a and phycobilins
done
clear
B)
chlorophyll-a and carotenoids
done
clear
C)
chlorophyll-a and chlorophyll-b
done
clear
D)
chlorophyll-b and carotenoids
done
clear
View Answer play_arrow
question_answer 174) An antitranspirant is
A)
cobalt chloride
done
clear
B)
mercury
done
clear
C)
potassium
done
clear
D)
phenyl mercuric acetate
done
clear
View Answer play_arrow
question_answer 175) Crossing over in diploid organisms is responsible for
A)
dominance of genes
done
clear
B)
linkage between genes
done
clear
C)
recombination of linked genes
done
clear
D)
segregation of alleles
done
clear
View Answer play_arrow
question_answer 176) Active \[{{K}^{+}}\]exchange mechanism for opening and closing of stomata was given by
A)
Darwin
done
clear
B)
Levit
done
clear
C)
Scarth
done
clear
D)
Khorana
done
clear
View Answer play_arrow
question_answer 177) First geneticist, father of genetics was
A)
de Vries
done
clear
B)
Mendel
done
clear
C)
Darwin
done
clear
D)
Morgan
done
clear
View Answer play_arrow
question_answer 178) Quiescent centre is found in root apex and acts as
A)
permanent source of active initiates
done
clear
B)
reservoir of resistant cells
done
clear
C)
reservoir of passive cells to form new root apex if root apex is damaged/killed
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 179) Which type of meristems allow the fallen stem of cereals/grasses to become erect?
A)
Apical meristem
done
clear
B)
Lateral meristem
done
clear
C)
Intercalary meristem
done
clear
D)
Secondary meristem
done
clear
View Answer play_arrow
question_answer 180) A simple technology has been developed in India for plant breeders and fanners to use two plants as biofertilizers for growing rice. These are
A)
Azotobacter and Rhizobium
done
clear
B)
Chlorella and Spirulina
done
clear
C)
Azolla and nitrogen fixing blue-green algae
done
clear
D)
Riccia and Marc/ianria
done
clear
View Answer play_arrow
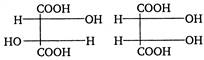
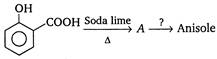 Reagent in second step should be
Reagent in second step should be
